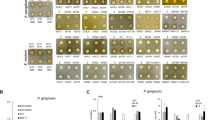
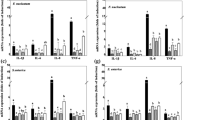
Abstract
Previously, we showed that the growth, antibiotic resistance, and biofilm formation properties of the pathogens Pseudomonas aeruginosa and Klebsiella pneumonia were tremendously inhibited by the cell-free supernatant of the oral probiotic Streptococcus salivarius M18. These anti-pathogenic activities of the supernatant were more efficient under acidic conditions. The present approach takes advantage of the acidic nature of the tumor microenvironment to evaluate the effect of the S. salivarius M18 postbiotics on colon cancer cells. In both two-dimensional (2D) and three-dimensional (3D) cell culture models, S. salivarius M18 cell-free supernatant showed anti-cancer actions in the pH conditions mimicking the acidity of the tumor. The inhibitory effect was more prominent when the colon cancer cells have been treated with the cell-free supernatant obtained from the inulin incubated S. salivarius M18. The results of this study point out the potential of the S. salivarius M18 functional probiotic products to be used for targeting low pH environments including the unique acidic microenvironment of tumors.






Similar content being viewed by others
Data Availability
All data generated during this study are available from the corresponding author on reasonable request.
References
Górska A, Przystupski D, Niemczura MJ, Kulbacka J (2019) Probiotic bacteria: a promising tool in cancer prevention and therapy. Curr Microbiol 76:939–949. https://doi.org/10.1007/s00284-019-01679-8
Molska M, Reguła J (2019) Potential mechanisms of probiotics action in the prevention and treatment of colorectal cancer. Nutrients 11:2453. https://doi.org/10.3390/nu11102453
Raman M, Ambalam P, Kondepudi KK, Pithva S, Kothari C, Patel AT, Vyas BRM (2013) Potential of probiotics, prebiotics and synbiotics for management of colorectal cancer. Gut microbes 4:181–192. https://doi.org/10.4161/gmic.23919
Hendler R, Zhang Y (2018) Probiotics in the treatment of colorectal cancer. Medicines (Basel) 5:101. https://doi.org/10.3390/medicines5030101
Delia P, Sansotta G, Donato V, Frosina P, Messina G, De Renzis C, Famularo G (2007) Use of probiotics for prevention of radiation-induced diarrhea. World J Gastroenterol 13:912. https://doi.org/10.3748/wjg.v13.i6.912
Mego M, Chovanec J, Vochyanova-Andrezalova I, Konkolovsky P, Mikulova M, Reckova M, Drgona L (2015) Prevention of irinotecan induced diarrhea by probiotics: a randomized double blind, placebo controlled pilot study. Complement Ther Med 23:356–362. https://doi.org/10.1016/j.ctim.2015.03.008
Pino A, Angelis MDE, Chieppa M, Caggia C, Randazzo CL (2020) Gut microbiota, probiotics and colorectal cancer: a tight relation. World Cancer Res J 7:e1456.https://doi.org/10.32113/wcrj_20201_1456
Rad AH, Aghebati-Maleki L, Kafil HS, Abbasi A (2020) Molecular mechanisms of postbiotics in colorectal cancer prevention and treatment. Crit Rev Food Sci Nutr 1–17. Online ahead of print. https://doi.org/10.1080/10408398.2020.1765310
Lerner A, Shoenfeld Y, Matthias T (2019) Probiotics: if it does not help it does not do any harm. Really? Microorganisms 7:104. https://doi.org/10.3390/microorganisms7040104
Sivieri K, Bedani R, Umbelino Cavallini DC, AE (2013) Probiotics and intestinal microbiota: implications in colon cancer prevention. probiotics and intestinal microbiota: implications in colon cancer prevention. Lactic Acid Bacteria - R & D for Food, Health and Livestock Purposes, Marcelino Kongo, IntechOpen, https://doi.org/10.5772/51696
Dreher-Lesnick SM, Schreier JE, Stibitz S (2015) Development of phage lysin LysA2 for use in improved purity assays for live biotherapeutic products. Viruses 7:6675–6688. https://doi.org/10.3390/v7122965
Doron S, Snydman DR (2015) Risk and safety of probiotics. Clin Infect Dis 60(Suppl 2):129–134. https://doi.org/10.1093/cid/civ085
Nataraj BH, Ali SA, Behare PV, Yadav H (2020) Postbiotics-parabiotics: the new horizons in microbial biotherapy and functional foods. Microb Cell Fact 19:1–22. https://doi.org/10.1186/s12934-020-01426-w
Sanders ME, Merenstein DJ, Ouwehand AC, Reid G, Salminen S, Cabana MD, Leyer G (2016) Probiotic use in at-risk populations. J Am Pharm Assoc 56:680–686. https://doi.org/10.1016/j.japh.2016.07.001
FAO/WHO (2006) Probiotics in food: health and nutritional properties and guidelines for evaluation. FAO/WHO, Rome
Legesse Bedada T, Feto TK, Awoke KS, Garedew AD, Yifat FT, Birri DJ (2020) Probiotics for cancer alternative prevention and treatment. Biomed Pharmacother 129:110409. https://doi.org/10.1016/j.biopha.2020.110409
Adams CA (2010) The probiotic paradox: live and dead cells are biological response modifiers. Nutr Res Rev 23:37–46. https://doi.org/10.1017/S0954422410000090
Gagliardi A, Totino V, Cacciotti F, Iebba V, Neroni B, Bonfiglio G, Schippa S (2018) Rebuilding the gut microbiota ecosystem. Int J Environ Res Public Health 15:1679. https://doi.org/10.3390/ijerph15081679
Martín R, Langella P (2019) Emerging health concepts in the probiotics field: streamlining the definitions. Front Microbiol 10:1047. https://doi.org/10.3389/fmicb.2019.01047
Fong W, Li Q, Yu J (2020) Gut microbiota modulation: a novel strategy for prevention and treatment of colorectal cancer. Oncogene 39:4925–4943. https://doi.org/10.1038/s41388-020-1341-1
Homayouni Rad A, Aghebati Maleki L, Samadi Kafil H, Abbasi A (2020) Postbiotics: a novel strategy in food allergy treatment. Crit Rev Food Sci Nutr 1:8. https://doi.org/10.1080/10408398.2020.1738333
Stowik TA (2016) Contribution of probiotics Streptococcus salivarius strains K12 and M18 to oral health in humans: a review. Honors Scholar Theses 488:1–27. https://opencommons.uconn.edu/srhonors_theses/488
Park HK, Shim SS, Kim SY, Park JH, Park SE, Kim HJ, Kim CM (2005) Molecular analysis of colonized bacteria in a human newborn infant gut. J Microbiol 43:345–353
Urbaniak C, Burton JP, Reid G (2012) Breast, milk and microbes: a complex relationship that does not end with lactation. Womens Health (Lond) 8:385–398. https://doi.org/10.2217/WHE.12.23
Sallam M, Wali I, Attia AEF, Mehanna NS (2016) Relation between probiotic properties of isolates isolated from breast milk and infants’ stools. Nutr Food Sci 46:294–305. https://doi.org/10.1108/NFS-10-2014-0091
Dzidic M, Collado MC, Abrahamsson T, Artacho A, Stensson M, Jenmalm MC, Mira A (2018) Oral microbiome development during childhood: an ecological succession influenced by postnatal factors and associated with tooth decay. ISME J 12:2292–2306. https://doi.org/10.1038/s41396-018-0204-z
Ferretti P, Pasolli E, Tett A, Asnicar F, Gorfer V, Fedi S, Segata N (2018) Mother-to-infant microbial transmission from different body sites shapes the developing infant gut microbiome. Cell Host Microbe 24:133–145. https://doi.org/10.1016/j.chom.2018.06.005
Couvigny B, De Wouters T, Kaci G, Jacouton E, Delorme C, Dore J, Lapaque N (2015) Commensal Streptococcus salivarius modulates PPARγ transcriptional activity in human intestinal epithelial cells. PLoS One 10:e0125371. https://doi.org/10.1371/journal.pone.0125371
Di Pierro F, Zanvit A, Nobili P, Risso P, Fornaini C (2015) Cariogram outcome after 90 days of oral treatment with Streptococcus salivarius M18 in children at high risk for dental caries: results of a randomized, controlled study. Clin Cosmet Investig Dent 7:107. https://doi.org/10.2147/CCIDE.S93066
Burton JP, Drummond BK, Chilcott CN, Tagg JR, Thomson WM, Hale JD, Wescombe PA (2013) Influence of the probiotic Streptococcus salivarius strain M18 on indices of dental health in children: a randomized double-blind, placebo-controlled trial. J Med Microbiol 62:875–884. https://doi.org/10.1099/jmm.0.056663-0
Tunçer S, Karaçam S (2020) Cell-free supernatant of Streptococcus salivarius M18 impairs the pathogenic properties of Pseudomonas aeruginosa and Klebsiella pneumonia. Arch Microbiol 202:2825–2840. https://doi.org/10.1007/s00203-020-02005-8
Feng L, Dong Z, Tao D, Zhang Y, Liu Z (2018) The acidic tumor microenvironment: a target for smart cancer nano-theranostics. Natl Sci Rev 5:269–286. https://doi.org/10.1093/nsr/nwx062
Justus CR, Dong L, Yang LV (2013) Acidic tumor microenvironment and pH-sensing G protein-coupled receptors. Front Physiol 4:354. https://doi.org/10.3389/fphys.2013.00354
Andreucci E, Peppicelli S, Ruzzolini J, Bianchini F, Biagioni A, Papucci L, Calorini L (2020) The acidic tumor microenvironment drives a stem-like phenotype in melanoma cells. J Mol Med 98:1431–1446. https://doi.org/10.1007/s00109-020-01959-y
Huber V, Camisaschi C, Berzi A, Ferro S, Lugini L, Triulzi T, Rivoltini L (2017) Cancer acidity: an ultimate frontier of tumor immune escape and a novel target of immunomodulation. Semin Cancer Biol 43:74–89. https://doi.org/10.1016/j.semcancer.2017.03.001
Ward C, Meehan J, Gray ME, Murray AF, Argyle DJ, Kunkler IH, Langdon SP (2020) The impact of tumour pH on cancer progression: strategies for clinical intervention. Explor Target Anti Tumor Ther 1:71–100. https://doi.org/10.37349/etat.2020.00005
Avnet S, Di Pompo G, Lemma S, Baldini N (2019) Cause and effect of microenvironmental acidosis on bone metastases. Cancer Metastasis Rev 38:133–147. https://doi.org/10.1007/s10555-019-09790-9
Erra Díaz F, Dantas E, Geffner J (2018) Unravelling the interplay between extracellular acidosis and immune cells. Mediators Inflamm 1218297. https://doi.org/10.1155/2018/1218297
Zhang Y, Dang M, Tian Y, Zhu Y, Liu W, Tian W, Lu G (2017) Tumor acidic microenvironment targeted drug delivery based on phlip-modified mesoporous organosilica nanoparticles. ACS Appl Mater Interfaces 9:30543–30552. https://doi.org/10.1021/acsami.7b10840
Sulea T, Rohani N, Baardsnes J, Corbeil CR, Deprez C, Cepero-Donates Y, Zwaagstra JC (2020) Structure-based engineering of pH-dependent antibody binding for selective targeting of solid-tumor microenvironment. MAbs 12:1682866. https://doi.org/10.1080/19420862.2019.1682866
Gurbanov R, Karadağ H, Karaçam S, Samgane G (2020) Tapioca starch modulates cellular events in oral probiotic Streptococcus salivarius strains. Probiotics Antimicrob Proteins 13:195–207. https://doi.org/10.1007/s12602-020-09678-z
Swain JE, Stevens J, Schoolcraft WB (2016) Comparison of pH stability of bicarbonate buffered media within a gassed isolette versus MOPS buffered media in room atmosphere (#P-62). Fertil Steril 105:e39. https://doi.org/10.1016/j.fertnstert.2015.12.105
Quinn P, Cooke S (2004) Equivalency of culture media for human in vitro fertilization formulated to have the same pH under an atmosphere containing 5% or 6% carbon dioxide. Fertil Steril 81:1502–1506. https://doi.org/10.1016/j.fertnstert.2004.02.093
Tunçer S, Tunçay Çağatay S, Keşküş AG, Çolakoğlu M, Konu Ö, Banerjee S (2016) Interplay between 15-lipoxygenase-1 and metastasis-associated antigen 1 in the metastatic potential of colorectal cancer. Cell Prolif 49:448–459. https://doi.org/10.1111/cpr.12267
Lee GY, Kenny PA, Lee EH, Bissell MJ (2007) Three-dimensional culture models of normal and malignant breast epithelial cells. Nat Methods 4:359–365. https://doi.org/10.1038/nmeth1015
Bahuguna A, Khan I, Bajpai VK, Kang SC (2017) MTT assay to evaluate the cytotoxic potential of a drug. Bangladesh J Pharmacol 12:115–118. https://doi.org/10.3329/bjp.v12i2.30892
Tunçer S, Çolakoğlu M, Ulusan S, Ertaş G, Karasu Ç, Banerjee S (2019) Evaluation of colloidal platinum on cytotoxicity, oxidative stress and barrier permeability across the gut epithelium. Heliyon 5:e01336. https://doi.org/10.1016/j.heliyon.2019.e01336
Glahn R (2009) The use of Caco-2 cells in defining nutrient bioavailability: application to iron bioavailability of foods. In: McClements D, Decker E (Eds) Designing functional foods: measuring and controlling food structure breakdown and nutrient absorption. Woodhead Publishing Ltd., Cambridge Elsevier 340–361. https://doi.org/10.1533/9781845696603.2.340
Tunçer S, Banerjee S (2017) Determination of autophagy in the Caco-2 spontaneously differentiating model of intestinal epithelial cells. In: Turksen, K (Ed) Autophagy in differentiation and tissue maintenance. Humana Press, New York, NY. 55–70. https://doi.org/10.1007/7651_2017_6651
Torun A, Enayat S, Sheraj I, Tunçer S, Ülgen DH, Banerjee S (2019) Butyrate mediated regulation of RNA binding proteins in the post-transcriptional regulation of inflammatory gene expression. Cell Signal 64:109410. https://doi.org/10.1016/j.cellsig.2019.109410
Ramond MJ, Martinot-Peignoux M, Erlinger S (1985) Dome formation in the human colon carcinoma cell line Caco-2 in culture. Influence of ouabain and permeable supports. Biol Cell 54:89–92. https://doi.org/10.1111/j.1768-322X.1985.tb00383.x
Herzog P, Drosten C, Müller MA (2008) Plaque assay for human coronavirus NL63 using human colon carcinoma cells. Virol J 5:138. https://doi.org/10.1186/1743-422X-5-138
Matikeviciene V, Grikiškis S, Lubyte E, Dienys G (2017) Partial purification and characterization of bacteriocin-like peptide produced by Staphylococcus xylosus. Environ Technol Resour Proc Int Sci Pract Conf 3:213. https://doi.org/10.17770/etr2017vol3.2586
Wang T, Wang L, Wang G, Zhuang Y (2020) Leveraging and manufacturing in vitro multicellular spheroid-based tumor cell model as a preclinical tool for translating dysregulated tumor metabolism into clinical targets and biomarkers. Bioresour Bioprocess 7:35. https://doi.org/10.1186/s40643-020-00325-7
Roy V, Magne B, Vaillancourt-Audet M, Blais M, Chabaud S, Grammond E, Bolduc S (2020) Human organ-specific 3D cancer models produced by the stromal self-assembly method of tissue engineering for the study of solid tumors. Biomed Res Int. https://doi.org/10.1155/2020/6051210
Koltai T (2016) Cancer: fundamentals behind pH targeting and the double-edged approach. Onco Targets Ther 9:6343. https://doi.org/10.2147/OTT.S115438
Annan DA, Maishi N, Soga T, Dawood R, Li C, Kikuchi H, Hida K (2019) Carbonic anhydrase 2 (CAII) supports tumor blood endothelial cell survival under lactic acidosis in the tumor microenvironment. Cell Commun Signal 17:1–15. https://doi.org/10.1186/s12964-019-0478-4
Vinci MC, Visentin B, Cusinato F, Nardelli GB, Trevisi L, Luciani S (2004) Effect of vascular endothelial growth factor and epidermal growth factor on iatrogenic apoptosis in human endothelial cells. Biochem Pharmacol 67:277–284. https://doi.org/10.1016/j.bcp.2003.09.007
Gerber HP, McMurtrey A, Kowalski J, Yan M, Keyt BA, Dixit V, Ferrara N (1998) Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3’-kinase/Akt signal transduction pathway: requirement for Flk-1/KDR activation. J Biol Chem 273:30336–30343. https://doi.org/10.1074/jbc.273.46.30336
Shah P, Jogani V, Bagchi T, Misra A (2006) Role of Caco-2 cell monolayers in prediction of intestinal drug absorption. Biotechnol Prog 22:186–198. https://doi.org/10.1021/bp050208u
Kernéis S, Chauvière G, Darfeuille-Michaud A, Aubel D, Coconnier MH, Joly B, Servin AL (1992) Expression of receptors for enterotoxigenic Escherichia coli during enterocytic differentiation of human polarized intestinal epithelial cells in culture. Infect Immun 60:2572–2580. https://doi.org/10.1128/IAI.60.7.2572-2580.1992
Schreider C, Peignon G, Thenet S, Chambaz J, Pinçon-Raymond M (2002) Integrin-mediated functional polarization of Caco-2 cells through E-cadherin–actin complexes. J Cell Sci 115:543–552
Golub AL, Frost RW, Betlach CJ, Gonzalez MA (1986) Physiologic considerations in drug absorption from the gastrointestinal tract. J Allergy Clin Immunol 78:689–694. https://doi.org/10.1016/0091-6749(86)90047-3
Banfalvi G (2017) Methods to detect apoptotic cell death. Apoptosis 22:306–323. https://doi.org/10.1007/s10495-016-1333-3
Jamaluddin N, Ariff AB, Wong FWF (2019) Purification of a bacteriocin-like inhibitory substance derived from Pediococcus acidilactici Kp10 by an aqueous micellar two-phase system. Biotechnol Prog 35:e2719. https://doi.org/10.1002/btpr.2719
Voss NCS, Dreyer T, Henningsen MB, Vahl P, Honoré B, Boedtkjer E (2020) Targeting the acidic tumor microenvironment: unexpected pro-neoplastic effects of oral NAHCO3 therapy in murine breast tissue. Cancers (Basel) 12:891. https://doi.org/10.3390/cancers12040891
Pillai SR, Damaghi M, Marunaka Y, Spugnini EP, Fais S, Gillies RJ (2019) Causes, consequences, and therapy of tumors acidosis. Cancer Metastasis Rev 38:205–222. https://doi.org/10.1007/s10555-019-09792-7
Corbet C, Feron O (2017) Tumour acidosis: from the passenger to the driver’s seat. Nat Rev Cancer 17:577. https://doi.org/10.1038/nrc.2017.77
Śliżewska K, Markowiak-Kopeć P, Śliżewska W (2021) The role of probiotics in cancer prevention. Cancers (Basel) 13:20. https://doi.org/10.3390/cancers13010020
de Souza Oliveira RP, Perego P, de Oliveira MN, Converti A (2012) Effect of inulin on the growth and metabolism of a probiotic strain of Lactobacillus rhamnosus in co-culture with Streptococcus thermophilus. LWT-Food Sci Technol 47:358–363. https://doi.org/10.1016/j.lwt.2012.01.031
Kim WS, Lee JY, Singh B, Maharjan S, Hong L, Lee SM, Cho CS (2018) A new way of producing pediocin in Pediococcus acidilactici through intracellular stimulation by internalized inulin nanoparticles. Sci Rep 8:1–14. https://doi.org/10.1038/s41598-018-24227-z
Shi W, Kwon J, Huang Y, Tan J, Uhl CG, He R, Liu Y (2018) Facile tumor spheroids formation in large quantity with controllable size and high uniformity. Sci Rep 8:1–9. https://doi.org/10.1038/s41598-018-25203-3
Sodek KL, Ringuette MJ, Brown TJ (2009) Compact spheroid formation by ovarian cancer cells is associated with contractile behavior and an invasive phenotype. Int J Cancer 124:2060–2070. https://doi.org/10.1002/ijc.24188
Hamilton G, Rath B (2019) Role of circulating tumor cell spheroids in drug resistance. Cancer Drug Resist 2:762–772. https://doi.org/10.20517/cdr.2019.47
El Khoury F, Corcos L, Durand S, Simon B, Jossic-Corcos L (2016) Acquisition of anticancer drug resistance is partially associated with cancer stemness in human colon cancer cells. Int J Oncol 49:2558–2568. https://doi.org/10.3892/ijo.2016.3725
Balkwill FR, Capasso M, Hagemann T (2012) The tumor microenvironment at a glance. J Cell Sci 125:5591–5596. https://doi.org/10.1242/jcs.116392
Tunçer S, Keşküş AG, Çolakoğlu M, Çimen I, Yener C, Konu Ö, Banerjee S (2017) 15-Lipoxygenase-1 re-expression in colorectal cancer alters endothelial cell features through enhanced expression of TSP-1 and ICAM-1. Cell Signal 39:44–54. https://doi.org/10.1016/j.cellsig.2017.07.022
Joyce JA (2005) Therapeutic targeting of the tumor microenvironment. Cancer Cell 7:513–520. https://doi.org/10.1016/j.ccr.2005.05.024
Ivey JW, Bonakdar M, Kanitkar A, Davalos RV, Verbridge SS (2016) Improving cancer therapies by targeting the physical and chemical hallmarks of the tumor microenvironment. Cancer Lett 380:330–339. https://doi.org/10.1016/j.canlet.2015.12.019
Rodrigues G, Silva GGO, Buccini DF, Duque HM, Dias SC, Franco OL (2019) Bacterial proteinaceous compounds with multiple activities toward cancers and microbial infection. Front Microbiol 10:1690. https://doi.org/10.3389/fmicb.2019.01690
Chikindas ML, Weeks R, Drider D, Chistyakov VA, Dicks LM (2018) Functions and emerging applications of bacteriocins. Curr Opin Biotechnol 49:23–28. https://doi.org/10.1016/j.copbio.2017.07.011
Kaci G, Goudercourt D, Dennin V, Pot B, Doré J, Ehrlich SD, Delorme C (2014) Anti-inflammatory properties of Streptococcus salivarius, a commensal bacterium of the oral cavity and digestive tract. Appl Environ Microbiol 80:928–934. https://doi.org/10.1128/AEM.03133-13
Koliarakis I, Messaritakis I, Nikolouzakis TK, Hamilos G, Souglakos J, Tsiaoussis J (2019) Oral bacteria and intestinal dysbiosis in colorectal cancer. Int J Mol Sci 20:4146. https://doi.org/10.3390/ijms20174146
Kato I, Vasquez AA, Moyerbrailean G, Land S, Sun J, Lin HS, Ram JL (2016) Oral microbiome and history of smoking and colorectal cancer. J Epidemiol Res 2:92. https://doi.org/10.5430/jer.v2n2p92
Arora M, Weuve J, Fall K, Pedersen NL, Mucci LA (2010) An exploration of shared genetic risk factors between periodontal disease and cancers: a prospective co-twin study. Am J Epidemiol 171:253–259. https://doi.org/10.1093/aje/kwp340
Yen AMF, Lai H, Fann JCY, Chiu SH, Chen SS (2014) Relationship between community periodontal index and fecal hemoglobin concentration, an indicator for colorectal neoplasm. J Dent Res 93:760–766. https://doi.org/10.1177/0022034514539976
Olsen I, Yamazaki K (2019) Can oral bacteria affect the microbiome of the gut? J Oral Microbiol 11:1586422. https://doi.org/10.1080/20002297.2019.1586422
Kostic AD, Gevers D, Pedamallu CS, Michaud M, Duke F, Earl AM, Meyerson M (2012) Genomic analysis identifies association of Fusobacterium with colorectal carcinoma. Genome Res 22:292–298. https://doi.org/10.1101/gr.126573.111
Komiya Y, Shimomura Y, Higurashi T, Sugi Y, Arimoto J, Umezawa S, Nakajima A (2019) Patients with colorectal cancer have identical strains of Fusobacterium nucleatum in their colorectal cancer and oral cavity. Gut 68:1335–1337. https://doi.org/10.1136/gutjnl-2018-316661
Kostic AD, Chun E, Robertson L, Glickman JN, Gallini CA, Michaud M, Garrett WS (2013) Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microbe 14:207–215. https://doi.org/10.1016/j.chom.2013.07.007
Tsuzuno T, Takahashi N, Yamada-Hara M, Yokoji-Takeuchi M, Sulijaya B, Aoki-Nonaka Y, Yamazaki K (2021) Ingestion of Porphyromonas gingivalis exacerbates colitis via intestinal epithelial barrier disruption in mice. J Periodontal Res 56:275–288. https://doi.org/10.1111/jre.12816
Liu XB, Gao ZY, Sun CT, Wen H, Gao B, Li SB, Tong Q (2019) The potential role of P.gingivalis in gastrointestinal cancer: a mini review. Infect Agent Cancer 14:23. https://doi.org/10.3389/fcimb.2020.585917
Yang Y, Cai Q, Shu X, Steinwandel MD, Blot WJ, Zheng W, Long J (2019) Prospective study of oral microbiome and colorectal cancer risk in low-income and African American populations. Int J Cancer 144:2381–2389. https://doi.org/10.1002/ijc.31941
Vesty A, Gear K, Boutell S, Taylor MW, Douglas RG, Biswas K (2020) Randomised, double-blind, placebo-controlled trial of oral probiotic Streptococcus salivarius M18 on head and neck cancer patients post-radiotherapy: a pilot study. Sci Rep 10(1):13201. https://doi.org/10.1038/s41598-020-70024-y
Vesty A, Gear K, Biswas K, Radcliff FJ, Taylor MW, Douglas RG (2018) Microbial and inflammatory-based salivary biomarkers of head and neck squamous cell carcinoma. Clin Exp Dent Res 4:255–262. https://doi.org/10.1002/cre2.139
Burton JP, Wescombe PA, Macklaim JM, Chai MH, MacDonald K, Hale JD, Cadieux PA (2013) Persistence of the oral probiotic Streptococcus salivarius M18 is dose dependent and megaplasmid transfer can augment their bacteriocin production and adhesion characteristics. PLoS One 8:e65991. https://doi.org/10.1371/journal.pone.0065991
Yoo HJ, Jwa SK, Kim DH, Ji YJ (2020) Inhibitory effect of Streptococcus salivarius K12 and M18 on halitosis in vitro. Clin Exp Dent Res 6:207–214. https://doi.org/10.1002/cre2.269
Acknowledgements
The authors sincerely acknowledge Dr. Sreeparna Banerjee (Middle East Technical University) and Dr. Sabahattin Cömertpay (Kahramanmaraş Sütçü İmam University) for sharing cell lines and Dr. Rafig Gurbanov (Bilecik Şeyh Edebali University) for sharing bacterial strains and for providing some reagents used in this study. We also thank Bilecik Şeyh Edebali University, Biotechnology Application and Research Center for the provision of the laboratory facilities.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Karaçam, S., Tunçer, S. Exploiting the Acidic Extracellular pH: Evaluation of Streptococcus salivarius M18 Postbiotics to Target Cancer Cells. Probiotics & Antimicro. Prot. 14, 995–1011 (2022). https://doi.org/10.1007/s12602-021-09806-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-021-09806-3