Abstract
Objective
Asthma, a well-known disease with high morbidity, is characterized by chronic airway inflammation. However, the allergic inflammation mechanisms of follistatin-like protein 1 (FSTL1) have not been elucidated. This study aims to investigate the effects of FSTL1 in ovalbumin (OVA)-induced mice and macrophages on nucleotide-binding domain and leucine-rich repeat protein 3 (NLRP3)/interleukin-1β (IL-1β) signaling pathway.
Methods
Mice were randomly divided into control-WT, OVA-WT, control-Fstl1±, OVA-Fstl1±. Histological changes were assessed by HE and PAS staining. The protein levels of Muc-5AC, FSTL1, NLRP3, and IL-1β in lung tissue were detected by immunohistochemistry and ELISA. The bronchoalveolar lavage fluid (BALF) in mice and human serum samples were detected by ELISA. Then, mice were grouped into control, FSTL1, MCC950 + FSTL1 to further investigate the relationship between FSTL1 and NLRP3/IL-1β. Alveolar macrophage cells (MH-S cells) were separated into control, OVA, FSTL1, OVA + FSTL1, OVA + siNC, OVA + siFSTL1, MCC950, and FSTL1 + MCC950 groups to explore the effect of FSTL1 on the NLRP3/IL-1β signaling. The protein expression of NLRP3 and IL-1β in MH-S cells was detected by Western blot analysis.
Results
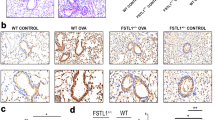
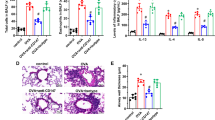
The present results uncovered that Fstl1± significantly ameliorated OVA-induced Muc-5AC production and mucus hypersecretion. Fstl1± was also found to decrease the production of inflammatory cytokines and inflammatory cell infiltration in OVA-induced asthmatic mice. Meanwhile, the serum concentrations of FSTL1 and IL-1β were higher in asthma subjects than the health subjects, and Fstl1± ameliorated the production of NLRP3 and IL-1β in OVA-induced asthmatic mice. Furthermore, mice by injected FSTL1 substantially stimulated the expression of NLRP3 and IL-1β, while pretreatment with MCC950 in mice significantly weakened the production of NLRP3 and IL-1β induced by injection FSTL1. Pretreatment with siFSTL1 or MCC950 significantly reduced the production of NLRP3 and IL-1β induced by OVA or FSTL1 in MH-S cells.
Conclusions
The study results showed that FSTL1 played an important role in allergic airway inflammation by activating NLRP3/IL-1β. Hence, inhibition FSTL1 could be applied as a therapeutic agent against asthma.






Similar content being viewed by others
Availability of data and materials
The data generated during the study are available from the corresponding author on reasonable request.
References
Liao Z, Xiao HT, Zhang Y, Tong RS, Zhang LJ, Bian Y, et al. IL-1beta: a key modulator in asthmatic airway smooth muscle hyper-reactivity. Expert Rev Respir Med. 2015;9:429–36.
Liu Y, Liu T, Wu J, Li T, Jiao X, Zhang H, et al. The Correlation between FSTL1 Expression and Airway Remodeling in Asthmatics. Mediators Inflamm. 2017;2017:7918472.
Chaly Y, Hostager B, Smith S, Hirsch R. Follistatin-like protein 1 and its role in inflammation and inflammatory diseases. Immunol Res. 2014;59:266–72.
Ogura Y, Ouchi N, Ohashi K, Shibata R, Kataoka Y, Kambara T, et al. Therapeutic impact of follistatin-like 1 on myocardial ischemic injury in preclinical models. Circulation. 2012;126:1728–38.
Zheng X, Qi C, Zhang S, Fang Y, Ning W. TGF-beta1 induces fstl1 via the smad3-c-jun pathway in lung fibroblasts. Am J Physiol Lung Cell Mol Physiol. 2017;313:L240–51.
Kudo-Saito C, Ishida A, Shouya Y, Teramoto K, Igarashi T, Kon R, et al. Blocking the FSTL1-DIP2A axis improves anti-tumor immunity. Cell Rep. 2018;24:1790–801.
Johnson VJ, Yucesoy B, Luster MI. Prevention of IL-1 signaling attenuates airway hyperresponsiveness and inflammation in a murine model of toluene diisocyanate-induced asthma. J Allergy Clin Immunol. 2005;116:851–8.
Besnard AG, Guillou N, Tschopp J, Erard F, Couillin I, Iwakura Y, et al. NLRP3 inflammasome is required in murine asthma in the absence of aluminum adjuvant. Allergy. 2011;66:1047–57.
Simpson JL, Phipps S, Baines KJ, Oreo KM, Gunawardhana L, Gibson PG. Elevated expression of the NLRP3 inflammasome in neutrophilic asthma. Eur Respir J. 2014;43:1067–76.
Li R, Wang J, Li R, Zhu F, Xu W, Zha G, et al. ATP/P2X7-NLRP3 axis of dendritic cells participates in the regulation of airway inflammation and hyper-responsiveness in asthma by mediating HMGB1 expression and secretion. Exp Cell Res. 2018;366:1–15.
Besnard AG, Togbe D, Couillin I, Tan Z, Zheng SG, Erard F, et al. Inflammasome-IL-1-Th17 response in allergic lung inflammation. J Mol Cell Biol. 2012;4:3–10.
Zhang Y, Xu CB, Cardell LO. Long-term exposure to IL-1beta enhances Toll-IL-1 receptor-mediated inflammatory signaling in murine airway hyperresponsiveness. Eur Cytokine Netw. 2009;20:148–56.
Coll RC, Robertson AA, Chae JJ, Higgins SC, Munoz-Planillo R, Inserra MC, et al. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat Med. 2015;21:248–55.
Li KC, Zhang FX, Li CL, Wang F, Yu MY, Zhong YQ, et al. Follistatin-like 1 suppresses sensory afferent transmission by activating Na+, K+-ATPase. Neuron. 2011;69(5):974–87.
Nie H, Wang A, He Q, Yang Q, Liu L, Zhang G, et al. Phenotypic switch in lung interstitial macrophage polarization in an ovalbumin-induced mouse model of asthma. Exp Ther Med. 2017;14:1284–92.
Miller M, Beppu A, Rosenthal P, Pham A, Das S, Karta M, et al. Fstl1 promotes asthmatic airway remodeling by inducing oncostatin M. J Immunol. 2015;195:3546–56.
Chong L, Zhang W, Nie Y, Yu G, Liu L, Lin L, et al. Protective effect of curcumin on acute airway inflammation of allergic asthma in mice through Notch1-GATA3 signaling pathway. Inflammation. 2014;37:1476–85.
Ritter M, Straubinger K, Schmidt S, Busch DH, Hagner S, Garn H, et al. Functional relevance of NLRP3 inflammasome-mediated interleukin (IL)-1beta during acute allergic airway inflammation. Clin Exp Immunol. 2014;178:212–23.
Myou S, Leff AR, Myo S, Boetticher E, Tong J, Meliton AY, et al. Blockade of inflammation and airway hyperresponsiveness in immune-sensitized mice by dominant-negative phosphoinositide 3-kinase-TAT. J Exp Med. 2003;198:1573–82.
Miyamae T, Marinov AD, Sowders D, Wilson DC, Devlin J, Boudreau R, et al. Follistatin-like protein-1 is a novel proinflammatory molecule. J Immunol. 2006;177:4758–62.
Chaly Y, Marinov AD, Oxburgh L, Bushnell DS, Hirsch R. FSTL1 promotes arthritis in mice by enhancing inflammatory cytokine/chemokine expression. Arthritis Rheum. 2012;64:1082–8.
Clutter SD, Wilson DC, Marinov AD, Hirsch R. Follistatin-like protein 1 promotes arthritis by up-regulating IFN-gamma. J Immunol. 2009;182:234–9.
Ni S, Miao K, Zhou X, Xu N, Li C, Zhu R, et al. The involvement of follistatin-like protein 1 in osteoarthritis by elevating NF-kappaB-mediated inflammatory cytokines and enhancing fibroblast like synoviocyte proliferation. Arthritis Res Ther. 2015;17:91.
Wang Y, Li D, Xu N, Tao W, Zhu R, Sun R, et al. Follistatin-like protein 1: a serum biochemical marker reflecting the severity of joint damage in patients with osteoarthritis. Arthritis Res Ther. 2011;13:R193.
Wilson DC, Marinov AD, Blair HC, Bushnell DS, Thompson SD, Chaly Y, et al. Follistatin-like protein 1 is a mesenchyme-derived inflammatory protein and may represent a biomarker for systemic-onset juvenile rheumatoid arthritis. Arthritis Rheum. 2010;62:2510–6.
Widera C, Horn-Wichmann R, Kempf T, Bethmann K, Fiedler B, Sharma S, et al. Circulating concentrations of follistatin-like 1 in healthy individuals and patients with acute coronary syndrome as assessed by an immunoluminometric sandwich assay. Clin Chem. 2009;55:1794–800.
Liu Y, Wei J, Zhao Y, Zhang Y, Han Y, Chen B, et al. Follistatin-like protein 1 promotes inflammatory reactions in nucleus pulposus cells by interacting with the MAPK and NFkappaB signaling pathways. Oncotarget. 2017;8:43023–34.
Fan N, Sun H, Wang Y, Wang Y, Zhang L, Xia Z, et al. Follistatin-like 1: a potential mediator of inflammation in obesity. Mediators Inflamm. 2013; 2013:752519.
Chaly Y, Fu Y, Marinov A, Hostager B, Yan W, Campfield B, et al. Follistatin-like protein 1 enhances NLRP3 inflammasome-mediated IL-1beta secretion from monocytes and macrophages. Eur J Immunol. 2014;44:1467–79.
Lee YG, Jeong JJ, Nyenhuis S, Berdyshev E, Chung S, Ranjan R, et al. Recruited alveolar macrophages, in response to airway epithelial-derived monocyte chemoattractant protein 1/CCl2, regulate airway inflammation and remodeling in allergic asthma. Am J Respir Cell Mol Biol. 2015;52:772–84.
Gwyer Findlay E, Hussell T. Macrophage-mediated inflammation and disease: a focus on the lung. Mediators Inflamm. 2012; 2012:140937.
Kim RY, Pinkerton JW, Essilfie AT, Robertson AAB, Baines KJ, Brown AC, et al. Role for NLRP3 Inflammasome-mediated, IL-1beta-dependent responses in severe, steroid-resistant asthma. Am J Respir Crit Care Med. 2017;196:283–97.
Fu JJ, McDonald VM, Baines KJ, Gibson PG. Airway IL-1beta and systemic inflammation as predictors of future exacerbation risk in asthma and COPD. Chest. 2015;148:618–29.
Mahmutovic Persson I, Menzel M, Ramu S, Cerps S, Akbarshahi H, Uller L. IL-1beta mediates lung neutrophilia and IL-33 expression in a mouse model of viral-induced asthma exacerbation. Respir Res. 2018;19:16.
Arae K, Morita H, Unno H, Motomura K, Toyama S, Okada N, et al. Chitin promotes antigen-specific Th2 cell-mediated murine asthma through induction of IL-33-mediated IL-1beta production by DCs. Sci Rep. 2018;8:11721.
Kim SR, Kim DI, Kim SH, Lee H, Lee KS, Cho SH, et al. NLRP3 inflammasome activation by mitochondrial ROS in bronchial epithelial cells is required for allergic inflammation. Cell Death Dis. 2014; 5:e1498.
Acknowledgements
We would like to thank Xiang Gao and Wen Ning for their generous help in Fstl1+/- mice.
Funding
This work was supported by grants from the National Natural Science Foundation of China (81770029), National Key Research and Development Project (2017YFC1310601).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
This study was approved by the Ethics Review Committee for Human Studies at Qilu Hospital of Shandong University and Institutional Animal Care and Use Committee of Shandong University (Grant NO. KYLL-2017[ks]-112).
Additional information
Responsible Editor: John Di Battista.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, Y., Zhang, D., Liu, T. et al. FSTL1 aggravates OVA-induced inflammatory responses by activating the NLRP3/IL-1β signaling pathway in mice and macrophages. Inflamm. Res. 70, 777–787 (2021). https://doi.org/10.1007/s00011-021-01475-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-021-01475-w