Abstract
Investigations of flow boiling in highly viscous fluids show that heat transfer mechanisms in such fluids are different from those in fluids of low viscosity like refrigerants or water. To gain a better understanding, a modified standard apparatus was developed; it was specifically designed for fluids of high viscosity up to 1000 Pa∙s and enables heat transfer measurements with a single horizontal test tube over a wide range of heat fluxes. Here, we present measurements of the heat transfer coefficient at pool boiling conditions in highly viscous binary mixtures of three different polydimethylsiloxanes (PDMS) and n-pentane, which is the volatile component in the mixture. Systematic measurements were carried out to investigate pool boiling in mixtures with a focus on the temperature, the viscosity of the non-volatile component and the fraction of the volatile component on the heat transfer coefficient. Furthermore, copper test tubes with polished and sanded surfaces were used to evaluate the influence of the surface structure on the heat transfer coefficient. The results show that viscosity and composition of the mixture have the strongest effect on the heat transfer coefficient in highly viscous mixtures, whereby the viscosity of the mixture depends on the base viscosity of the used PDMS, on the concentration of n-pentane in the mixture, and on the temperature. For nucleate boiling, the influence of the surface structure of the test tube is less pronounced than observed in boiling experiments with pure fluids of low viscosity, but the relative enhancement of the heat transfer coefficient is still significant. In particular for mixtures with high concentrations of the volatile component and at high pool temperature, heat transfer coefficients increase with heat flux until they reach a maximum. At further increased heat fluxes the heat transfer coefficients decrease again. Observed temperature differences between heating surface and pool are much larger than for boiling fluids with low viscosity. Temperature differences up to 137 K (for a mixture containing 5% n-pentane by mass at a heat flux of 13.6 kW/m2) were measured.
Similar content being viewed by others
1 Introduction
The demands on polymer products concerning quality and environmental legislation have significantly increased during the last years. To reach high qualities and to meet the environmental regulations, undesirable side products of the polymerization process must be separated from the product by extruders or evaporators. In food technologies, falling film evaporators and other technologies are used to thicken highly viscous intermediate products. The design of corresponding apparatuses is based on specific experiments and on experience. Both, in polymer and in food technologies, the boiling highly viscous mixtures typically consist of at least one low and one very high boiling component, whereby the high boiling component is temperature sensitive in most cases. Attempts to develop predictive relations for flow boiling of such highly viscous mixtures showed that the foundations are still missing – yet there is no correlation available that describes pool boiling of highly viscous mixtures and that properly considers all effects relevant for this most fundamental form of boiling of highly viscous mixtures [1]. In correlations for convective boiling the heat transfer coefficient α usually depends on Grashof and Prandtl number; with a number of simplified assumptions both parameters can be calculated for the mixtures considered in this article. Compared to experimental data and depending on the assumptions made, the results are in the right order of magnitude. However, relations for nucleate boiling of mixtures commonly require heat transfer coefficients for nucleate boiling of the pure components at given temperature and / or reduced pressure. This information is not available for common highly viscous components. Nucleate boiling of these pure components cannot be observed for reasons of chemical stability and reduced temperatures and pressures cannot be defined, because critical parameters are unknown. Consequently, experimental results could not be correlated properly, see e.g. [2]. To establish a data base for the development of such correlations, systematic heat transfer measurements of pool boiling heat transfer in highly viscous mixtures are required.
Against this background, a modified standard apparatus was developed and set up [3] at the thermodynamics institute of Ruhr University Bochum to improve the fundamental understanding of heat transfer processes and to establish a database for correlation needs. We describe the apparatus and the experimental procedure in detail in an article [4] published in parallel to this article, which is focused on the experimental results. The heat transfer measurements discussed in the present paper were carried out with binary mixtures of polydimethylsiloxane (PDMS)Footnote 1 of three viscosities and n-pentane over a wide range of heat fluxes and temperatures. The influence of the temperature, the composition of the mixture, the surface roughness of the horizontal copper test tube, and of the different types of PDMS (with a significant variation in viscosity) on the heat transfer coefficient are systematically investigated and evaluated. With this, the impact of different parameters on the heat transfer can be separated from each other and assessed.
In literature, studies on heat transfer at pool boiling in refrigerants with small mass fractions of oil can be found. The effects of lubricant mass fraction in the refrigerant R143a, its viscosity and miscibility on the heat transfer coefficient was investigated in twelve mixtures [5]. Small lubricant mass fraction, high lubricant viscosity and large critical solution temperature improve the heat transfer coefficient. Nucleate pool boiling of mixtures of R134a and oil on two heating surfaces was investigated [6]. Two types of oil were used, medium and high viscosity, with a content up to 5% mass fraction. The heat transfer coefficients in mixtures with more than 3% mass fraction oil are always lower than in pure R134a.
The effect of oil on pool boiling of oil/R245fa mixtures up to 5% lubricant mass fraction was investigated [7]. At pool boiling, the heat transfer coefficient for the smooth tube decreases with increasing oil concentration. On the other hand, the heat transfer coefficient increases for the fin tube with 0.4 mm fin high because of a more significant bubbly foam.
2 Experimental section
2.1 Apparatus description
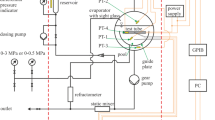
The so-called standard apparatus [2, 3] was modified to carry out heat transfer measurements in highly viscous mixtures, see [4] for details on the used apparatus and on experimental procedures. Mixtures of PDMS of significantly different viscositiesFootnote 2 (PDMS M10k with ηPDMS M10k = 9.7 Pa∙s, PDMS M100k with ηPDMS M100k = 97 Pa∙s, and PDMS M1000k with ηPDMS M1000k = 970 Pa∙s at T = 298.15 K) and n-pentane (ηn-pentane = 0.22 mPa∙s) were selected as model fluids. The volatile component, n-pentane, evaporates from the surface of an exchangeable test tube inside the evaporator and is subsequently condensed. By means of a dosing pump, the n-pentane is pumped to the static mixer. Inside the evaporator, the depleted PDMS flows over a guide plate and is pumped through the static mixer by a gear pump, where it is mixed with the condensed n-pentane. Downstream of the static mixer, the refractive index of the mixture is measured. The composition is calculated applying a correlation, which considers the refractive index and the temperature [4]. Ultimately, the mixture is pumped back into the evaporator. The temperature difference between the tube surface and the bulk fluid as well as the temperature distribution in the pool are measured with thermocouples. The modified standard apparatus is mounted inside a thermal chamber. Characteristic temperatures are measured with nine platinum resistance thermometers. The relative combined expanded uncertainty in measured heat transfer coefficients is 16% (k = 2), see the more detailed discussion on uncertainty of the measured values given in [4]. Pure n-pentane was used to validate the apparatus. The influence of the flow in the pool caused by remixing of the components on the heat transfer coefficient was studied in pretests [4].
The heat transfer coefficient α is defined as α = q / ΔT = Q / (A ∙ ΔT) with the heat flow Q, the heated area A and the superheat ΔT (temperature difference between heated wall and bulk liquid). The heated area is calculated with A = do ∙ π ∙ l where do is the outer diameter of the tube and l its heated length. The heat flux q is calculated with
and with
where R1 and R2 are high-precision resistors. The current Itube is calculated with
where R3 is a third high-precision resistor. The resistance Rcold causes a voltage drop at the inactive end of the heating element and is given by the manufacturer with 0.0345 Ω.
The superheat ΔTexp = (Tsurface − Tfluid) is measured directly with each of 12 thermocouples in the test tube, which each have a reference junction in the bulk of the fluid below the heating tube. The experimental superheat values ΔTexp = (Tsurface − Tfluid) are calculated with the arithmetical mean of the voltages measured for all 12 thermocouples, corrected by the voltage measured in equilibrium at q = 0 (ΔTexp = 0). ΔTexp is corrected for radial heat conduction within the tube from the groove to the surface by ΔT = ΔTexp − ΔTr,th with the temperature correction factor ΔTr,th. This factor depends on the construction of the tube and the thermal conductivity λ of its material (λcopper = 360 W/mK, λmagnesium oxid = 50 W/mK, λInconel = 15 W/mK, λthermal grease = 3 W/mK). It is calculated with
The thickness of every layer is defined by the outer and inner diameter di and do of each layer. The copper tubes have a correction factor of rth,copper = 5.88·10–5 m2K/W.
3 Nucleate boiling heat transfer in highly viscous fluids
The photographs in Fig. 1 show the difference in nucleate boiling in mixtures with 84 wt.-% (row A) and 95 wt.-% PDMS M10k (row B), i.e., with viscosities different by a factor of about three and with different content of n-pentane. The experiments were conducted using a polished copper test tube at similar temperatures (T = 323 K and 321 K), and the heat flux was varied from q = 2 kW/m2 to q = 16 kW/m2 in both cases. Measurement series were generally run from high to low heat fluxes.
For the mixture with 84 wt.-% PDMS M10k, the superheat ∆T (the temperature difference between heated wall and bulk liquid) decreases from ∆T = 94 K to 22 K with decreasing heat fluxes. Visual video and photo observations show that nucleate boiling remains stable down to q = 2 kW/m2. At q = 2 kW/m2, bubbles formed at the heating surface recondense in the pool before they reach the phase boundary. Up to q = 8 kW/m2 the number of nucleation sites increases with increasing heat fluxes. From q = 10 kW/m2 to 16 kW/m2, the number of bubbles does not increase further; small areas of the test tube surface are covered by gaseous n-pentane. With increasing heat fluxes, the shape of bubbles changes from spherical to drop shaped. At each heat flux, some bubbles collapsed before they reached the phase boundary. Coalescens was observed at heat fluxes higher than 4 kW/m2. At heat fluxes between 14 kW/m2 and 16 kW/m2, small bubbles occurred in the fluid away from the heated surface, which is probably caused by spontaneous evaporation when hot depleted mixture from close to the test tube mixes with fluid still rich in n-pentane.
Superheat temperatures ∆T in the mixture with 95 wt.-% PDMS M10k (Fig. 1, row B) are even higher; they increase up to ∆T = 129 K at q = 16 kW/m2. The predominant heat transfer mechanism changes from convective heat transfer to nucleate boiling at heat fluxes between 1 kW/m2 and 2 kW/m2. In comparison to row A, the photographs in row B show a significantly lower number of bubbles, especially at higher heat fluxes. For the more viscous mixture containing less n-pentane, the shape of the bubbles is continuously spherical with a larger diameter. With increasing heat fluxes, the mass flow of evaporating n-pentane increases. The observed phenomena indicate that the turbulence in the pool is not sufficient to compensate for this effect in highly viscous mixtures – close to the heating surface the concentration of n-pentane is reduced, the concentration of PDMS increases.
Just like for refrigerant mixtures or other mixtures with low viscosity, convective boiling and nucleate boiling can be distinguished for boiling highly viscous mixtures. The heat and mass transfer mechanisms responsible for the characteristics of boiling are the same, but due to high viscosity turbulent mixing of the pool is limited. Concentration and temperature gradients are larger. And the viscosity of the mixture is influenced not only by the chosen PDMS, but also by temperature and by the n-pentane concentration, see [2]. Effects related to n-pentane depletion close to the heating surface will likely by less pronounced for higher average n-pentane concentrations in the pool. And boiling research for less viscous mixtures suggests that influences by surface structure and material of the heating surface have to be considered as well. The goal of this work is to establish an experimental data base that allows to distinguish between effects of the mentioned factors and to quantify the effects that are most relevant for heat transfer in boiling highly viscous mixtures.
4 Results and discussion
The results reported here address the variation of the heat transfer coefficient with increasing heat fluxes with a focus on the influence of the evaporator temperature (Sect. 3.1), the mass fraction of the volatile component (Sect. 3.2), the surface structure of the test tube (Sect. 3.3) and the viscosity of the non-volatile component (Sect. 3.4). The covered parameter range is summarized in Tables 1 and 2.
The dependence of the viscosity on the mixture composition and the temperature are discussed in detail in an article published in parallel in this journal [4].
5 Influence of the temperature
In fluids of low viscosity, the influence of the reduced pressure p* on boiling heat transfer.
was investigated [8]. The reduced pressure is commonly calculated as
with pc the critical pressure of the boiling fluid. At convective heat transfer, the variation of all parameters, e.g., reduced pressure, test tube diameter, surface structure and heat flux, changes the heat transfer coefficient only by a factor of two [9]. However, at nucleate boiling, the heat transfer coefficient increases significantly with increasing reduced pressure. The critical pressures of the mixtures studied in the present work are unknown; for PDMS the critical temperature is much higher than the limit of its chemical stability. Thus, reduced pressures cannot be calculated. As a corresponding property, the temperature in the evaporator was chosen. The influence of the temperature on the heat transfer coefficient in highly viscous mixtures was investigated over a temperature range from 319 to 380 K. All parameters were kept constant except for the temperature in the evaporator to separate different influences from each other. The temperature is regulated by four heating elements in a ventilation conduit, which is installed in the climatic chamber [4]. In Fig. 2, results of a measurement series in a mixture with 20 wt.-% M100k are plotted. The temperature was varied in ≈10 K steps from 319 to 379 K. Nucleate boiling is the main heat transfer mechanism for all data points.
The general observation is, that at all temperatures, the heat transfer coefficient increases with increasing heat flux. From a specific temperature on (351 K in the case shown in Fig. 2, the heat transfer coefficient reaches a maximum and decreases again at higher heat fluxes. Below this temperature, the increase in the heat transfer coefficient declines at the highest observed heat fluxes. From 0.15 kW/m2 to 10 kW/m2, the evaporator temperatures show an ascending order, which is in agreement with results on heat transfer in refrigerants [10]. At heat fluxes above 10 kW/m2, the temperature order changes because the α(q) maximum is reached first for the highest temperatures. At this point, all nucleation sites are activated, and the test tube is covered with bubbles; the heat transfer by nucleate boiling is hindered. For high evaporator temperatures, this stage is reached at lower heat fluxes than for low temperatures. In Fig. 3a, results are shown for heat transfer measurements in a mixture with 73 wt.-% M100k at three different temperatures. Compared to Fig. 2, the maximum heat transfer coefficients, and the slope of the α(q)—trends are lower because of the larger PDMS fraction. The ascending order of the evaporator temperatures holds from 0.5 kW/m2 to 4 kW/m2, the formation of a maximum can be observed again. For the measurement series at T = 379 K, all nucleation sites are activated at 4 kW/m2, and the heat transfer coefficient decreases again with further increasing heat flux, whereas the maximum heat transfer coefficient at T = 316 K is only reached at 8 kW/m2. At 0.15 kW/m2 the data points at 352 K and 379 K indicate a reversed order, but at this extremely low heat flux the difference between the data points is still considered within the mutual experimental uncertainty.
Log, log α(q)—diagram for a 73 wt.-% PDMS M100k and n-pentane at ∆ T = 316 K, × T = 352 K and + T = 379 K on a polished copper tube b 95 wt.-% PDMS M10k and n-pentane at ∆ T = 323 K, × T = 355 K and + T = 380 K on a polished copper tube c 95 wt.-% PDMS M1000k and n-pentane at ∆ T = 325 K, × T = 350 K and + T = 374 K on a sanded copper tube
Figure 3b presents the heat transfer coefficient as a function of the heat flux at three different temperatures for mixtures with 95 wt.-% PDMS M10k on a polished copper test tube. The transition from free convection to nucleate boiling is at approximately 1 kW/m2 ≤ q ≤ 2 kW/m2. The evaporator temperatures show an ascending order, whereby a significant temperature influence is observed only in the nucleate boiling regime. A flattening of the α(q)—trend cannot be observed in the range of measured heat fluxes. Experiments at higher heat flux were not conducted because the test tube could have been damaged due to extremely high heating surface temperatures.
The influence of temperature on the heat transfer coefficient in a mixture with 95 wt.-% M1000k was studied as well (see Fig. 3c). Here, the heat transfer coefficient increases only slightly with increasing heat fluxes; the temperature’s influence is visible but less pronounced. At low temperatures, the heat transfer coefficient is lowest, and it increases slightly with increasing temperature at constant heat flux.
The experimental data show that the heat transfer coefficient is larger at higher temperatures when being measured at constant heat flux; all data confirm this relation within their experimental uncertainty. Exceptions are possible for heat fluxes beyond the observed maximum in the heat transfer coefficient. Since this maximum is reached earlier at higher temperature, it may result in a reversed temperature dependence of the heat transfer coefficient. The evaporator temperature influences the nucleation and the bubble density on the surface of the test tube depending on the heat flux. The maximum observed for the heat transfer coefficient depends on the temperature and on the composition of the mixture. When all nucleation sites are activated and the heating surface is increasingly covered by vapor, the heat transfer coefficient does not increase further but decreases despite further increasing heat flux. At high PDMS concentrations, the impact of temperature on the heat transfer coefficient is lower. For high concentrations of PDMS, in particular for the highly viscous PDMS M1000k, the temperature related decrease in viscosity seems to become the main reason for the increase of the heat transfer coefficient with temperature.
6 Influence of the mass fraction of the volatile component
The composition of the mixture has a large impact on the heat transfer coefficient; with a larger n-pentane fraction, the viscosity of the mixture decreases significantly. Convective heat transfer is improved by a lower viscosity, and the bubbles must overcome less resistance while they nucleate and rise. A higher nucleation rate leads to a more turbulent boundary layer above the test tube, the effect of n-pentane depletion close to the heating surface is reduced. Therefore, a larger n-pentane fraction leads to a higher heat transfer coefficient.
In Fig. 4, experimental α(q) data for five different compositions covering the range from 7 wt.-% to 95 wt.-% M100k at T ≈ 352 K are presented. For the mixtures with 7 wt.-%, 20 wt.-% and 30 wt.-% M100k, nucleate boiling is the main heat transfer mechanism at all heat fluxes. Values of the heat transfer coefficient are significantly higher in mixtures with a larger n-pentane fraction and decrease with an increasing fraction of PDMS. Maxima in α(q) are fully developed and shift to lower heat fluxes with increasing fraction of PDMS up to 73 wt.-% PDMS. For mixtures with 73 wt.-% and 95 wt.-% PDMS M100k, the transition from convective boiling to nucleate boiling is in the range 1 kW/m2 ≤ q ≤ 2 kW/m2. The experimental heat transfer coefficients of these measurement series are very similar and overlap partly. The slope of the α(q) data trend is smaller compared to the mixtures containing less PDMS. At 73 wt.-% PDMS, a weak maximum in α(q) is still visible.
An increase of the PDMS fraction in mixtures with a small concentration of PDMS has a much higher impact on the heat transfer coefficient than it has on mixtures with a large fraction of PDMS. Increasing the PDMS fraction from 7 wt.-% to 20 wt.-% PDMS M100k reduces the heat transfer coefficient by up to 80%. On the contrary, a change in composition from 73 wt.-% to 95 wt.-% reduces the heat transfer coefficient by only 20% to 30%.
In mixtures with M10k and n-pentane, heat transfer measurements for three different compositions were conducted at T ≈ 354 K (Fig. 5). The measured heat transfer coefficients in a fluid with 7 wt.-% PDMS M10k are considerable higher than in fluids with 84 wt.-% or 95 wt.-%. The α(q)—trend forms a maximum at q = 20 kW/m2 for 7 wt.-% PDMS M10k and decreases with further increasing heat flux. PDMS enrichment close to the test tube and increasing vapor coverage of the test tube lead to a decreasing heat transfer coefficient. Up to a heat flux of q = 1 kW/m2, convective boiling was observed for 84 wt.-% and 95 wt.-% PDMS M10k. At higher heat fluxes, in the range of nucleate boiling, the heat transfer coefficient is systematically larger for the mixture with 84 wt.-% PDMS M10k. These measurement series confirm the deterioration of the heat transfer coefficient with a decreasing fraction of n-pentane.
7 Influence of the surface structure of the test tube
The influence of the surface structure on the heat transfer coefficient can be calculated for pure fluids of low viscosity such as water or other refrigerants with empirical correlations given, e.g., in [10]. However, there are no published data and therefore no empirical correlations available for highly viscous mixtures. For this research, a sanded and a polished surface structure of copper test tubes were produced and analyzed by Prof. A. Luke and her team at University Kassel. The surface roughness can be described by the parameter Pa, which represents the arithmetic mean of the absolute values of the profile height within a single measuring section in relation to the primary profile [11]; for a more detailed description of the used profiles see [4].
Experiments with these test tubes were conducted in mixtures with PDMS M10k and M100k. In Fig. 5, the measured heat transfer coefficients are plotted over the heat flux for a mixture with 84 wt.-% M10k at T ≈ 327 K (a) and T ≈ 359 K (b) for a sanded and a polished test tube.
As expected, the surface structure has no significant influence on the heat transfer for convective boiling; differences between both surfaces are stochastic and within the combined uncertainty of the measured values. The transition to nucleate boiling was observed at heat fluxes 0.5 kW/m2 ≤ q ≤ 1 kW/m2. At nucleate boiling, the heat transfer coefficient on the sanded copper tube is larger than on the polished tube for T ≈ 327 K. At heat fluxes 10 ≤ q ≤ 16 kW/m2, the difference between the polished and the surface is largest with Δα = 16 W/m2K (10%). At a higher evaporator temperature (T ≈ 359 K, Fig. 6b), the effect of the surface structure becomes smaller. The reinforcing effect of the sanded surface can be observed for q ≥ 4 kW/m2, but less strong than at T ≈ 327 K.
The influence on the surface structure was investigated for a mixture with 95 wt.-% M10k at three different temperatures as well. The results are plotted in Fig. 7a-c.
At all three temperatures, the heat transfer coefficients measured for both surfaces at convective boiling (q ≤ 2 kW/m2) agree within the uncertainty of the measurements. For nucleate boiling, the heat transfer coefficient on the sanded copper tube is significantly higher at T ≈ 323 K and T ≈ 355 K. At T = 380 K, the surface structure appears to have no influence on the heat transfer coefficient. For T ≈ 323 K, the maximum effect of the surface structure was determined at q = 8 kW/m2 and q = 10 kW/m2. The maximum difference in the heat transfer coefficient is Δα = 16 W/m2K. At T ≈ 355 K, the effect shifts to lower heat fluxes with a maximum of Δα = 18 W/m2K at q = 4 kW/m2.
In Fig. 8 the influence of the surface structure is shown based on two measurement series with mixtures with 94/95 wt.-% M100k at T = 321 K.
The sanded surface improves the heat transfer, which results in larger heat transfer coefficients. Especially at nucleate boiling between 4 kW/m2 ≤ q ≤ 10 kW/m2, the enhancement is Δα = 16 to 18 W/m2K, which is equivalent to findings for mixtures with PDMS M10k. For very high heat fluxes, the effect is reduced because the test tube is completely covered with insulating bubbles.
The discussed observations suggest that even in highly viscous mixtures the surface structure has an influence on heat transfer. For nucleate boiling, the higher surface roughness of the sanded copper test tube improves the heat transfer coefficient in all fluids (84 wt.-% and 94/95 wt.-% M10k and M100k, respectively) and at various evaporator temperatures (322 K ≤ T ≤ 380 K). The absolute enhancements of the heat transfer coefficient are lower in highly viscous mixtures than in refrigerants [10], but the relative enhancements of the heat transfer coefficient are significant with a maximum of 19%.
7.1 Influence of the viscosity of the different types of PDMS
To investigate and quantify the influence of viscosity on the heat transfer coefficient, mixtures with PDMS M10k, M100k, and M1000k, but with the same or a similar fraction of n-pentane were prepared, and heat transfer measurements were conducted. In Fig. 9 the α(q)—trends for similar mixtures with 84 wt.-% M10k and 88 wt.-% M100k at a) T ≈ 324 K and at b) T ≈ 357 K are plotted.
In Fig. 9, there is only a small difference between the measured values at convective boiling up to q ≤ 1 kW/m2. With the onset of nucleate boiling and with increasing heat flux, the heat transfer coefficient increases significantly more for the mixture with M10k than for the mixture with M100k. The difference increases constantly with increasing heat fluxes from Δα = 36 W/m2K for q = 4 kW/m2 to Δα = 73 W/m2K for q = 12 kW/m2. A flattening or even a decrease in the α(q)-trends can be recognized for the last three measuring points of each measurement series. The maximum heat transfer coefficient in the mixture with M100k was measured at lower heat fluxes than in the mixture with M10k. The overheating ΔT between the test tube and the fluid is ΔT = 124 K at q = 12 kW/m2 for the mixture with M100k. In contrast, the overheating for the fluid with M10k is ΔT = 92 K at q = 16 kW/m2. The higher viscosity inhibits the formation of bubbles, inhibits the mass-transport processes and thus reduces the heat transfer coefficient.
The results of heat transfer measurements in these mixtures (84 wt.-% M10k and 88 wt.-% M100k) at T ≈ 357 K are shown in Fig. 9b. The trend of the measurement series in Fig. 9b resembles the one in Fig. 9a. The flattening in the range of high heat fluxes is also well visible. This measurement series indicates that lower viscosity results in larger heat transfer coefficients for convective boiling as well. An effect that was expected, but the difference hardly exceeds the combined uncertainty of the data for both mixtures. The transition from convective boiling to nucleate boiling takes place between 1 kW/m2 ≤ q ≤ 2 kW/m2. Compared to an evaporator temperature of T ≈ 324 K, the difference between the heat transfer coefficients is slightly lower with Δα = 55 W/m2K at q = 12 kW/m2. The difference Δα = 42 W/m2K at a heat flux of q = 4 kW/m2 is in a similar order of magnitude as the one observed for T ≈ 324 K.
When the fraction of PDMS is increased from 84/88 wt.-% to 94/95 wt.-%, respectively, at T ≈ 323 K the transition from free convection to nucleate boiling shifts to higher heat fluxes, namely to 2 kW/m2 ≤ q ≤ 4 kW/m2 (Fig. 10). Over the entire measuring range, the heat transfer coefficient for the mixture with M10k is higher than for the fluid with M100k. For q = 12 kW/m2, the difference of the heat transfer coefficients is Δα = 30 W/m2K; for q = 4 kW/m2 it is Δα = 20 W/m2K. Measurements in a mixture with 95 wt.-% M1000k were carried out as well. For M1000k, all measuring points are in the range of nucleate boiling. At low heat fluxes, a slight increase of α with increasing heat flux can be observed. From q = 4 kW/m2 on, the heat transfer coefficient stagnates despite an increasing heat flux.
A comparison of Figs. 9a and 10 shows that the values measured for the investigated mixtures with M100k are very similar (see Fig. 11). The larger PDMS fraction (88 wt.-% to 94 wt.-%) has hardly any influence on the heat transfer coefficient. For mixtures with M10k, however, a clear decrease of the heat transfer coefficient is observed due to the higher concentration of PDMS M10k. From a certain viscosity onwards, a further increase in viscosity seems to have little effect on the heat transfer coefficient. At T ≈ 323 K, the differences in the heat transfer coefficients Δα of the mixtures with 94/95 wt.-% M10k/M100k are much smaller than for the mixtures with 84/88 wt.-% M10k/M100k. The viscosities of the mixtures with 94/95 wt.-% M10k/M100k are higher due to the larger fraction of PDMS. The discussed data series are plotted in Fig. 11.
In Fig. 12, 13, 14 further data series are presented. The mixtures have the same composition, but the kind of PDMS (PDMS M10k, M100k and M1000k) varies. All diagrams confirm that the heat transfer coefficient significantly decreases with a higher viscosity at all heat flux densities, temperatures, and compositions. The trend of the measured data is similar, especially the flattening of the α(q)—trend at high heat fluxes, which is observed for several measurement series.
In Fig. 12a) two measurement series for the polished copper tube are plotted additionally to the data measured for the sanded tube shown in Fig. 9a). The trends are very similar, and the measuring points agree within their reproducibility. Although the temperature and the compositions are not the same, the influence of the viscosity clearly prevails the influence of the surface structure.
The heat transfer coefficient in these mixtures was investigated at T ≈ 352 K as well and compared with data for the polished tube. The results are plotted in Fig. 12b.
For 4 kW/m2 ≤ q ≤ 10 kW/m2, the difference of the heat transfer coefficient between the mixture with M10k and the mixture with M100k on the polished tube is Δα = 46 W/m2K. The temperature increase from T ≈ 326 K to 354 K has hardly any influence on the measurement results (not shown graphically). At nucleate boiling, the influence of the temperature is negligible in this case. Comparing the measurement series on the polished tube with those on the sanded tube (Fig. 12b), the statement made with reference to Fig. 12a is confirmed. The α(q)-trends are very similar. The influence of the viscosity on the heat transfer coefficient is significantly higher than the influence of the surface structure.
Heat transfer measurements were conducted in mixtures with 95 wt.-% M10k, M100k and M1000k at T ≈ 323 K (Figs. 10 and 13) and 354 K (Fig. 13). A reduction of the heat transfer coefficient with increasing nominal viscosity of the PDMS can be clearly observed. We conclude that the evaporator temperature has only a slight influence on the heat transfer coefficient for all heat fluxes in mixtures with a high fraction of PDMS. Effects such as higher pressure and density in the bubbles are at least almost negligible in this case. The remaining influence of the pool temperature is related to its influence on viscosity and the influence of viscosity on the heat transfer coefficient.
The heat transfer was additionally investigated in mixtures with 7 wt.-% PDMS M10k and M100k at an evaporator temperature of T ≈ 350 K (Fig. 14). In mixtures with a large n-pentane fraction, nucleate boiling occurs at much lower heat fluxes. For the mixture containing M10k, nucleate boiling was observed for q > 0.4 kW/m2; for the mixture containing M100k, nucleate boiling was observed over the entire range of heat fluxes.
The transition to nucleate boiling shifts towards lower heat fluxes with increasing viscosity. A clear maximum of the heat transfer coefficient, αmax, is formed for both fluids. At higher heat fluxes the heat transfer coefficient becomes smaller again. The offset of the maxima is clearly based on the different viscosities of M10k and M100k. The measured heat transfer coefficients in the fluid with M100k are expected to be below those in the fluid with M10k at all heat fluxes. The atypical shift at 0.4 kW/m2 ≤ q ≤ 6 kW/m2 could be due to different settings of the condenser temperature and not to an effect of the viscosity.
8 Conclusion
Heat transfer measurements at pool boiling conditions in highly viscous mixtures over a wide temperature range were carried out utilizing a modified so-called standard apparatus. A comprehensive set of data was generated. The focus was set on the systematic investigation of the influence of the temperature, the surface structure, the viscosity and the fraction of the volatile component on the heat transfer coefficient.
All measurement series show that a higher evaporator temperature enhances the heat transfer coefficient at constant heat flux. In general, α(q) increases with the heat flux. However, the α(q)-trend forms a maximum and declines with further increasing heat fluxes again in particular for mixtures with low PDMS content. It was observed that the characteristics of the maxima depend on the temperature and the composition. The impact of the temperature on the heat transfer coefficient is smaller in mixtures with a large PDMS fraction. To evaluate the influence of the fraction of the volatile component, six different binary mixtures were prepared with M10k and M100k. The measurement series show that a lager n-pentane fraction generally leads to a higher heat transfer coefficient. The α(q)-trend is significantly influenced by the composition of the mixture. Changing the fraction of PDMS has a much higher impact on the heat transfer coefficient in mixtures with mainly n-pentane. Measurement series on sanded and polished test tubes show that the surface structure has an influence on the heat transfer coefficient in highly viscous fluids. The absolute enhancements by the sanded surface is lower than in mixtures of lower viscosity, but the relative enhancements are significant. To evaluate the influence of the viscosity, mixtures of the same composition but with different nominal viscosities were produced. All measurement series confirm that a larger viscosity reduces the heat transfer coefficient, but the reduction seems to be limited. Overall, the heat transfer coefficient is significantly more influenced by the viscosity than by the surface structure.
Notes
Polydimethylsiloxane is a silicon oil and is frequently referred to as such in literature. However, since there are different silicon oils available on the market and since we cannot be sure that our results are completely independent of the type of silicone oil, we stick to the more specific denomination PDMS in this article.
The numbers 10, 100 and 1000 in M10k, M100k and M100k refer to the viscosity at 298.15 K in [Pa∙s], e.g. η PDMS M10k 10 Pa∙s.
Abbreviations
- A:
-
area [M2]
- d:
-
diameter [M]
- I:
-
current [A]
- k:
-
extension factor for measurement uncertainties [-]
- l:
-
heated length [M]
- P:
-
mean value of the primary profile coordinates [M]
- p:
-
pressure [Pa]
- Q:
-
heat flow [W]
- q:
-
heat flux [W/m2]
- R:
-
resistance [Ω]
- r:
-
thermal correction factor [M2K/W]
- T:
-
temperature in the Kelvin scale (ITS-90) [K]
- U:
-
voltage [V]
- α:
-
heat transfer coefficient [W/m2K]
- ∆:
-
difference [-]
- η:
-
dynamic viscosity [Pa∙s]
- λ:
-
thermal conductivity [W/mK]
- a:
-
unfiltered arithmetic mean [-]
- c:
-
critical [-]
- exp:
-
experimental [-]
- i:
-
inner [-]
- mx:
-
maximum [-]
- o:
-
outer [-]
- r, th:
-
corrected by rth [-]
- th:
-
themal [-]
- * :
-
reduced [-]
References
Adib T, Vasseur J (2008) Bibliographic analysis of predicting heat transfer coefficients in boiling for applications in designing liquid food evaporators. J of Food Engineering 87:149–161. https://doi.org/10.1016/j.jfoodeng.2007.12.013
Wienecke M, Luke A, Gorenflo D, Span R (2005) Flow boiling of highly viscous fluids in a vertical annular tube. J Chem Eng Res and Design 83:1044–1051.
Buljina I (2010) Entwicklung und Aufbau zweier Siedeapparaturen zur Untersuchung des Wärmeübergangs weitsiedender binärer niedrig- und hochviskoser Gemische. Dissertation, Ruhr University Bochum.
Fleer A, Richter M, Span R (2020) Development of a modified standard apparatus for heat transfer measurements in highly viscous binary fluids. Heat Mass Transf. submitted.
Kedzierski M (2001) The effect of lubricant concentration, miscibility, and viscosity on R134a pool boiling. Int J of Ref 24:348–266.
Spindler K, Hahne E (2009) The influence of oil on nucleate pool boiling heat transfer. Heat Mass Transfer 45:979–990.
Cien LH, Tsai YL, Chang CH (2019) A study of pool boiling and falling-film vaporization with R-245fa/oil mixtures on horizontal tubes Int. J of Heat and Mass Transfer 133:940–950. https://doi.org/10.1016/j.ijheatmasstransfer.2018.12.176
Gorenflo D, Goetz J, Bier K (1982) Proposal of a standard apparatus for the measurement of pool boiling heat transfer. Wärme- und Stoffübertragung 16:69.
Baehr H, Stephan K (2019) Wärme- und Stoffübertragung, 10th edn. Springer, Berlin Heidelberg.
Gorenflo D (2013) H2. In: VDI-Wärmeatlas, 11th edn. Springer, Berlin Heidelberg, 853–894.
DIN EN ISO 4287:2010–07 Geometrical Product Specifications (GPS) - Surface texture: Profile method - Terms, definitions and surface texture parameters. 2010–07.
Acknowledgements
The authors are grateful to all who contributed to this work, especially to Prof. A. Luke and her team at University of Kassel. We thank the Deutsche Forschungsgemeinschaft (DFG) for funding this project under Grant No. SP 507/6-2. We are thankful to Stanislaw Derksen for supporting the present project within the scope of his bachelor’s and master’s theses and to Jan Humbach, a student research assistant. Moreover, we thank Christian Gramann and the team of the mechanical workshop at Ruhr University Bochum for their support.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fleer, AC., Richter, M. & Span, R. Systematic heat transfer measurements in highly viscous binary fluids. Heat Mass Transfer 57, 2067–2080 (2021). https://doi.org/10.1007/s00231-021-03087-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00231-021-03087-w