Abstract
Adverse peer experiences, such as social exclusion, are known risks for socioemotional problems among shy youth. Yet, little is known about how shy children and adolescents process social exclusion in the brain and whether these responses are amplified in adolescence. Using the Cyberball task, we examined mediofrontal theta (4-7 Hz) event-related EEG spectral power during conditions of fair play and social exclusion in 122 participants (58 children, ages 10-12 years, and 64 adolescents, ages 14-17 years). Age effects of the task showed that adolescents displayed heightened theta power to both outright rejection and baseline “not my turn” events, whereas children showed higher theta power to rejection compared with “not my turn” events. Further results on individual differences showed that children with relatively higher levels of shyness displayed enhanced theta power to both rejection and “not my turn” events—a pattern that also was observed in adolescents. These findings suggest that a pattern of heightened neural sensitivity to both outright social exclusion and threats of exclusion, which is the norm by adolescence, also is observed in children with higher levels of shyness. The similar neural response pattern might be driven by salient social motivations that similarly modify the social cognition and behaviors of these groups and might reflect neural antecedents of rejection sensitivity.
Similar content being viewed by others
Shyness is a personality trait characterized by an anxious preoccupation with the self in social interactions (Cheek & Melchior, 1990). During social interactions, shy children often express more anxious behaviors (Asendorpf & Meier, 1993; Coplan et al., 2004) compared with non-shy children. Shyness is associated with adjustment problems, including social withdrawal and internalizing symptoms, in childhood and adolescence. Yet, studies examining peer experiences suggest that not all shy youth develop these problems and that peer victimization and exclusion experiences serve as potent risk factors (Boivin et al., 1995; Booth-LaForce et al., 2012; Booth-LaForce & Oxford, 2008; Gazelle & Ladd, 2003; Oh et al., 2008; Tang et al., 2017). However, relatively little is known about how shy youth process these social challenges. The extant literature has used sociometric ratings and parent and teacher reports to describe individual differences in rejected children, who often tend to be shy and socially withdrawn (Burgess et al., 2006; Chen et al., 2019; Gazelle & Ladd, 2003; Harrist et al., 1997). Few studies have measured real-time neural processes to social rejection. To gain insight into the individual differences and motivations underlying these neural processes, the present study examined whether shyness was associated with neural responses to social exclusion, and whether these associations differed across development in children and adolescents.
Prior work using laboratory-based social exclusion and evaluation tasks suggest that youth who are shy and behaviorally inhibited ([BI]—a temperament reflecting cautious and fearful behaviors to social situations; Tang et al., 2020) show heightened emotional and neural responses to negative evaluative and exclusion events, as well as unpredictable events that signal potential negative social feedback (Guyer et al., 2014; Howarth et al., 2013; Jarcho et al., 2016; Walker et al., 2014). Specifically, compared with other children, shy and BI children report being more emotionally upset when unexpectedly rejected (Howarth et al., 2013) and express more withdrawn and less assertive behaviors when excluded (Walker et al., 2014). Also, youth characterized by BI show greater activation in brain regions (dorsal anterior cingulate cortex, insula, and amygdala) for processing distress, uncertainty, and salience, when anticipating unpredictable social feedback and receiving negative evaluation (Jarcho et al., 2016). Although these prior studies have located brain regions for processing social rejection related to individual differences, these studies have only used functional magnetic resonance imaging (fMRI) (Guyer et al., 2014; Jarcho et al., 2016). The blood oxygen level-dependent (BOLD) signals in fMRI are delayed and dispersed in time and consequently cannot capture early and later cognitive stages of processing. To provide additional information about the temporal sequence of neural processes underlying different cognitive-affective functions, in this study, we relied on the superior temporal resolution of electroencephalogram (EEG), acquired during the widely used social exclusion task: Cyberball (Hartgerink et al., 2015).
Cyberball is a ball-toss game that uses a gaming format to mimic the experience of social inclusion and exclusion to capture associated cognitive-affective processes. Participants are told that they will play a ball-toss game online with two other players. In fact, there are no real players, because all the ball throws are programmed to include or exclude the participant. The game starts with an inclusion block, in which the ball can either be thrown to the participant (i.e., the “favor” condition) or to another player, meaning the participants would wait for their turn (i.e., the “not my turn” condition). The inclusion block runs for several minutes, leading participants to build up the expectation that they are included. Seamlessly, the game transitions into an exclusion block during which the ball will not be thrown to the participant again; therefore the participant is excluded (i.e., the “rejection” condition). While behavioral ratings of emotional distress are typically used to quantify the effect of social exclusion, as participants across hundreds of studies report being excluded in Cyberball is mildly distressing (Hartgerink et al., 2015), further applying EEG methods can provide at least two important insights: (a) the identification of neurophysiological correlates at early and later processing stages of social exclusion, which have different cognitive-affective functions; and (b) how individual differences (e.g., personality, age, and clinical features) shape these neurophysiological correlates, which might explain why some individuals are hypersensitive to social exclusion (Kujawa & Burkhouse, 2017; McPartland et al., 2011).
Prior Cyberball studies have identified a sequence of event-related potentials (ERPs) in processing social exclusion, including an early negativity (i.e., an N2 at ~200 ms) involved in the detection of exclusion, followed by positive potentials (i.e., a P3 ~300 ms, and a late positive potential ~450 ms) involved in the appraisal and regulation of subjective emotional distress (Crowley et al., 2010; Themanson et al., 2013; van Noordt et al., 2015; Wang et al., 2017; White et al., 2012). Further studies using event-related EEG oscillations examining spectral power of the theta (4-7 Hz) frequency band for its involvement in emotion processing (Bekkedal et al., 2011; Knyazev, 2007; Luu et al., 2003) also suggest that distinct cognitive-affective functions underly theta across the time course in processing social exclusion. Specifically, theta power in mediofrontal sites at later processing stages (400-800 ms) is thought to underly the appraisal of and regulation to social exclusion, because it is increased during exclusion compared to inclusion conditions (Cristofori et al., 2012; Tang et al., 2019; van Noordt et al., 2015). In contrast, at an earlier stage of processing (200-400 ms), mediofrontal theta is involved in monitoring social involvement and tracking expectancy violation as this earlier theta is increased when children and adults receive unexpected rejection feedback (Harrewijn et al., 2018; Morales et al., 2019; Van der Molen et al., 2017). This earlier theta underlying expectancy violation is relevant to Cyberball, because participants’ expectations of inclusion in the ball-tosses are violated when they no longer receive the ball in the exclusion block (Crowley et al., 2010; Themanson et al., 2013).
Beyond neural correlates of social exclusion, the developmental transition to adolescence is important to consider. Adolescence is a period in which peers have strong influence on adolescents’ emotions and behaviors; during this time, peer rejection is highly salient (Foulkes & Blakemore, 2018; Guyer et al., 2016; Michiel Westenberg et al., 2004). Indeed, studies report that adolescents show exaggerated emotional, neural, and physiological responses when rejected during social evaluation and exclusion tasks compared with children and adults (Abrams et al., 2011; Sebastian et al., 2010; Stroud et al., 2009; Tang et al., 2019; van den Bos et al., 2014). This research includes prior work comparing theta EEG oscillations using Cyberball in typical children, adolescents, and adults (Tang et al., 2019). Specifically, adolescents show heightened theta power to both rejection and “not my turn” compared to inclusion events. Such heightened responses reflect the considerable sensitivity to contexts involving outright exclusion and potential exclusion during adolescence. In contrast, children are not sensitive to potential exclusion, as they show higher theta power to rejection events compared to “not my turn” events. Nevertheless, this prior work was based on a group-approach analysis, which focused on age differences. It remains unknown whether salient motivations in adolescence, particularly the need for peer acceptance, would modulate exaggerated responses to social exclusion among shy individuals or whether all adolescents would display similar responses because of a normative peak in the need for peer acceptance.
In the present study, we extended a recent previous report in which the authors examined age-related differences in the neural and behavioral responses to social exclusion in Cyberball, independent of individual differences (Tang et al., 2019). The present study used the same sample of children (ages 10-12 years) and adolescents (ages 14-17 years) from Tang et al. (2019) to examine associations between individual differences in shyness and theta EEG oscillations during social exclusion and whether these relations were moderated by age. We focused on children and adolescents and collected the same shyness measures, as these groups would capture transitional changes from late childhood to adolescence. Also, we focused on mediofrontal theta event-related spectral perturbation (ERSP) to rejection events, representing outright exclusion, and “not my turn” events, representing potential exclusion. Both events involve processing information about one’s involvement in the game and could reveal whether individual differences in shyness and age modulate similar or different neural responses across these situations. We hypothesized that shy children and adolescents would both show greater theta ERSP to rejection and not my turn events, and report greater distress to Cyberball (Coplan et al., 2004; Howarth et al., 2013; Jarcho et al., 2016; Walker et al., 2014), although these relations would be stronger in adolescence compared with childhood.
Method
Participants
This report included 122 participants: 58 children (age range = 10-12 years; M age = 10.83 years; SD = 0.82; 20 males) and 64 adolescents (age range = 14-17 years; M age = 15.02 years; SD = 0.98; 25 males). The discreet age groups were determined before participant recruitment to capture distinct developmental periods; as such, there were no 13-year-olds. Participants were recruited from a database containing birth records of children born at the McMaster University Medical Center and St. Joseph’s Hospital. Three 17-year-olds were undergraduate students recruited from Psychology courses. This sample of youth was primarily White, 91.8%. Participants reported no history of neurological disorders or previous brain injuries. Participant sex was proportionately distributed across age groups, χ2(1) = 0.86, p = 0.354. Consent was obtained from parents, and assent was obtained from children and adolescents. This study was conducted with approval from the McMaster University Research Ethics Board. Participants received $30 for their participation.
Sample size was determined using Monte Carlo simulation-based power analyses in MPlus, version 8 (Muthén & Muthén, 2002). For a simple moderation model with two covariates and an interaction term as predictors of a continuous outcome, 10,000 replications were drawn, and sample size attrition (20% missingness of EEG data) was taken into account. Results indicated that the secured sample size has a minimum power of 0.81 to detect standardized moderation effects of at least β = 0.30. A prior fMRI study, which examined 128 adolescents (ages 8-18 years) using a social evaluation task, suggested that the interactive effect between age and social anxiety on brain activity to negative evaluative feedback was small to moderate (e.g., r’s = 0.32 to 0.36 in younger adolescents and r’s = −0.22 in older adolescents), whereas the effect on behavioral responses in that task was near zero (i.e., ηp2 = 0.003-0.008) (Smith et al., 2020).
Shyness Measures
Children and adolescents’ shyness were self- and parent-reported using the revised Early Adolescent Temperament Questionnaire (Capaldi & Rothbart, 1992). Items in the shyness scale include, "I tend to be shy." Items were scored on a 5-point scale (1 = almost always untrue; 5 = almost always true). Internal consistency for the shyness scale in this sample was α = 0.79 for self-report and α = 0.88 for parent-report. Because self- and parent reports of shyness were correlated, r(117) = 0.47, p < 0.001, the two scores were averaged to provide a multi-informant score of shyness used in further analyses. Shyness scores ranged from 1.23 to 4.57 in children and from 1.00 to 4.28 in adolescents. A nonparametric Mann-Whitney U test suggested that the distributions of shyness scores do not differ between the age groups, p = 0.702.
Social Exclusion Task: Cyberball
Participants were led to believe that they would play an online ball-toss game, Cyberball, with two other players, who were playing in other laboratories. In this game, the participant and players passed a ball amongst themselves, but unbeknownst to the participant, the game was computerized with two other virtual players. Before the game, an experimenter took a photograph of the participant to use in the game. While the participant sat in front of a computer, one experimenter pretended to telephone call other laboratories and told the second experimenter that the other players were ready. The participant provided his/her age, sex, and ethnicity, which were automatically used by the computer program to match the other players on the screen with similar age, ethnic appearance, and sex by drawing from a bank of stock photos of opponent pictures. Next, a Google™ webpage appeared, followed by a "Cyberball" webpage, and a screen with a “loading” status bar. A female voice narrated the instructions, asking the participant to choose one of the six gloves and two opponents.
When the game began, the participant's glove was at the bottom center of the screen, whereas the gloves of the other two players were at the top left and right corners of the screen. Pictures of the participant and other players appeared above their names and gloves. Participants were instructed to use their right index and middle fingers to respond by pressing 1 and 4 on the number panel of the keyboard to pass to the right- and left-side players on the screen. Each trial began with the ball in the glove of one of the two players for 1,000 to 3,000 ms. Before each throw event, the ball disappeared for 500 ms. Then, a yellow outline cue around the glove was displayed for 500 ms, signaling that a throw would occur. EEG was time-locked to the reappearance of the ball en route to the receiving player and its color changed to yellow or orange, differentiating throws to the participant or to the other players. Visualization of the sequence of events have been reported in van Noordt et al. (2015).
A total of 155 trials were divided into fair play and exclusion blocks. The fair play block consisted of 108 trials divided into three types of events: (1) in "not my turn" events (36 times), the virtual players threw the ball at each other but not to the participant; (2) in "favor" events (36 times), the virtual players threw the ball to the participant; and (3) the participant threw back to the other players (36 times). The frequency in which the ball was thrown to the participant during any trial was pseudo-random and predetermined, such that the participant waited 0 to 3 throws by the other players before receiving the ball again. Seamlessly, the fair play block transitioned into the exclusion block with 47 trials, in which the virtual players passed the ball to each other and not to the participant. During the exclusion block, the ball was passed to the participant only three times to maintain attention to task. Only 36 "rejection" events in the exclusion block were used in EEG analyses (excluding the first 5 trials and 3 throws to the participant).
Cyberball Distress
Immediately after the game, participants followed instructions given by the female voice that narrated items from the Need Threat Scale (Van Beest & Williams, 2006) to select their answers using the mouse. This scale is a reliable and valid measure that included 21 items to evaluate feelings of distress to being ostracized with four dimensions: belonging, self-esteem, meaningful existence, and control (Crowley et al., 2010; Van Beest & Williams, 2006). Items include: "I felt rejected" and "I felt invisible." Items were rated on a 5-point scale (1 = not at all; 5 = extremely/a lot). The sum of the four scales provided an index of ostracism distress during Cyberball. Internal consistency for this measure in this sample was α = 0.93.
EEG Data Collection and Analyses
EEG recording and preprocessing
Continuous EEG data were collected using a high-density 128 electrode Hydrocel net (Electrical Geodesics, Inc. (EGI)) with Netstation (EGI) and high impedance amplifiers, sampled at 250 Hz (0.1-Hz high pass, 100-Hz low pass). All electrodes were referenced to the central (Cz) scalp site for recording. Impedances at or below 40 kΩ were considered acceptable. Stimulus presentation was delivered using E-prime (Psychology Software Tools, Inc.).
EEG data were preprocessed offline in Netstation through a 0.1-Hz high-pass filter and a 30-Hz low-pass filter. Continuous EEG data were segmented into 2,000-ms epochs with a 700-ms prestimulus baseline and a 1,300-ms poststimulus interval. ERPs were time-locked to the reappearance of the ball on the path to the participant or the player after leaving the other player’s glove. Eye channels were visually inspected, and channels that likely had poor connection to the scalp, either flat (no EEG signal) or those that likely reflected white noise, were manually marked as contaminated and interpolated by surrounding channels. Automatic artifact rejection removed segments that contained extreme voltage fluctuations (>200 μV). Epochs with eye blinks or eye movements (>150 μV) were rejected. Epochs with more than 10 bad channels (40% or more segments marked as bad) also were rejected. The remaining bad channels were replaced by interpolating from surrounding channels. Statistical eye-blink removal was applied (blink slope threshold = 14 μV/ms) (Gratton et al., 1983). The single trial data were re-referenced from Cz to an average reference of all electrodes (Junghöfer et al., 1999).
Children’s EEG responses are more susceptible to artifacts for various reasons, including more body movements, less patience, and attention span for repetitive trials and the EEG net (Brooker et al., 2020). In this sample, children had less artifact-free trials in every condition (M number of artifact-free trials: 21.76 favor events, 21.33 not my turn events, 17.16 rejection events) compared to adolescents (M number of artifact-free trials: 27.13 favor events, 26.81 not my turn events, 25.13 rejection events), p’s < 0.05. However, the number of artifact-free trials was not related to shyness in all conditions, p’s > 0.05. Participants who had at least 10 artifact-free trials per condition were included in further EEG analysis; 12 children and 6 adolescents were excluded due to inadequate number of trials. Additionally, 2 participants did not have EEG recordings due to technical problems, 2 participants did not complete the task, and 2 participants were excluded for left-handedness. This resulted in 98 participants (42 children and 56 adolescents) in further EEG analyses. Our previous report showed that the usable EEG data have reliable signal-to-noise ratios (>2) (Tang et al., 2019).
EEG Time-frequency Decomposition
Theta (4-7 Hz) ERSP was examined in EEGLab version 13.4.4b, MATLab version R2013a. The "new timef" function in EEGLab uses algorithms for implementing a time X frequency spectrogram (Delorme & Makeig, 2004). ERSP calculations relied on fast Fourier Transform at the lowest frequency and wavelet decomposition at the highest frequency. The standard settings in EEGLab were used, cycles set as 3, 0.5, cycles linearly increase with frequency from 0 for fast Fourier Transform (same window width at all frequencies) to 1 for wavelet (same number of cycles at all frequencies). The software uses 3 cycles at the lowest frequency to 11.25 cycles at the highest frequency. The time-frequency decomposition yields a time X frequency transform with a complex number for every time point, frequency, and trial. ERSP was obtained at 53 linear-spaced frequencies, 4 to 30 Hz, and 290 linear-spaced time points from −280 to 880 ms relative to the stimulus onset. ERSP measures were derived every 4 ms and every 0.5 Hz.
Based on the spatial and temporal locations of theta oscillations to social exclusion/rejection reported in prior studies of children and adolescents (Morales et al., 2019; Tang et al., 2019; van Noordt et al., 2015), we extracted mean ERSP values from the precomputed matrices between 200 to 400 and 400 to 600 ms for the theta (4-7 Hz) frequency band in a frontocentral cluster (FCz, mean of electrodes 6, 7, 106, 129) (Fig. 1a). We focused on theta power to rejection and used not my turn events as a baseline, because both types of events are locked to the same stimuli and require no motor response. The only difference is the change in context from inclusion to exclusion. In sensitivity analyses, we also tested theta power in a left frontal cluster, (F3, mean of electrodes 18, 19, 22, 23, 24) and a right parietal cluster P4 (mean of electrodes 86, 92, 93, 98), which have been observed in our prior work (Tang et al., 2019).Footnote 1
Data Analyses
Main Analysis
Multiple-group univariate latent change score models (Kievit et al., 2018) were performed to test the study’s hypotheses in the R software package, Lavaan (Rosseel, 2012). Multiple-group analysis is a well-established method to examine moderation effects in structural equation modeling. Figure 2a shows the conceptual model and separate models were conducted for theta power at the early (200-400 ms) and later (400-600 ms) processing stages. A latent factor measured the condition difference in theta power to rejection and theta power to “not my turn” as the baseline. To measure the difference, the path from rejection theta to the latent factor and the autoregressive path between rejection and not my turn theta were fixed to one; the intercept of rejection theta was set to zero. The covariance between not my turn theta (i.e., the baseline) and the latent factor captures the degree to which change is dependent on the baseline (i.e., not my turn). Shyness was added into the model as a predictor of the latent difference score. To examine whether the associations between shyness and theta power were moderated by age, age group was modeled as a grouping variable.
We used χ2 difference tests to evaluate whether age groups differed in the path of interest (i.e., the effect of shyness on the theta difference score). This involved comparing χ2 values between a null model, in which the path coefficients of interest were constrained to be equal between age groups, and an alternative model, in which all path coefficients were freely estimated. A significant difference in χ2 values (Δχ2) between the null and alternative models is evidence that the effect of shyness on neural responses was moderated by age group. Furthermore, to examine whether the association between shyness and ratings of distress was moderated by age, a multiple-group regression model was performed, with distress regressed on shyness and age group as the grouping variable to examine the moderation by age (Fig. 2b). To test the effect of shyness on distress, a χ2 difference test on that key path was followed.
In all models, we adjusted for sex and mother’s highest level of education, and allowed predictors to covary. All models are just-identified; thus, we do not report fit indices. Full information maximum likelihood (FIML) estimation was used to handle missing data. FIML reduces potential bias in parameter estimates due to missing data and includes all available data (Enders & Bandalos, 2001). Robust standard errors were used to account for potential skewness and kurtosis in variables (Yuan & Bentler, 2000).
Sensitivity analysis
To test whether shyness and age group interacted to predict theta power in other scalp clusters (F3 and P4), we repeated the multiple-group univariate latent change score models using theta power extracted from these scalp clusters.
Exploratory analysis
Given that anxiety tends to be more prevalent among females than males, we also used χ2 difference tests to test two-way interactions between sex and age group in the multiple-group latent change score and regression models for theta power and distress ratings. Additionally, to test three-way interactions among sex, shyness, and age group, we repeated our multiple group latent change score and regression models by adding a sex X shyness term. The interaction was tested with χ2 difference tests.
Of the total sample (N = 122), 119 participants (97.5%) had data on shyness. For Cyberball measures, 120 participants (98.4%) had complete subjective responses of distress, and 98 participants (80.3%) had usable EEG responses. Compared with participants with complete data, those with missing data were not different in shyness (p = 0.685), sex (p = 0.400), mothers’ education level (p = 0.712), or age group (p = 0.112).
Results
Preliminary Analyses
Descriptive Statistics
Sample characteristics of children and adolescents are shown in Table 1. The mothers of children were more highly educated compared with those of adolescents. There were no age group differences in shyness or ratings of distress to the task.
Task Effect on Mediofrontal Theta Power
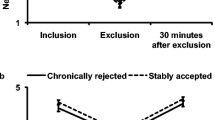
As previously reported in this sample (Tang et al., 2019), a repeated-measures ANOVA with within-subject factors of condition (not my turn, rejection) and time (200-400 ms; 400-600 ms) and between subjects factors of age groups (children, adolescents) showed a three-way condition X time X age group interaction, F(1,96) = 4.98, p = 0.028, ηp2 = 0.05. Follow-up repeated-measures ANOVAs showed a condition X time interaction in children, F(1,41) = 6.14, p = 0.017, ηp2 = 0.13, but not in adolescents, F(1,55) = 0.21, p = 0.653, ηp2 = 0.004. Figure 1b shows the within- and between-group differences. Within-group contrasts suggest that children elicit greater theta power to rejection compared with not my turn events at 400 to 600 ms, but no differences at 200 to 400 ms. Adolescents showed comparable levels of theta power to both conditions at 200 to 400 and 400 to 600 ms. Further between-group contrasts showed that adolescents elicited greater theta power to rejection at 200-400 ms compared with children, t(96) = −1.97, p = 0.052. These results suggest that adolescents show similar sensitivity, manifest as heightened theta power, to both not my turn and rejection conditions. In contrast, children distinguish the two conditions with greater theta power at later processing stages elicited to rejection compared with not my turn events.
Age as a Moderator on the Relation between Shyness and Responses to Social Exclusion
Mediofrontal Theta power
Regression coefficients from the latent change score models for latent theta differences between rejection and not my turn conditions at FCz in earlier (200-400 ms) and later (400-600 ms) processing stages are shown in Tables 2 and 3, respectively. In the earlier processing stage, no interaction between shyness and age group emerged, Δχ2(1) = 0.36, p = 0.551 (Table 2; Fig. 3). Also, within-group estimates revealed no effect of shyness on latent mediofrontal theta difference scores in children (β = −0.12, p = 0.291) or adolescents (β = −0.03, p = 0.808). In the later processing stage, shyness interacted with age group to predict latent mediofrontal theta difference scores, Δχ2(1) = 5.18, p = 0.023 (Table 3; Fig. 3). Within-group estimates showed that shyness was inversely associated with theta difference scores in children (β = −0.33, p = 0.007), but not in adolescents (β = −0.01, p = 0.958). Figure 3 shows the interaction, whereby children with higher levels of shyness elicited a lower theta difference score that approximated zero (i.e., no difference in theta power between rejection and not my turn conditions) and the confidence intervals overlapped with adolescents. Contrasting with our hypothesis that shyness in adolescence would amplify neural responses to potential and outright exclusion, these results instead suggest a maturational shift in shy children who displayed similar levels of theta power across rejection and not my turn events, a pattern that was observed in the group of adolescents.
Distress
Table 4 shows regression coefficients in predicting distress. Contrasting with our hypothesis that shyness would be associated with higher ratings of distress, there were no significant main effects of shyness on distress in children (β = −0.02, p = 0.903) or adolescents (β = 0.08, p = 0.454). Also, no significant interaction between shyness and age group emerged, Δχ2(1) = 0.35, p = 0.553 (Fig. 3).
Associations between Neural and Behavioral Responses and Shyness
To further examine whether theta power was associated with subjective ratings of distress and shyness, latent difference scores between mediofrontal theta to rejection and not my turn events were extracted from the models and Spearman correlations were performed. As seen in Table 5, within the adolescents, distress correlated with latent mediofrontal theta difference scores at 200 to 400 ms (r = 0.27, p = 0.046), but not at 400-600 ms (r = 0.17, p = 0.214). In contrast, these relations were not observed in children (r’s = 0.12, p’s = 0.462-0.464). In terms of relations with shyness, the results showed that shyness was related to lower latent difference scores in theta at FCz at 400-600 ms in children, (r = −0.32, p = 0.040), but not in adolescents (r = −0.01, p = 0.968). These results are consistent with the prior latent change score analysis. Again, inconsistent with our hypothesis that distress would be related to shyness, shyness was not significantly correlated with distress in either age groups, although this correlation in adolescents was in the positive direction (r = 0.15, p = 0.255).
Sensitivity Analyses
Sensitivity analyses further tested our hypotheses for theta power in other scalp sites, including a F3 and a P4 cluster. In terms of age differences in task effects, separate repeated measures ANOVAs, including within-subject factors of time (200-400 and 400-600 ms) and condition (not my turn, rejection) and a between-subjects factor of age group (children, adolescents), indicated no three-way interaction for theta at F3, F(1, 96) = 1.03, p = 0.313, ηp2 = 0.01, and P4, F(1,96) = 0.05, p = 0.824, ηp2 = 0.001, clusters. The latent change score models examining interactions between shyness and age group also did not reveal significant interactions for theta at F3 or P4 clusters at 200-400 ms (Table 2) or 400-600 ms (Table 3). Correlations between latent theta difference scores did not show significant correlations with shyness and distress, with the exception that latent theta difference scores at 200-400 ms at P4 was related to distress ratings in children (r = 0.31, p = 0.047).
Exploratory Analyses
Exploratory analyses further tested potential sex interactions. In testing two-way sex X age group interactions, the results showed no significant interactions for latent theta difference scores at FCz, nor at F3 and P4 (Tables 2 and 3), although a significant interaction appeared for distress Δχ2(1) = 4.15, p = 0.041 (Table 4). This interaction showed that female adolescents reported higher levels of distress than male adolescents, β = 0.37, p = 0.002. In contrast, sex had no effect in children, β = 0.06, p = 0.664. Furthermore, there were no significant three-way sex X shyness X age group interactions on theta power or distress (Supplemental Tables 1-3).
Discussion
Our recent prior report showed that adolescents were sensitive to both social exclusion and events that signal potential exclusion, as they display high theta power to both rejection and not my turn events (Tang et al., 2019). In contrast, children process these events differently in the brain with higher theta power elicited to rejection compared to not my turn events. The current study extended these age-related differences by examining whether individual differences in shyness were related to neural responses to social exclusion in children and adolescents. Contrary to our hypothesis, our results showed that shyness had no effect on adolescents’ theta responses to social exclusion. However, children with higher levels of shyness elicited similar levels of mediofrontal theta power to rejection and not my turn events at the later processing stage (400-600 ms), manifesting as a lower theta difference score across conditions. This pattern of brain activity was similarly observed in the group of adolescents. Altogether, these findings suggest that shyness by late childhood (ages 10-12 years) modulates heighted neural sensitivity to both outright social exclusion and potential exclusion—a normative pattern that is present by adolescence.
The finding that children with higher levels of shyness showed similar mediofrontal theta power at 400 to 600 ms to both rejection and not my turn events extends prior work examining the influences of shyness on social cognition by showing this relation in the context of social exclusion. This finding is consistent with studies reporting that shy children and preadolescents engage in heightened physiological and neural processing of ambiguous social cues, situations that signal potential negative social feedback, as well as situations involving outright rejection (Buzzell et al., 2017; Gazelle & Druhen, 2009; Jarcho et al., 2016; Morales et al., 2015; Pérez-Edgar et al., 2010; Poole & Schmidt, 2021; Wilson & Henderson, 2020). This result also converges with studies reporting cognitive biases in shy children, because they tend to negatively interpret and ruminate about social interactions (Coplan et al., 2013; LoBue & Pérez-Edgar, 2014).
Interestingly, the pattern of mediofrontal theta power to rejection and not my turn events in children with higher levels of shyness was also observed in adolescents. This similarity might reflect similar social fears that adolescents and children with higher levels of shyness are preoccupied with. In that, heightened social fears, such as fear of negative evaluation, are features of shyness. Similarly, studies suggest that adolescence is characterized by a normative developmental peak in social fears (Westenberg et al., 2004; Sumter, Bokhorst, & Westenberg, 2009) and the need for social acceptance (Steinberg & Monahan, 2007), which parallel with the growth of social networks (Lamblin et al., 2017) and functional neurodevelopment linked to changes in social cognition (Blakemore & Mills, 2014; Crone & Dahl, 2012). We speculate that similar theta response patterns at the later processing stage (400-600 ms) observed in the group of adolescents and children with relatively higher levels of shyness might have functional roles in appraising negative cues, and/or regulating distress. Support for this interpretation also is based on studies using other experimental tasks that indicate theta power at later processing stages is enhanced during aversive experiences, including undesirable outcomes (Cohen et al., 2007), unpleasant feelings (Luu et al., 2003; Vecchiato et al., 2011), unsuccessful avoidance of unpleasant stimuli (van Noordt et al., 2018), and during cognitive reappraisal of negative emotional states (Ertl et al., 2013). As such, both children who are shyer and the group of adolescents might be appraising potential and outright exclusion events as negative events.
In contrast, we did not observe significant interactive effects between age groups and shyness on mediofrontal theta at the earlier processing stage (200-400 ms). These findings highlight the functional differences in theta across the time course, as theta at the earlier processing stage reflects attentional systems that monitor social involvement in Cyberball and has been observed when expectations of social acceptance are violated (Harrewijn et al., 2018; Morales et al., 2019; Van der Molen et al., 2017). We speculate this earlier attentional process might facilitate further appraisal of and behavioral adaptations to social exclusion. Our results also showed that mediofrontal latent theta difference scores were significantly correlated with distress at 200-400 ms, but not 400-600 ms, in adolescents. This suggests that greater attentional resources directed at differentiating rejection from not my turn events are related to emotional distress in the adolescents. In children, however, there were no significant correlations between distress and mediofrontal theta, although sensitivity analyses suggested that distress was related to latent theta difference scores at the P4 cluster at 200-400 ms. Presently, it is unclear whether this posterior scalp region reflects the same neural process in monitoring social involvement or a different neural process in children.
Moreover, we did not find significant relations between shyness and distress nor interactions between shyness and age group for distress, which contrast with our hypothesis. Considering that distress is linked to neural processes underlying latent mediofrontal theta differences at 200-400 ms, these null associations between shyness and distress could mean that shyness is not sensitive to neural monitoring of social involvement at these ages. Alternatively, the null associations might be due to a lack of elements that threaten how the participant is perceived, because the online ball-toss game did not involve introductions to other players or knowing anything about the other players and vice versa. As such, there might be less social incentive for shy individuals to monitor their social involvement, producing little variability in distress ratings and theta differences at the earlier processing stage.
Furthermore, we found no evidence to support the hypothesis that shyness might be more strongly related to heightened responses to social exclusion in adolescence compared with childhood. Instead, the null effect of shyness on neural and behavioral responses in adolescents suggests that salient social motivations during adolescence might induce similar responses to social exclusion across individuals and mask the effect of shyness. These findings are consistent with a recent study that found socially anxious youth younger than age 13 years were more sensitive to unpredictable rejection feedback, manifested as greater activation in brain regions for social cognition (caudate, medial, and superior temporal gyri) (Smith et al., 2020). In contrast, social anxiety in older adolescents (older than age 13 years) had no effect as adolescents’ brain responses were similar. Alternatively, these null effects of shyness in adolescence could be because our modest sample size was unable to capture enough variability in our shyness measure or enough individuals who were extremely shy. Nevertheless, studies examining individual differences in combination with developmental differences in the processing of social exclusion are lacking. The work on social exclusion/rejection related to shyness and BI have been largely limited to childhood or preadolescence (Howarth et al., 2013; Jarcho et al., 2016; Walker et al., 2014), and other work has focused on the relevance of negative peer environments, such as a history of peer rejection experiences (Rudolph et al., 2016; Will et al., 2016). Future work is needed to understand how individual differences in personality and positive aspects of the peer environment, such as supportive friendships, might modulate brain function to these social challenges across development. Given that peer exclusion experiences and rejection sensitivity are linked to the development of internalizing problems among shy and socially withdrawn children (Boivin et al., 1995; Booth-LaForce et al., 2012; Booth-LaForce & Oxford, 2008; Gazelle & Ladd, 2003; Oh et al., 2008; Tang et al., 2017), future longitudinal work also should examine whether mediofrontal theta power in processing social exclusion might be a mechanism that contributes to later social and emotional functioning. For example, one study using fMRI found that heightened insular activity to unpredictable social evaluative feedback mediates the relation between stable childhood social reticence and social anxiety symptoms in adolescence (Clarkson et al., 2019). Heightened neural sensitivity to social rejection feedback also has been observed in adolescents with depression compared to controls (Silk et al., 2014).
Our study should be considered in light of its strengths and limitations. Strengths include a social exclusion task mirroring real-life rejection conducted in a controlled laboratory setting to assess changes in real-time brain processes and examination of different age groups at salient social developmental stages. There were, however, several limitations. First, although we tried to reduce informant bias by using reports from both parents and youth, we used subjective measures of shyness. Second, we used a cross-sectional design and collected concurrent measures of shyness and neural responses. This design limits inferences on causal relations and mechanisms. Hence, our findings should be interpreted as nondirectional associations. Third, our participants were demographically homogenous in terms of ethnicity as they were primarily white, which limits the generalizability of our results. Fourth, although we determined that our sample size can detect modest two-way interaction effects (β = 0.30), we may not have power to detect smaller effect sizes. Future studies using a longitudinal design with observational measures of shyness and a demographically diverse sample would increase the generalizability of the current findings. Future studies also should use larger sample sizes to increase variability on shyness measures and examine potential interactions with sex.
Conclusions
This study provides evidence demonstrating that the influence of shyness on neural processing of social exclusion is different across development. Older adolescents and shy preadolescents demonstrate a similar pattern of enhanced neural responses to situations that involve outright social exclusion and situations that signal potential social exclusion. These similar neural response patterns might be driven by salient social fears and motivations that are apparent by adolescence in general but also are present among preadolescents who are shyer, suggesting that earlier shyness might be predictive of later behavioral and physiological responses to social exclusion observed in adolescence.
Notes
In the prior study (Tang et al., 2019), we also examined theta temporal phase coherence. Because this measure was not related to distress in the task, we focused on theta ERSP only in the current study.
References
Abrams, D., Weick, M., Thomas, D., Colbe, H., & Franklin, K. M. (2011). On-line ostracism affects children differently from adolescents and adults. British Journal of Developmental Psychology, 29(1), 110–123.
Asendorpf, J. B., & Meier, G. H. (1993). Personality effects on children's speech in everyday life: Sociability-mediated exposure and shyness-mediated reactivity to social situations. Journal of Personality and Social Psychology, 64(6), 1072–1083.
Bekkedal, M. Y., Rossi III, J., & Panksepp, J. (2011). Human brain EEG indices of emotions: delineating responses to affective vocalizations by measuring frontal theta event-related synchronization. Neuroscience & Biobehavioral Reviews, 35(9), 1959–1970.
Blakemore, S.-J., & Mills, K. L. (2014). Is adolescence a sensitive period for sociocultural processing? Annual review of psychology, 65, 187-207.
Boivin, M., Hymel, S., & Bukowski, W. M. (1995). The roles of social withdrawal, peer rejection, and victimization by peers in predicting loneliness and depressed mood in childhood. Development and Psychopathology, 7(4), 765–785.
Booth-LaForce, C., Oh, W., Kennedy, A. E., Rubin, K. H., Rose-Krasnor, L., & Laursen, B. (2012). Parent and peer links to trajectories of anxious withdrawal from grades 5 to 8. Journal of Clinical Child & Adolescent Psychology, 41(2), 138–149.
Booth-LaForce, C., & Oxford, M. L. (2008). Trajectories of social withdrawal from grades 1 to 6: Prediction from early parenting, attachment, and temperament. Developmental Psychology, 44(5), 1298–1313.
Brooker, R. J., Bates, J. E., Buss, K. A., Canen, M. J., Dennis-Tiwary, T. A., Gatzke-Kopp, L. M., ... Schmidt, L.A. (2020). Conducting event-related potential (ERP) research with young children: A review of components, special considerations and recommendations for research on cognition and emotion. Journal of Psychophysiology, 34, 137–158.
Burgess, K. B., Wojslawowicz, J. C., Rubin, K. H., Rose-Krasnor, L., & Booth-LaForce, C. (2006). Social information processing and coping strategies of shy/withdrawn and aggressive children: Does friendship matter? Child Development, 77(2), 371–383.
Buzzell, G. A., Troller-Renfree, S. V., Barker, T. V., Bowman, L. C., Chronis-Tuscano, A., Henderson, H. A., ... Fox, N. A. (2017). A neurobehavioral mechanism linking behaviorally inhibited temperament and later adolescent social anxiety. Journal of the American Academy of Child & Adolescent Psychiatry, 56(12), 1097–1105.
Capaldi, D. M., & Rothbart, M. K. (1992). Development and validation of an early adolescent temperament measure. Journal of Early Adolescence, 12(2), 153–173.
Cheek, J. M., & Melchior, L. (1990). Shyness and anxious self-preoccupation during a social interaction. Journal of Social Behaviour and Personality, 5, 117–130.
Chen, X., Fu, R., Li, D., & Liu, J. (2019). Developmental trajectories of shyness-sensitivity from middle childhood to early adolescence in China: Contributions of peer preference and mutual friendship. Journal of Abnormal Child Psychology, 47(7), 1197–1209.
Clarkson, T., Eaton, N. R., Nelson, E. E., Fox, N. A., Leibenluft, E., Pine, D. S., ... Jarcho, J. M. (2019). Early childhood social reticence and neural response to peers in preadolescence predict social anxiety symptoms in midadolescence. Depression and Anxiety, 36(8), 676–689.
Cohen, M. X., Elger, C. E., & Ranganath, C. (2007). Reward expectation modulates feedback-related negativity and EEG spectra. NeuroImage, 35(2), 968–978.
Coplan, R. J., Prakash, K., O'Neil, K., & Armer, M. (2004). Do you" want" to play? Distinguishing between conflicted shyness and social disinterest in early childhood. Developmental Psychology, 40(2), 244–258.
Coplan, R. J., Rose-Krasnor, L., Weeks, M., Kingsbury, A., Kingsbury, M., & Bullock, A. (2013). Alone is a crowd: Social motivations, social withdrawal, and socioemotional functioning in later childhood. Developmental Psychology, 49(5), 861–875.
Cristofori, I., Moretti, L., Harquel, S., Posada, A., Deiana, G., Isnard, J., ... Sirigu, A. (2012). Theta signal as the neural signature of social exclusion. Cerebral Cortex, 23(10), 2437–2447.
Crone, E. A., & Dahl, R. E. (2012). Understanding adolescence as a period of social–affective engagement and goal flexibility. Nature Reviews Neuroscience, 13(9), 636.
Crowley, M. J., Wu, J., Molfese, P. J., & Mayes, L. C. (2010). Social exclusion in middle childhood: rejection events, slow-wave neural activity, and ostracism distress. Social Neuroscience, 5(5-6), 483–495.
Delorme, A., & Makeig, S. (2004). EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. Journal of Neuroscience Methods, 134(1), 9–21.
Enders, C. K., & Bandalos, D. L. (2001). The relative performance of full information maximum likelihood estimation for missing data in structural equation models. Structural Equation Modeling, 8(3), 430–457.
Ertl, M., Hildebrandt, M., Ourina, K., Leicht, G., & Mulert, C. (2013). Emotion regulation by cognitive reappraisal—the role of frontal theta oscillations. NeuroImage, 81, 412-421.
Foulkes, L., & Blakemore, S.-J. (2018). Studying individual differences in human adolescent brain development. Nature Neuroscience, 21(3), 315–323.
Gazelle, H., & Druhen, M. J. (2009). Anxious solitude and peer exclusion predict social helplessness, upset affect, and vagal regulation in response to behavioral rejection by a friend. Developmental Psychology, 45(4), 1077–1096.
Gazelle, H., & Ladd, G. W. (2003). Anxious solitude and peer exclusion: A diathesis–stress model of internalizing trajectories in childhood. Child Development, 74(1), 257–278.
Gratton, G., Coles, M. G., & Donchin, E. (1983). A new method for off-line removal of ocular artifact. Electroencephalography and Clinical Neurophysiology, 55(4), 468–484.
Guyer, A. E., Benson, B., Choate, V. R., Bar-Haim, Y., Perez-Edgar, K., Jarcho, J. M., ... Nelson, E. E. (2014). Lasting associations between early-childhood temperament and late-adolescent reward-circuitry response to peer feedback. Development and Psychopathology, 26(1), 229–243.
Guyer, A. E., Silk, J. S., & Nelson, E. E. (2016). The neurobiology of the emotional adolescent: From the inside out. Neuroscience & Biobehavioral Reviews, 70, 74–85.
Harrewijn, A., van der Molen, M. J., van Vliet, I. M., Tissier, R. L., & Westenberg, P. M. (2018). Behavioral and EEG responses to social evaluation: a two-generation family study on social anxiety. NeuroImage: Clinical, 17, 549–562.
Harrist, A. W., Zaia, A. F., Bates, J. E., Dodge, K. A., & Pettit, G. S. (1997). Subtypes of social withdrawal in early childhood: Sociometric status and social-cognitive differences across four years. Child Development, 68(2), 278–294.
Hartgerink, C. H., Van Beest, I., Wicherts, J. M., & Williams, K. D. (2015). The ordinal effects of ostracism: A meta-analysis of 120 Cyberball studies. PLoS One, 10(5), e0127002.
Howarth, G. Z., Guyer, A. E., & Pérez-Edgar, K. (2013). Young children's affective responses to acceptance and rejection from peers: A computer-based task sensitive to variation in temperamental shyness and gender. Social Development, 22(1), 146–162.
Jarcho, J. M., Davis, M. M., Shechner, T., Degnan, K. A., Henderson, H. A., Stoddard, J., ... Nelson, E. E. (2016). Early-childhood social reticence predicts brain function in preadolescent youths during distinct forms of peer evaluation. Psychological Science, 27(6), 821–835.
Junghöfer, M., Elbert, T., Tucker, D. M., & Braun, C. (1999). The polar average reference effect: a bias in estimating the head surface integral in EEG recording. Clinical Neurophysiology, 110(6), 1149–1155.
Kievit, R. A., Brandmaier, A. M., Ziegler, G., Van Harmelen, A.-L., de Mooij, S. M., Moutoussis, M., ... Fonagy, P. (2018). Developmental cognitive neuroscience using latent change score models: A tutorial and applications. Developmental Cognitive Neuroscience, 33, 99–117.
Knyazev, G. G. (2007). Motivation, emotion, and their inhibitory control mirrored in brain oscillations. Neuroscience & Biobehavioral Reviews, 31(3), 377–395.
Kujawa, A., & Burkhouse, K. L. (2017). Vulnerability to depression in youth: advances from affective neuroscience. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 2(1), 28–37.
Lamblin, M., Murawski, C., Whittle, S., & Fornito, A. (2017). Social connectedness, mental health and the adolescent brain. Neuroscience and Biobehavioral Reviews, 80, 57–68.
LoBue, V., & Pérez-Edgar, K. (2014). Sensitivity to social and non-social threats in temperamentally shy children at-risk for anxiety. Developmental Science, 17(2), 239–247.
Luu, P., Tucker, D. M., Derryberry, D., Reed, M., & Poulsen, C. (2003). Electrophysiological responses to errors and feedback in the process of action regulation. Psychological Science, 14(1), 47–53.
McPartland, J. C., Crowley, M. J., Perszyk, D. R., Naples, A. J., Mukerji, C. E., Wu, J., ... Mayes, L. C. (2011). Temporal dynamics reveal atypical brain response to social exclusion in autism. Developmental Cognitive Neuroscience, 1(3), 271–279.
Michiel Westenberg, P., Drewes, M. J., Goedhart, A. W., Siebelink, B. M., & Treffers, P. D. (2004). A developmental analysis of self-reported fears in late childhood through mid-adolescence: Social-evaluative fears on the rise? Journal of Child Psychology and Psychiatry, 45(3), 481–495.
Morales, S., Pérez-Edgar, K. E., & Buss, K. A. (2015). Attention biases towards and away from threat mark the relation between early dysregulated fear and the later emergence of social withdrawal. Journal of Abnormal Child Psychology, 43(6), 1067–1078.
Morales, S., Vallorani, A., & Pérez-Edgar, K. (2019). Young children’s behavioral and neural responses to peer feedback relate to internalizing problems. Developmental Cognitive Neuroscience, 36, 100610.
Muthén, L. K., & Muthén, B. O. (2002). How to use a Monte Carlo study to decide on sample size and determine power. Structural Equation Modeling, 9(4), 599–620.
Oh, W., Rubin, K. H., Bowker, J. C., Booth-LaForce, C., Rose-Krasnor, L., & Laursen, B. (2008). Trajectories of social withdrawal from middle childhood to early adolescence. Journal of Abnormal Child Psychology, 36(4), 553–566.
Pérez-Edgar, K., Bar-Haim, Y., McDermott, J. M., Chronis-Tuscano, A., Pine, D. S., & Fox, N. A. (2010). Attention biases to threat and behavioral inhibition in early childhood shape adolescent social withdrawal. Emotion, 10(3), 349–357.
Poole, K. L., & Schmidt, L. A. (2021). Vigilant or avoidant? Children's temperamental shyness, patterns of gaze, and physiology during social threat. Developmental Science, 1–13. https://doi.org/10.1111/desc.13118
Rosseel, Y. (2012). Lavaan: An R package for structural equation modeling and more. Version 0.5–12 (BETA). Journal of Statistical Software, 48(2), 1–36.
Rudolph, K. D., Miernicki, M. E., Troop-Gordon, W., Davis, M. M., & Telzer, E. H. (2016). Adding insult to injury: neural sensitivity to social exclusion is associated with internalizing symptoms in chronically peer-victimized girls. Social Cognitive and Affective Neuroscience, 11(5), 829–842.
Sebastian, C., Viding, E., Williams, K. D., & Blakemore, S.-J. (2010). Social brain development and the affective consequences of ostracism in adolescence. Brain and Cognition, 72(1), 134–145.
Silk, J. S., Siegle, G. J., Lee, K. H., Nelson, E. E., Stroud, L. R., & Dahl, R. E. (2014). Increased neural response to peer rejection associated with adolescent depression and pubertal development. Social Cognitive and Affective Neuroscience, 9(11), 1798–1807.
Smith, A., Nelson, E., Kircanski, K., Rappaport, B., Do, Q., Leibenluft, E., ... Jarcho, J. (2020). Social anxiety and age are associated with neural response to social evaluation during adolescence. Developmental Cognitive Neuroscience, 42, 100768.
Steinberg, L., & Monahan, K. C. (2007). Age differences in resistance to peer influence. Developmental Psychology, 43(6), 1531–1543.
Stroud, L. R., Foster, E., Papandonatos, G. D., Handwerger, K., Granger, D. A., Kivlighan, K. T., & Niaura, R. (2009). Stress response and the adolescent transition: Performance versus peer rejection stressors. Development and Psychopathology, 21(1), 47–68.
Sumter, S. R., Bokhorst, C. L., & Westenberg, P. M. (2009). Social fears during adolescence: Is there an increase in distress and avoidance? Journal of Anxiety Disorders, 23(7), 897–903.
Tang, A., Van Lieshout, R. J., Lahat, A., Duku, E., Boyle, M. H., Saigal, S., & Schmidt, L. A. (2017). Shyness trajectories across the first four decades predict mental health outcomes. Journal of Abnormal Child Psychology, 45(8), 1621–1633.
Tang, A., Lahat, A., Crowley, M. J., Wu, J., & Schmidt, L. A. (2019). Neurodevelopmental differences to social exclusion: An event-related neural oscillation study of children, adolescents, and adults. Emotion, 19(3), 520–532.
Tang, A., Crawford, H., Morales, S., Degnan, K. A., Pine, D. S., & Fox, N. A. (2020). Infant behavioral inhibition predicts personality and social outcomes three decades later. Proceedings of the National Academy of Sciences, 117(18), 9800–9807.
Themanson, J. R., Khatcherian, S. M., Ball, A. B., & Rosen, P. J. (2013). An event-related examination of neural activity during social interactions. Social Cognitive and Affective Neuroscience, 8(6), 727–733.
Van Beest, I., & Williams, K. D. (2006). When inclusion costs and ostracism pays, ostracism still hurts. Journal of Personality and Social Psychology, 91(5), 918.
van den Bos, E., de Rooij, M., Miers, A. C., Bokhorst, C. L., & Westenberg, P. M. (2014). Adolescents' increasing stress response to social evaluation: Pubertal effects on cortisol and alpha-amylase during public speaking. Child Development, 85(1), 220–236.
Van der Molen, M., Dekkers, L. M., Westenberg, P. M., van der Veen, F. M., & Van der Molen, M. (2017). Why don't you like me? Midfrontal theta power in response to unexpected peer rejection feedback. NeuroImage, 146, 474–483.
van Noordt, S. J., White, L. O., Wu, J., Mayes, L. C., & Crowley, M. J. (2015). Social exclusion modulates event-related frontal theta and tracks ostracism distress in children. NeuroImage, 118, 248–255.
van Noordt, S. J., Wu, J., Thomas, C., Schlund, M. W., Mayes, L. C., & Crowley, M. J. (2018). Medial frontal theta dissociates unsuccessful from successful avoidance and is modulated by lack of perseverance. Brain Research, 1694, 29-37.
Vecchiato, G., Toppi, J., Astolfi, L., Fallani, F. D. V., Cincotti, F., Mattia, D., ... Babiloni, F. (2011). Spectral EEG frontal asymmetries correlate with the experienced pleasantness of TV commercial advertisements. Medical & Biological Engineering & Computing, 49(5), 579–583.
Walker, O. L., Henderson, H. A., Degnan, K. A., Penela, E. C., & Fox, N. A. (2014). Associations between behavioral inhibition and children's social problem-solving behavior during social exclusion. Social Development, 23(3), 487–501.
Wang, H., Braun, C., & Enck, P. (2017). How the brain reacts to social stress (exclusion)–A scoping review. Neuroscience & Biobehavioral Reviews, 80, 80–88.
Westenberg, MP., Drewes, M. J., Goedhart, A. W., Siebelink, B. M., & Treffers, P. D. (2004). A developmental analysis of self‐reported fears in late childhood through mid‐adolescence: Social‐evaluative fears on the rise? Journal of Child Psychology and Psychiatry, 45(3), 481–495.
White, L. O., Wu, J., Borelli, J. L., Rutherford, H. J., David, D. H., Kim-Cohen, J., ... Crowley, M. J. (2012). Attachment dismissal predicts frontal slow-wave ERPs during rejection by unfamiliar peers. Emotion, 12(4), 690–700.
Will, G.-J., van Lier, P. A., Crone, E. A., & Güroğlu, B. (2016). Chronic childhood peer rejection is associated with heightened neural responses to social exclusion during adolescence. Journal of Abnormal Child Psychology, 44(1), 43–55.
Wilson, M., & Henderson, H. (2020). Shyness and perceived monitoring by peers affect children’s performance in a divided attention task. Journal of Experimental Child Psychology, 198, 104882.
Yuan, K.-H., & Bentler, P. M. (2000). 5. Three likelihood-based methods for mean and covariance structure analysis with nonnormal missing data. Sociological Methodology, 30(1), 165–200.
Acknowledgments
This research was supported by a Social Sciences and Humanities Research Council of Canada (SSHRC) Doctoral Award to A.T., a SSHRC Banting Postdoctoral Award to A.L., and a Natural Sciences and Engineering Research Council of Canada operating grant to L.A.S. The authors thank the participants and their families for their participation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Open practice statement
Data used in this study are available on Open Science, https://osf.io/smdfv/. The experiment was not pre-registered.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 23 kb)
Rights and permissions
About this article
Cite this article
Tang, A., Lahat, A., Crowley, M.J. et al. Children’s shyness and neural responses to social exclusion: Patterns of midfrontal theta power usually not observed until adolescence. Cogn Affect Behav Neurosci 21, 1262–1275 (2021). https://doi.org/10.3758/s13415-021-00916-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13415-021-00916-7