Abstract
Introduction
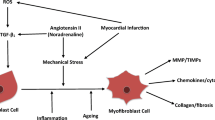
Tissue fibrosis is characterized by progressive extracellular matrix (ECM) stiffening and loss of viscoelasticity that ultimately impairs organ functionality. Cells bind to the ECM through integrins, where αv integrin engagement in particular has been correlated with fibroblast activation into contractile myofibroblasts that drive fibrosis progression. There is a significant unmet need for in vitro hydrogel systems that deconstruct the complexity of native tissues to better understand the individual and combined effects of stiffness, viscoelasticity, and integrin engagement on fibroblast behavior.
Methods
We developed hyaluronic acid hydrogels with independently tunable cell-instructive properties (stiffness, viscoelasticity, ligand presentation) to address this challenge. Hydrogels with mechanics matching normal or fibrotic lung tissue were synthesized using a combination of covalent crosslinks and supramolecular interactions to tune viscoelasticity. Cell adhesion was mediated through incorporation of either RGD peptide or engineered fibronectin fragments promoting preferential integrin engagement via αvβ3 or α5β1.
Results
On fibrosis-mimicking stiff elastic hydrogels, preferential αvβ3 engagement promoted increased spreading, actin stress fiber organization, and focal adhesion maturation as indicated by paxillin organization in human lung fibroblasts. In contrast, preferential α5β1 binding suppressed these metrics. Viscoelasticity, mimicking the mechanics of healthy tissue, largely curtailed fibroblast spreading and focal adhesion organization independent of adhesive ligand type, highlighting its role in reducing fibroblast-activating behaviors.
Conclusions
Together, these results provide new insights into how mechanical and adhesive cues collectively guide disease-relevant cell behaviors.






Similar content being viewed by others
References
Aida, T., E. W. Meijer, and S. I. Stupp. Functional supramolecular polymers. Science 335:813–817, 2012.
Arora, P. D., N. Narani, and C. A. G. McCulloch. The compliance of collagen gels regulates transforming growth factor-β induction of α-smooth muscle actin in fibroblasts. Am. J. Pathol. 154:871–882, 1999.
Asano, S., S. Ito, K. Takahashi, K. Furuya, and M. Kondo. Matrix stiffness regulates migration of human lung fibroblasts. Physiol. Rep. 5:1–11, 2017.
Baker, B. M., and C. S. Chen. Deconstructing the third dimension—how 3D culture microenvironments alter cellular cues. J. Cell Sci. 125:3015–3024, 2012.
Balestrini, J. L., S. Chaudhry, V. Sarrazy, A. Koehler, and B. Hinz. The mechanical memory of lung myofibroblasts. Integr. Biol. 4:410, 2012.
Berginski, M.E., and S.M. Gomez. The focal adhesion analysis server: a web tool for analyzing focal adhesion dynamics. F1000Research, 2013.
Booth, A. J., et al. Acellular normal and fibrotic human lung matrices as a culture system for in vitro investigation. Am. J. Respir. Crit. Care Med. 186:866–876, 2012.
Brown, A. C., J. A. Rowe, and T. H. Barker. Guiding epithelial cell phenotypes with engineered integrin-specific recombinant fibronectin fragments. Tissue Eng. A 17:139–150, 2011.
Burdick, J. A., and G. D. Prestwich. Hyaluronic acid hydrogels for biomedical applications. Adv. Mater. 23:H41–H56, 2011.
Caliari, S. R., and J. A. Burdick. A practical guide to hydrogels for cell culture. Nat. Methods 13:405–414, 2016.
Caliari, S. R., M. Perepelyuk, E. M. Soulas, G. Y. Lee, R. G. Wells, and J. A. Burdick. Gradually softening hydrogels for modeling hepatic stellate cell behavior during fibrosis regression. Integr. Biol. 8:720–728, 2016.
Caliari, S. R., S. L. Vega, M. Kwon, E. M. Soulas, and J. A. Burdick. Dimensionality and spreading influence MSC YAP/TAZ signaling in hydrogel environments. Biomaterials 103:314–323, 2016.
Caliari, S. R., et al. Stiffening hydrogels for investigating the dynamics of hepatic stellate cell mechanotransduction during myofibroblast activation. Sci. Rep. 6:1–10, 2016.
Cameron, A. R., J. E. Frith, and J. J. Cooper-White. The influence of substrate creep on mesenchymal stem cell behaviour and phenotype. Biomaterials 32:5979–5993, 2011.
Cao, L., et al. Detection of an integrin-binding mechanoswitch within fibronectin during tissue formation and fibrosis. ACS Nano 11:7110–7117, 2017.
Charrier, E. E., K. Pogoda, R. G. Wells, and P. A. Janmey. Control of cell morphology and differentiation by substrates with independently tunable elasticity and viscous dissipation. Nat. Commun. 1–13, 2018.
Chaudhuri, O., J. Cooper-white, P. A. Janmey, D. J. Mooney, and V. B. Shenoy. Effects of extracellular matrix viscoelasticity on cellular behaviour. Nature 584:535–546, 2020.
Chaudhuri, O., et al. Substrate stress relaxation regulates cell spreading. Nat. Commun. 6:6365, 2015.
Chaudhuri, O., et al. Hydrogels with tunable stress relaxation regulate stem cell fate and activity. Nat. Mater. 15:326–334, 2016.
Conroy, K.P., L.J. Kitto, N.C. Henderson, and N.C. Henderson. αv integrins: key regulators of tissue fibrosis. Cell Tissue Res. 511–519, 2016.
Deng, X., K. Jin, Y. Li, W. Gu, and M. Liu. Platelet-derived growth factor and transforming growth factor β1 regulate ARDS-associated lung fibrosis through distinct signaling pathways. Cell Physiol. Biochem. 201199:937–946, 2015.
Dicker, K. T., L. A. Gurski, S. Pradhan-Bhatt, R. L. Witt, M. C. Farach-Carson, and X. Jia. Hyaluronan: a simple polysaccharide with diverse biological functions. Acta Biomater. 10:1558–1570, 2014.
Driscoll, T. P., S. J. Ahn, B. Huang, A. Kumar, and M. A. Schwartz. Actin flow-dependent and -independent force transmission through integrins. Proc. Natl. Acad. Sci. 202010292, 2020.
Duscher, D., et al. Mechanotransduction and fibrosis. J. Biomech. 47:1997–2005, 2014.
Fernandez, I. E., and O. Eickelberg. The impact of TGF-b on lung fibrosis from targeting to biomarkers. Proc. Am. Thorac. Soc. 9:111–116, 2012.
Fiore, V. F., et al. Integrin avb3 drives fibroblast contraction and strain stiffening of soft provisional extracellular matrix during progressive fibrosis. JCI Insight 3:1–35, 2018.
Gardel, M. L., I. C. Schneider, Y. Aratyn-Schaus, and C. M. Waterman. Mechanical integration of actin and adhesion dynamics in cell migration. Annu. Rev. Cell Dev. Biol. 26:315–333, 2010.
Gong, Z., et al. Matching material and cellular timescales maximizes cell spreading on viscoelastic substrates. Proc. Natl. Acad. Sci. 115:E2686–E2695, 2018.
Gramlich, W. M., I. L. Kim, and J. A. Burdick. Synthesis and orthogonal photopatterning of hyaluronic acid hydrogels with thiol-norbornene chemistry. Biomaterials 34:9803–9811, 2013.
Guvendiren, M., M. Perepelyuk, R. G. Wells, and J. A. Burdick. Hydrogels with differential and patterned mechanics to study stiffness-mediated myofibroblastic differentiation of hepatic stellate cells. J. Mech. Behav. Biomed. Mater. 38:198–208, 2014.
Harjanto, D., and M. H. Zaman. Matrix mechanics and receptor-ligand interactions in cell adhesion. Org. Biomol. Chem. 8:299–304, 2010.
Hautanen, A., J. Gailit, D. M. Mann, and E. Ruoslahti. Effects of modifications of the RGD sequence and its context on recognition by the fibronectin receptor. J. Biol. Chem. 264:1437–1442, 1989.
Henderson, N. C., F. Rieder, and T. A. Wynn. Fibrosis: from mechanisms to medicines. Nature 587:555–566, 2020.
Henderson, N. C., and D. Sheppard. Integrin-mediated regulation of TGFβ in fibrosis. Biochim. Biophys. Acta. 891–896:2013, 1832.
Henderson, N. C., et al. Targeting of αv integrin identifies a core molecular pathway that regulates fibrosis in several organs. Nat. Med. 19:1617–1624, 2013.
Hilster, R. H. J. De, P. K. Sharma, E. S. White, E. A. Gercama, and M. Roobeek. Human lung extracellular matrix hydrogels resemble the stiffness and viscoelasticity of native lung tissue. Am. J. Physiol. Lung Cell Mol. Physiol. 318(4):L698-L704, 2020.
Hinz, B., and G. Gabbiani. Mechanisms of force generation and transmission by myofibroblasts. Curr. Opin. Biotechnol. 14:538–546, 2003.
Hui, E., K. I. Gimeno, G. Guan, and S. R. Caliari. Spatiotemporal control of viscoelasticity in phototunable hyaluronic acid hydrogels. Biomacromolecules 20:4126–4134, 2019.
Humphrey, J. D., E. R. Dufresne, and M. A. Schwartz. Mechanotransduction and extracellular matrix homeostasis. Nat. Rev. Mol. Cell Biol. 15:802–812, 2014.
Ingber, D. E. Cellular mechanotransduction: putting all the pieces together again. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 20:811–827, 2006.
Islam, M. R., J. Virag, and M. L. Oyen. Micromechanical poroelastic and viscoelastic properties of ex vivo soft tissues. J. Biomech. 113:2020.
Jansen, K. A., P. Atherton, and C. Ballestrem. Mechanotransduction at the cell-matrix interface. Semin. Cell Dev. Biol. 71:75–83, 2017.
Kechagia, J. Z., J. Ivaska, and P. Roca-Cusachs. Integrins as biomechanical sensors of the microenvironment. Nat. Rev. Mol. Cell Biol. 20:457–473, 2019.
Kishi, T., T. Mayanagi, S. Iwabuchi, and T. Akasaka. Myocardin-related transcription factor A (MRTF-A) activity-dependent cell adhesion is correlated to focal adhesion kinase (FAK) activity. Oncotarget 7, 2016.
Kloxin, A. M., J. A. Benton, and K. S. Anseth. In situ elasticity modulation with dynamic substrates to direct cell phenotype. Biomaterials 31:1–8, 2010.
Kwon, M. Y., C. Wang, J. H. Galarraga, E. Puré, L. Han, and J. A. Burdick. Influence of hyaluronic acid modification on CD44 binding towards the design of hydrogel biomaterials. Biomaterials 222, 2019.
Leiphart, R. J., D. Chen, A. P. Peredo, A. E. Loneker, and P. A. Janmey. Mechanosensing at cellular interfaces. Langmuir 35:7509–7519, 2019.
Li, S., et al. Hydrogels with precisely controlled integrin activation dictate vascular patterning and permeability. Nat. Mater. 16:953–961, 2017.
Liu, F., et al. Feedback amplification of fibrosis through matrix stiffening and COX-2 suppression. J. Cell Biol. 190:693–706, 2010.
López-Colomé, A. M., I. Lee-Rivera, R. Benavides-Hidalgo, and E. López. Paxillin: A crossroad in pathological cell migration. J. Hematol. Oncol. 10:1–15, 2017.
Markowski, M. C., A. C. Brown, and T. H. Barker. Directing epithelial to mesenchymal transition through engineered microenvironments displaying orthogonal adhesive and mechanical cues. J. Biomed. Mater. Res. A 100 A:2119–2127, 2012.
Marozas, I. A., K. S. Anseth, and J. J. Cooper-White. Adaptable boronate ester hydrogels with tunable viscoelastic spectra to probe timescale dependent mechanotransduction. Biomaterials 223:2019.
Martinez, F. J., et al. Idiopathic pulmonary fibrosis. Nat. Rev. Dis. Primer 3:17074, 2017.
Martino, M. M., M. Mochizuki, D. A. Rothenfluh, S. A. Rempel, J. A. Hubbell, and T. H. Barker. Controlling integrin specificity and stem cell differentiation in 2D and 3D environments through regulation of fibronectin domain stability. Biomaterials 30:1089–1097, 2009.
Mcbeath, R., D. M. Pirone, C. M. Nelson, K. Bhadriraju, and C. S. Chen. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev. Cell 6:483–495, 2004.
McKinnon, D. D., D. W. Domaille, J. N. Cha, and K. S. Anseth. Biophysically defined and cytocompatible covalently adaptable networks as viscoelastic 3D cell culture systems. Adv. Mater. 26:865–872, 2014.
Mohammadi, H., P. D. Arora, C. A. Simmons, P. A. Janmey, and C. A. McCulloch. Inelastic behaviour of collagen networks in cell-matrix interactions and mechanosensation. J. R. Soc. Interface 12, 2015.
Nam, S., K. H. Hu, M. J. Butte, and O. Chaudhuri. Strain-enhanced stress relaxation impacts nonlinear elasticity in collagen gels. Proc. Natl. Acad. Sci. 113:1–6, 2016.
Olsen, A. L., et al. Hepatic stellate cells require a stiff environment for myofibroblastic differentiation. Am J Physiol Gastroinstest Liver Physiol 301:110–118, 2011.
Panciera, T., L. Azzolin, M. Cordenonsi, and S. Piccolo. Mechanobiology of YAP and TAZ in physiology and disease. Nat. Rev. Mol. Cell Biol. 18:758–770, 2017.
Paszek, M. J., D. Boettiger, V. M. Weaver, and D. A. Hammer. Integrin Clustering Is Driven by Mechanical Resistance from the Glycocalyx and the Substrate. PLoS Comput. Biol. 5, 2009.
Perepelyuk, M., et al. Normal and fibrotic rat livers demonstrate shear strain softening and compression stiffening: a model for soft tissue mechanics. PLoS ONE 11:1–18, 2016.
Roca-Cusachs, P., T. Iskratsch, and M. P. Sheetz. Finding the weakest link—exploring integrin-mediated mechanical molecular pathways. J. Cell Sci. 125:3025–3038, 2012.
Rodell, C. B., N. N. Dusaj, C. B. Highley, and J. A. Burdick. Injectable and cytocompatible tough double-network hydrogels through tandem supramolecular and covalent crosslinking. Adv. Mater. 28:8419–8424, 2016.
Rodell, C. B., A. L. Kaminski, and J. A. Burdick. Rational design of network properties in guest-host assembled and shear-thinning hyaluronic acid hydrogels. Biomacromolecules 14:4125–4134, 2013.
Rodell, C. B., et al. Shear-thinning supramolecular hydrogels with secondary autonomous covalent crosslinking to modulate viscoelastic properties in vivo. Adv. Funct. Mater. 25:636–644, 2015.
Ruoslahti, E. RGD and other recognition sequences for integrins. Annu. Rev. Cell Dev. Biol. 12:697–715, 1996.
Rustad, K. C., V. W. Wong, and G. C. Gurtner. The role of focal adhesion complexes in fibroblast mechanotransduction during scar formation. Differentiation 86:87–91, 2013.
Scaffidi, A. K., Y. P. Moodley, M. Weichselbaum, P. J. Thompson, and D. A. Knight. Regulation of human lung fibroblast phenotype and function by vitronectin and vitronectin integrins. J. Cell Sci. 114:3507–3516, 2001.
Schanté, C. E., G. Zuber, C. Herlin, and T. F. Vandamme. Chemical modifications of hyaluronic acid for the synthesis of derivatives for a broad range of biomedical applications. Carbohydr. Polym. 85:469–489, 2011.
Seong, J., N. Wang, and Y. Wang. Mechanotransduction at focal adhesions: from physiology to cancer development. J Cell Mol Med 17:597–604, 2013.
Sero, J. E., A. E. German, A. Mammoto, and D. E. Ingber. Paxillin controls directional cell motility in response to physical cues. Cell Adhes. Migr. 6:502–508, 2012.
Shinde, A. V, C. Humeres, and N.G. Frangogiannis. The role of α-smooth muscle actin in fibroblast-mediated matrix contraction and remodeling. Biochim. Biophys. Acta 1863:298–309, 2017.
Sun, Z., S. S. Guo, and R. Fässler. Integrin-mediated mechanotransduction. J. Cell Bio. 215:445–456, 2016.
Tang, S., H. Ma, H.-C. Tu, H.-R. Wang, P.-C. Lin, and K. S. Anseth. Adaptable fast relaxing boronate-based hydrogels for probing cell-matrix interactions. Adv. Sci. 1800638:1800638, 2018.
Travers, J. G., F. A. Kamal, J. Robbins, K. E. Yutzey, and B. C. Blaxall. The fibroblast awakens. Circ. Res. 118(6):1021–1040, 2016.
Turner, C. E. Paxillin interactions. J. Cell Sci. 113:4139–4140, 2000.
Turner, C. E. Paxillin and focal adhesion signalling. Nat. Cell Biol. 2:231–236, 2000.
van de Manakker, F., L. M. J. Kroon-Batenburg, T. Vermonden, C. F. van Nostrum, and W. E. Hennink. Supramolecular hydrogels formed by β-cyclodextrin self-association and host–guest inclusion complexes. Soft Matter 6:187–194, 2010.
Waisberg, D.R., E.R. Parra, V. Barbas-filho, S. Fernezlian, and V. Luiza Capelozzi. Increased fibroblast telomerase expression precedes myofibroblast a-smooth muscle actin expression in idiopathic pulmonary fibrosis. Clinics 67:1039–1046, 2012.
Wells, R. G. Tissue mechanics and fibrosis. Biochim. Biophys. Acta 884–890:2014, 1832.
Wells, R. G. The role of matrix stiffness in regulating cell behavior. Hepatology 47:1394–1400, 2008.
Wen, J. H., et al. Interplay of matrix stiffness and protein tethering in stem cell differentiation. Nat. Mater. 13:979–987, 2014.
Wynn, T. Cellular and molecular mechanisms of fibrosis. J. Pathol. 214:199–210, 2008.
Wynn, T. A., and T. R. Ramalingam. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat. Med. 18:1028–1040, 2012.
Yeh, Y. C., et al. Mechanically dynamic PDMS substrates to investigate changing cell environments. Biomaterials 145:23–32, 2017.
Zhao, X., N. Huebsch, D. J. Mooney, and Z. Suo. Stress-relaxation behavior in gels with ionic and covalent crosslinks. J. Appl. Phys. 107:1–5, 2010.
Zhu, M., et al. Spatial mapping of tissue properties in vivo reveals a 3D stiffness gradient in the mouse limb bud. Proc. Natl. Acad. Sci. 117:4781, 2020.
Acknowledgments
This work was supported by the UVA Fibrosis Initiative and the NIH (R35GM138187, T32GM008715). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
S.R.C., E.H., and T.H.B. have filed a provisional patent application related to this work.
Ethical standards
No animal or human studies were carried out by the authors for this article.
Additional information
Associate Editor Sanjay Kumar oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Steven R. Caliari joined the faculty of the University of Virginia in fall 2016 as an Assistant Professor in the Department of Chemical Engineering with a secondary appointment in the Department of Biomedical Engineering. Prior to joining UVA he was an NIH Postdoctoral Fellow in the Department of Bioengineering at the University of Pennsylvania. Steven completed his B.S. in Chemical Engineering at the University of Florida and received both his M.S. and Ph.D. in Chemical Engineering from the University of Illinois at Urbana-Champaign. His lab designs biomaterials to study the dynamic reciprocity between cells and their microenvironment, applying these platforms to address fundamental human health challenges in understanding disease and engineering tissues. Steven recently received the NIH (NIGMS) Maximizing Investigators’ Research Award (MIRA) and NSF CAREER Award. His lab is grateful for generous support from the NIH, DoD, NSF, V Foundation, and UVA-Coulter Translational Research Partnership. To learn more about the lab’s work, please follow @Caliari_Lab on Twitter.

This article is part of the 2021 CMBE Young Innovators special issue.
Supplementary Information
Below is the link to the electronic supplementary material.
12195_2021_672_MOESM1_ESM.pdf
Supplementary material 1 1H NMR spectra for NorHA and CD-HA, MALDI spectra for the adamantane peptide, additional hydrogel mechanical characterization, and additional cell analysis can be found in the Supporting Information. (PDF 1197 kb)
Rights and permissions
About this article
Cite this article
Hui, E., Moretti, L., Barker, T.H. et al. The Combined Influence of Viscoelastic and Adhesive Cues on Fibroblast Spreading and Focal Adhesion Organization. Cel. Mol. Bioeng. 14, 427–440 (2021). https://doi.org/10.1007/s12195-021-00672-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12195-021-00672-1