Abstract
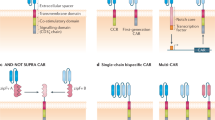
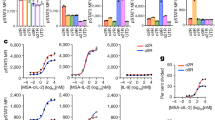
The efficacy of adoptive cell therapy for solid tumours is hampered by the poor accumulation of the transferred T cells in tumour tissue. Here, we show that forced expression of C-X-C chemokine receptor type 6 (whose ligand is highly expressed by human and murine pancreatic cancer cells and tumour-infiltrating immune cells) in antigen-specific T cells enhanced the recognition and lysis of pancreatic cancer cells and the efficacy of adoptive cell therapy for pancreatic cancer. In mice with subcutaneous pancreatic tumours treated with T cells with either a transgenic T-cell receptor or a murine chimeric antigen receptor targeting the tumour-associated antigen epithelial cell adhesion molecule, and in mice with orthotopic pancreatic tumours or patient-derived xenografts treated with T cells expressing a chimeric antigen receptor targeting mesothelin, the T cells exhibited enhanced intratumoral accumulation, exerted sustained anti-tumoral activity and prolonged animal survival only when co-expressing C-X-C chemokine receptor type 6. Arming tumour-specific T cells with tumour-specific chemokine receptors may represent a promising strategy for the realization of adoptive cell therapy for solid tumours.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The main data supporting the results of this study are available within the paper and its Supplementary Information. The raw and analysed datasets generated during this study are too large to be publicly shared, but they are available for research purposes from the corresponding author upon reasonable request. They also contain personal and patient data and are available for research purposes pending completion of adequate paper work ensuring personal data protection and ethical approval. RNA-sequencing data in this study have been published previously and are accessible through the NCBI Gene Expression Omnibus (accession codes GSE84133 and GSE122960), NCBI BioProject database (accession code PRJEB31843), Genome Sequence Archive (accession number CRA001160) and Synapse (https://www.synapse.org/#!Synapse:syn21041850/files/).
References
Rosenberg, S. A. & Restifo, N. P. Adoptive cell transfer as personalized immunotherapy for human cancer. Science 348, 62–68 (2015).
Kobold, S. et al. Immunotherapy in tumors. Dtsch. Ärztebl. Int. 112, 809–815 (2015).
Sheridan, C. First approval in sight for Novartis’ CAR-T therapy after panel vote. Nat. Biotechnol. 35, 691–693 (2017).
Ahmed, N. et al. Human epidermal growth factor receptor 2 (HER2)—specific chimeric antigen recpetor-modified T cells for the immunotherapy of HER2-positive sarcoma. J. Clin. Oncol. 33, 1688–1696 (2015).
Adusumilli, P. S. et al. Regional delivery of mesothelin-targeted CAR T cell therapy generates potent and long-lasting CD4-dependent tumor immunity. Sci. Transl. Med. 6, 261ra151 (2014).
Brown, C. E. et al. Regression of glioblastoma after chimeric antigen receptor T cell therapy. N. Engl. J. Med. 375, 2561–2569 (2016).
O’Rourke, D. M. et al. A single dose of peripherally infused EGFRvIII-directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma. Sci. Transl. Med. 9, eaaa0984 (2017).
Tchou, J. et al. Safety and efficacy of intratumoral injections of chimeric antigen receptor (CAR) T cells in metastatic breast cancer. Cancer Immunol. Res. 5, 1152–1161 (2017).
Akbay, E. A. et al. Interleukin-17A promotes lung tumor progression through neutrophil attraction to tumor sites and mediating resistance to PD-1 blockade. J. Thorac. Oncol. 12, 1268–1279 (2017).
Bauer, C. A. et al. Dynamic Treg interactions with intratumoral APCs promote local CTL dysfunction. J. Clin. Invest. 124, 2425–2440 (2014).
Linke, B. et al. CXCL16/CXCR6-mediated adhesion of human peripheral blood mononuclear cells to inflamed endothelium. Cytokine 122, 154081 (2019).
Polański, K. et al. BBKNN: fast batch alignment of single cell transcriptomes. Bioinformatics 36, 964–965 (2020).
Tokarew, N., Ogonek, J., Endres, S., von Bergwelt-Baildon, M. & Kobold, S.Teaching an old dog new tricks: next-generation CAR T cells. Br. J. Cancer 120, 26–37 (2019).
Grosser, R., Cherkassky, L., Chintala, N. & Adusumilli, P. S. Combination immunotherapy with CAR T cells and checkpoint blockade for the treatment of solid tumors. Cancer Cell 36, 471–482 (2019).
Rapp, M. et al. C-C chemokine receptor type-4 transduction of T cells enhances interaction with dendritic cells, tumor infiltration and therapeutic efficacy of adoptive T cell transfer. OncoImmunology 5, e1105428 (2016).
Hughes, C. E. & Nibbs, R. J. B. A guide to chemokines and their receptors. FEBS J. 285, 2944–2971 (2018).
Curiel, T. J. et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat. Med. 10, 942–949 (2004).
Lim, W. A. & June, C. H. The principles of engineering immune cells to treat cancer. Cell 168, 724–740 (2017).
Garetto, S. et al. Tailored chemokine receptor modification improves homing of adoptive therapy T cells in a spontaneous tumor model. Oncotarget 7, 43010–43026 (2016).
Siddiqui, I., Erreni, M., van Brakel, M., Debets, R. & Allavena, P. Enhanced recruitment of genetically modified CX3CR1-positive human T cells into Fractalkine/CX3CL1 expressing tumors: importance of the chemokine gradient. J. Immunother. Cancer 4, 21 (2016).
Muller, N. et al. Engineering NK cells modified with an EGFRvIII-specific chimeric antigen receptor to overexpress CXCR4 improves immunotherapy of CXCL12/SDF-1α-secreting glioblastoma. J. Immunother. 38, 197–210 (2015).
Moon, E. K. et al. Expression of a functional CCR2 receptor enhances tumor localization and tumor eradication by retargeted human T cells expressing a mesothelin-specific chimeric antibody receptor. Clin. Cancer Res. 17, 4719–4730 (2011).
Peng, W. et al. Transduction of tumor-specific T cells with CXCR2 chemokine receptor improves migration to tumor and antitumor immune responses. Clin. Cancer Res. 16, 5458–5468 (2010).
Shimaoka, T. et al. Cell surface-anchored SR-PSOX/CXC chemokine ligand 16 mediates firm adhesion of CXC chemokine receptor 6-expressing cells. J. Leukoc. Biol. 75, 267–274 (2004).
Kobold, S. et al. Impact of a new fusion receptor on PD-1-mediated immunosuppression in adoptive T cell therapy. J. Natl Cancer Inst. 107, djv146 (2015).
Li, K. et al. Impact of chemokine receptor CXCR3 on tumor-infiltrating lymphocyte recruitment associated with favorable prognosis in advanced gastric cancer. Int. J. Clin. Exp. Pathol. 8, 14725–14732 (2015).
Madissoon, E. et al. scRNA-seq assessment of the human lung, spleen, and esophagus tissue stability after cold preservation. Genome Biol. 21, 1 (2019).
Deng, L., Chen, N., Li, Y., Zheng, H. & Lei, Q. CXCR6/CXCL16 functions as a regulator in metastasis and progression of cancer. Biochim. Biophys. Acta 1806, 42–49 (2010).
Wente, M. N. et al. Expression and potential function of the CXC chemokine CXCL16 in pancreatic ductal adenocarcinoma. Int. J. Oncol. 33, 297–308 (2008).
Heydtmann, M. et al. CXC chemokine ligand 16 promotes integrin-mediated adhesion of liver-infiltrating lymphocytes to cholangiocytes and hepatocytes within the inflamed human liver. J. Immunol. 174, 1055–1062 (2005).
Rataj, F. et al. PD1–CD28 fusion protein enables CD4+ T cell help for adoptive T cell therapy in models of pancreatic cancer and non-Hodgkin lymphoma. Front. Immunol. https://doi.org/10.3389/fimmu.2018.01955 (2018).
Sato, T. et al. Role for CXCR6 in recruitment of activated CD8+ lymphocytes to inflamed liver. J. Immunol. 174, 277–283 (2005).
Unutmaz, D. et al. The primate lentiviral receptor Bonzo/STRL33 is coordinately regulated with CCR5 and its expression pattern is conserved between human and mouse. J. Immunol. 165, 3284–3292 (2000).
Karches, C. H. et al. Bispecific antibodies enable synthetic agonistic receptor-transduced T cells for tumor immunotherapy. Clin. Cancer Res. 25, 5890–5900 (2019).
Paulos, C. M. et al. Microbial translocation augments the function of adoptively transferred self/tumor-specific CD8+ T cells via TLR4 signaling. J. Clin. Invest. 117, 2197–2204 (2007).
Kobold, S. et al. Selective bispecific T cell recruiting antibody and antitumor activity of adoptive T cell transfer. J. Natl Cancer Inst. 107, 364 (2015).
Chinnasamy, D. et al. Local delivery of interleukin-12 using T cells targeting VEGF receptor-2 eradicates multiple vascularized tumors in mice. Clin. Cancer Res. 18, 1672–1683 (2012).
Jin, L. et al. CXCR1- or CXCR2-modified CAR T cells co-opt IL-8 for maximal antitumor efficacy in solid tumors. Nat. Commun. 10, 4016 (2019).
Bailey, P. et al. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature 531, 47–52 (2016).
Schizas, D. et al. Immunotherapy for pancreatic cancer: a 2020 update. Cancer Treat. Rev. 86, 102016 (2020).
Hartmann, N. et al. Prevailing role of contact guidance in intrastromal T-cell trapping in human pancreatic cancer. Clin. Cancer Res. 20, 3422–3433 (2014).
Kocher, H. M. et al. Phase I clinical trial repurposing all-trans retinoic acid as a stromal targeting agent for pancreatic cancer. Nat. Commun. 11, 4841 (2020).
Alvarez, R. et al. Stromal disrupting effects of nab-paclitaxel in pancreatic cancer. Br. J. Cancer 109, 926–933 (2013).
Lo, A. et al. Tumor-promoting desmoplasia is disrupted by depleting FAP-expressing stromal cells. Cancer Res. 75, 2800–2810 (2015).
Matloubian, M., David, A., Engel, S., Ryan, J. E. & Cyster, J. G. A transmembrane CXC chemokine is a ligand for HIV-coreceptor Bonzo. Nat. Immunol. 1, 298–304 (2000).
Linke, B. et al. CXCL16/CXCR6-mediated adhesion of human peripheral blood mononuclear cells to inflamed endothelium. Cytokine 122, 154081 (2019).
Collado, A. et al. Functional role of endothelial CXCL16/CXCR6–platelet–leucocyte axis in angiotensin II-associated metabolic disorders. Cardiovasc. Res. 114, 1764–1775 (2018).
Sackstein, R., Schatton, T. & Barthel, S. R. T-lymphocyte homing: an underappreciated yet critical hurdle for successful cancer immunotherapy. Lab. Invest. 97, 669–697 (2017).
Agostini, C. et al. Role for CXCR6 and its ligand CXCL16 in the pathogenesis of T-cell alveolitis in sarcoidosis. Am. J. Respir. Crit. Care Med. 172, 1290–1298 (2005).
Oldham, K. A. et al. T lymphocyte recruitment into renal cell carcinoma tissue: a role for chemokine receptors CXCR3, CXCR6, CCR5, and CCR6. Eur. Urol. 61, 385–394 (2012).
La Porta, C. A. CXCR6: the role of environment in tumor progression. Challenges for therapy. Stem Cell Rev. 8, 1282–1285 (2012).
Allaoui, R. et al. Cancer-associated fibroblast-secreted CXCL16 attracts monocytes to promote stroma activation in triple-negative breast cancers. Nat. Commun. 7, 13050 (2016).
Chalabi-Dchar, M. et al. Loss of somatostatin receptor subtype 2 promotes growth of KRAS-induced pancreatic tumors in mice by activating PI3K signaling and overexpression of CXCL16. Gastroenterology 148, 1452–1465 (2015).
Elyada, E. et al. Cross-species single-cell analysis of pancreatic ductal adenocarcinoma reveals antigen-presenting cancer-associated fibroblasts. Cancer Discov. 9, 1102–1123 (2019).
Hu, W., Liu, Y., Zhou, W., Si, L. & Ren, L. CXCL16 and CXCR6 are coexpressed in human lung cancer in vivo and mediate the invasion of lung cancer cell lines in vitro. PLoS ONE 9, e99056 (2014).
Slaga, D. et al. Avidity-based binding to HER2 results in selective killing of HER2-overexpressing cells by anti-HER2/CD3. Sci. Transl. Med. 10, eaat5775 (2018).
Morello, A., Sadelain, M. & Adusumilli, P. S. Mesothelin-targeted CARs: driving T cells to solid tumors. Cancer Discov 6, 133–146 (2016).
Beatty, G. L. et al. Activity of mesothelin-specific chimeric antigen receptor T cells against pancreatic carcinoma metastases in a phase 1 trial. Gastroenterology 155, 29–32 (2018).
Fujita, K. et al. Prolonged disease-free period in patients with advanced epithelial ovarian cancer after adoptive transfer of tumor-infiltrating lymphocytes. Clin. Cancer Res. 1, 501–507 (1995).
Hall, M. et al. Expansion of tumor-infiltrating lymphocytes (TIL) from human pancreatic tumors. J. Immunother. Cancer 4, 61 (2016).
Nanki, T. et al. Pathogenic role of the CXCL16–CXCR6 pathway in rheumatoid arthritis. Arthritis Rheum. 52, 3004–3014 (2005).
Akce, M., Zaidi, M. Y., Waller, E. K., El-Rayes, B. F. & Lesinski, G. B. The potential of CAR T cell therapy in pancreatic cancer. Front. Immunol. 9, 2166 (2018).
Jacobs, C. et al. An ISCOM vaccine combined with a TLR9 agonist breaks immune evasion mediated by regulatory T cells in an orthotopic model of pancreatic carcinoma. Int. J. Cancer 128, 897–907 (2011).
Anz, D. et al. Suppression of intratumoral CCL22 by type I interferon inhibits migration of regulatory T cells and blocks cancer progression. Cancer Res. 75, 4483–4493 (2015).
Ghani, K. et al. Efficient human hematopoietic cell transduction using RD114- and GALV-pseudotyped retroviral vectors produced in suspension and serum-free media. Hum. Gene Ther. 20, 966–974 (2009).
Metzger, P. et al. Immunostimulatory RNA leads to functional reprogramming of myeloid-derived suppressor cells in pancreatic cancer. J. Immunother. Cancer 7, 288 (2019).
Larimer, B. M. et al. Granzyme B PET imaging as a predictive biomarker of immunotherapy response. Cancer Res. 77, 2318–2327 (2017).
Larimer, B. M. et al. The effectiveness of checkpoint inhibitor combinations and administration timing can be measured by granzyme B pet imaging. Clin. Cancer Res. 25, 1196–1205 (2019).
Rühland, S. et al. Quantification of in vitro mesenchymal stem cell invasion into tumor spheroids using selective plane illumination microscopy. J. Biomed. Opt. 20, 040501 (2015).
Schmohl, K. A. et al. Thyroid hormones and tetrac: new regulators of tumour stroma formation via integrin αvβ3. Endocr. Relat. Cancer 22, 941–952 (2015).
Renz, B. W. et al. β2 adrenergic–neurotrophin feedforward loop promotes pancreatic cancer. Cancer Cell. 33, 75–90.e7 (2018).
Renz, B. W. et al. Cholinergic signaling via muscarinic receptors directly and indirectly suppresses pancreatic tumorigenesis and cancer stemness. Cancer Discov. 8, 1458–1473 (2018).
Ruess, D. A. et al. Mutant KRAS-driven cancers depend on PTPN11/SHP2 phosphatase. Nat. Med. 24, 954–960 (2018).
Reichert, M. et al. Isolation, culture and genetic manipulation of mouse pancreatic ductal cells. Nat. Protoc. 8, 1354–1365 (2013).
Halama, N. et al. Tumoral immune cell exploitation in colorectal cancer metastases can be targeted effectively by anti-CCR5 therapy in cancer patients. Cancer Cell 29, 587–601 (2016).
Halama, N. et al. Localization and density of immune cells in the invasive margin of human colorectal cancer liver metastases are prognostic for response to chemotherapy. Cancer Res. 71, 5670–5677 (2011).
Goldman, M. et al. Visualizing and interpreting cancer genomics data via the Xena platform. Nat. Biotechnol. 38, 675–678 (2020).
Wolf, F. A., Angerer, P. & Theis, F. J. SCANPY: large-scale single-cell gene expression data analysis. Genome Biol. 19, 15–15 (2018).
Travaglini, K. J. et al. A molecular cell atlas of the human lung from single-cell RNA sequencing. Nature 587, 619–625 (2020).
Reyfman, P. A. et al. Single-cell transcriptomic analysis of human lung provides insights into the pathobiology of pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 199, 1517–1536 (2019).
Peng, J. et al. Single-cell RNA-seq highlights intra-tumoral heterogeneity and malignant progression in pancreatic ductal adenocarcinoma. Cell Res. 29, 725–738 (2019).
Baron, M. et al. A single-cell transcriptomic map of the human and mouse pancreas reveals inter- and intra-cell population structure. Cell Syst. 3, 346–360 (2016).
Lun, A. T. L., Bach, K. & Marioni, J. C. Pooling across cells to normalize single-cell RNA sequencing data with many zero counts. Genome Biol. 17, 75 (2016).
Zheng, G. X. Y. et al. Massively parallel digital transcriptional profiling of single cells. Nat. Commun. 8, 14049 (2017).
McInnes L., Healy J. & Melville J. UMAP: uniform manifold approximation and projection for dimension reduction. Preprint at https://arxiv.org/abs/1802.03426 (2020).
Muus, C. et al. Single-cell meta-analysis of SARS-CoV-2 entry genes across tissues and demographics. Nat. Med. 27, 546–559 (2021).
Acknowledgements
This study was supported by the Wilhelm Sander-Stiftung (grant number 2014.018.1 to S.E. and S. Kobold), international doctoral program ‘i-Target: immunotargeting of cancer’ (funded by the Elite Network of Bavaria; to S. Kobold and S.E.), Melanoma Research Alliance (grant number N269626 to S.E. and grant number 409510 to S. Kobold), Marie Sklodowska-Curie Training Network for the Immunotherapy of Cancer (IMMUTRAIN) (funded by the Horizon 2020 programme of the European Union; to S.E. and S. Kobold), Marie Sklodowska-Curie Training Network for Optimizing Adoptive T Cell Therapy of Cancer (funded by the Horizon 2020 programme of the European Union; grant 955575 to S. Kobold), Else Kröner-Fresenius-Stiftung (to S. Kobold), German Cancer Aid (to S. Kobold), Ernst Jung Stiftung (to S. Kobold), Institutional Strategy LMUexcellent of LMU Munich (within the framework of the German Excellence Initiative; to S.E. and S. Kobold), Bundesministerium für Bildung und Forschung (to S.E. and S. Kobold), European Research Council (Starting Grant 756017 to S. Kobold), Deutsche Forschungsgemeinschaft (DFG; to S. Kobold), Fritz-Bender Foundation (to S. Kobold), José Carreras Foundation (to S. Kobold) and Hector Foundation (to S. Kobold). R.T.A.M. is supported by the DFG (INST409/97-1 FUGG), SFB1123/Z1 and ERA-CVD (AtheroInside). Z.D. was supported by an AGA-Moti L. & Kamla Rustgi International Travel Award. M. Reichert was supported by German Cancer Aid (Max Eder Program; Deutsche Krebshilfe 111273) and the DFG (SFB1321 (Modeling and Targeting Pancreatic Cancer) and RE 3723/4-1). E.D. was supported by a grant from INSERM (HTE: chemotaxis in cancer). M.T. is funded by the Volkswagen Foundation (project OntoTime). C.M. has received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (grant agreement number 866411). M. Schnurr was supported by the DFG (SFB1321 (Modeling and Targeting Pancreatic Cancer); project number 329628492). We acknowledge the iFlow Core Facility of the University Hospital of Munich for assistance with the generation of flow cytometry data. Image processing using the Imaris 7.6.5 software was performed at the Core Facility for Bioimaging of the Biomedical Center of the Ludwig-Maximilians-Universität München.
Author information
Authors and Affiliations
Contributions
S.L., V.B., S.S., J.O., B.L.C., Z.D., F.R., K.D., J.L., C.H.K., C. Heise, M.K., B.M.L., S.G., M. Rapp, A.N., A.G., S. Kruger, N.T., P.M., C. Hoerth, M.-R.B., D.D., A.O., R.G., M. Seifert, S.J., Ö.U., L.V., M.T., T.T., T.H., T.B., D.H., R.T.A.M., K.-P.J., M.J., D.L., S. Ruehland, M.D.P., J.N.P., M.T., S.O., C.M., E.T., E.D., M.H., A.R., S. Rothenfusser, P.D., L.M.K. and M. Schnurr performed or assisted with the experiments, analysed the data and supported the project. S. Kobold and S.E. supervised the project and acquired the funding. S. Kobold, S.L., V.B., S.S., J.O., B.L.C., M. Subklewe, A.S.L., N.H., M. Reichert and T.R.M. designed the experiments. S. Kobold and S.L. wrote the manuscript. All authors critically read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
Parts of this work have been performed for the doctoral theses of S.L., V.B., S.S., K.D. and J.L. at the Ludwig-Maximilians-Universität München. M. Rapp, S.G., S.E. and S. Kobold are inventors on a patent application related to this work (PCT/EP2016/074644), filed by the Ludwig-Maximilians-Universität München. S.E. and S. Kobold received research support from TCR2 Therapeutics and Arcus Biosciences for work on T cell therapies unrelated to the present manuscript. The remaining authors declare no competing interests.
Additional information
Peer review information Nature Biomedical Engineering thanks Eduard Ryschich, Prasad Adusumilli and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–8 and Tables 1 and 2.
Rights and permissions
About this article
Cite this article
Lesch, S., Blumenberg, V., Stoiber, S. et al. T cells armed with C-X-C chemokine receptor type 6 enhance adoptive cell therapy for pancreatic tumours. Nat Biomed Eng 5, 1246–1260 (2021). https://doi.org/10.1038/s41551-021-00737-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41551-021-00737-6
This article is cited by
-
Tumor immune microenvironment-based therapies in pancreatic ductal adenocarcinoma: time to update the concept
Journal of Experimental & Clinical Cancer Research (2024)
-
How chemokines organize the tumour microenvironment
Nature Reviews Cancer (2024)
-
Metabolic targeting of cancer associated fibroblasts overcomes T-cell exclusion and chemoresistance in soft-tissue sarcomas
Nature Communications (2024)
-
Lymphatic vessels in the age of cancer immunotherapy
Nature Reviews Cancer (2024)
-
Ectopic CXCR2 expression cells improve the anti-tumor efficiency of CAR-T cells and remodel the immune microenvironment of pancreatic ductal adenocarcinoma
Cancer Immunology, Immunotherapy (2024)