Abstract
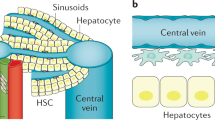
Liver regeneration is a complex process involving the crosstalk of multiple cell types, including hepatocytes, hepatic stellate cells, endothelial cells and inflammatory cells. The healthy liver is mitotically quiescent, but following toxic damage or resection the cells can rapidly enter the cell cycle to restore liver mass and function. During this process of regeneration, epithelial and non-parenchymal cells respond in a tightly coordinated fashion. Recent studies have described the interaction between inflammatory cells and a number of other cell types in the liver. In particular, macrophages can support biliary regeneration, contribute to fibrosis remodelling by repressing hepatic stellate cell activation and improve liver regeneration by scavenging dead or dying cells in situ. In this Review, we describe the mechanisms of tissue repair following damage, highlighting the close relationship between inflammation and liver regeneration, and discuss how recent findings can help design novel therapeutic approaches.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Bismuth, H. Surgical anatomy and anatomical surgery of the liver. World J. Surg. 6, 3–9 (1982).
Couinaud, C. Liver anatomy: portal (and suprahepatic) or biliary segmentation. Dig. Surg. 16, 459–467 (1999).
Trefts, E., Gannon, M. & Wasserman, D. H. The liver. Curr. Biol. 27, R1147–R1151 (2017).
Tabibian, J. H., Masyuk, A. I., Masyuk, T. V., O’Hara, S. P. & LaRusso, N. F. Physiology of cholangiocytes. Compr. Physiol. 3, 541–565 (2013).
Poisson, J. et al. Liver sinusoidal endothelial cells: physiology and role in liver diseases. J. Hepatol. 66, 212–227 (2017).
Michalopoulos, G. K. & DeFrances, M. C. Liver regeneration. Science 276, 60–66 (1997).
Michalopoulos, G. K. Liver regeneration. J. Cell. Physiol. 213, 286–300 (2007).
Higgins, G. M. & Anderson, R. M. Experimental pathology of liver I: restoration of the liver of the white rat following partial surgical removal. Arch. Pathol. 12, 186–202 (1931).
Forbes, S. J. & Newsome, P. N. Liver regeneration - mechanisms and models to clinical application. Nat. Rev. Gastroenterol. Hepatol. 13, 473–485 (2016).
Iredale, J. P. Models of liver fibrosis: exploring the dynamic nature of inflammation and repair in a solid organ. J. Clin. Invest. 117, 539–548 (2007).
Sakamoto, T. et al. Mitosis and apoptosis in the liver of interleukin-6-deficient mice after partial hepatectomy. Hepatology 29, 403–411 (1999).
Thomas, H. Senescence prevents regeneration after acute liver injury. Nat. Rev. Gastroenterol. Hepatol. 15, 582–582 (2018).
Zhang, D. Y. & Friedman, S. L. Fibrosis-dependent mechanisms of hepatocarcinogenesis. Hepatology 56, 769–775 (2012).
Forbes, S. J., Gupta, S. & Dhawan, A. Cell therapy for liver disease: from liver transplantation to cell factory. J. Hepatol. 62, S157–S169 (2015).
Ismail, A., Fouad, O., Abdelnasser, A., Chowdhury, A. & Selim, A. Stem cell therapy improves the outcome of liver resection in cirrhotics. J. Gastrointest. Cancer 41, 17–23 (2010).
Bartlett, D. C. & Newsome, P. N. Hepatocyte cell therapy in liver disease. Expert Rev. Gastroenterol. Hepatol. 9, 1261–1272 (2015).
Nicolas, C. T., Wang, Y. & Nyberg, S. L. Cell therapy in chronic liver disease. Curr. Opin. Gastroenterol. 32, 189–194 (2016).
Lu, W. Y. et al. Hepatic progenitor cells of biliary origin with liver repopulation capacity. Nat. Cell Biol. 17, 971–983 (2015).
He, J., Lu, H., Zou, Q. & Luo, L. Regeneration of liver after extreme hepatocyte loss occurs mainly via biliary transdifferentiation in zebrafish. Gastroenterology 146, 789–800 (2014).
Raven, A. et al. Cholangiocytes act as facultative liver stem cells during impaired hepatocyte regeneration. Nature 547, 350–354 (2017). Raven, Lu and co-workers show the potential regenerative capacity of biliary cells during chronic liver injury, when the native regenerative ability of the hepatocytes is exhausted.
Thomas, J. A. et al. Macrophage therapy for murine liver fibrosis recruits host effector cells improving fibrosis, regeneration, and function. Hepatology 53, 2003–2015 (2011).
Dangi, A. et al. Selective expansion of allogeneic regulatory T cells by hepatic stellate cells: role of endotoxin and implications for allograft tolerance. J. Immunol. https://doi.org/10.4049/jimmunol.1102460 (2012).
Dunham, R. M. et al. Hepatic stellate cells preferentially induce Foxp3+ regulatory T cells by production of retinoic acid. J. Immunol. https://doi.org/10.4049/jimmunol.1201937 (2013).
Langhans, B. et al. Regulatory CD4+ T cells modulate the interaction between NK cells and hepatic stellate cells by acting on either cell type. J. Hepatol. 62, 398–404 (2015).
Baeck, C. et al. Pharmacological inhibition of the chemokine C-C motif chemokine ligand 2 (monocyte chemoattractant protein 1) accelerates liver fibrosis regression by suppressing Ly-6C+ macrophage infiltration in mice. Hepatology 59, 1060–1072 (2014).
Lefebvre, E. et al. Antifibrotic effects of the dual CCR2/CCR5 antagonist cenicriviroc in animal models of liver and kidney fibrosis. PLoS ONE 11, e0158156 (2016).
Liedtke, C. et al. Experimental liver fibrosis research: update on animal models, legal issues and translational aspects. Fibrogenesis Tissue Repair. 6, 19 (2013).
Satyanarayana, A., Geffers, R., Manns, M. P., Buer, J. & Rudolph, K. L. Gene expression profile at the G1/S transition of liver regeneration after partial hepatectomy in mice. Cell Cycle 3, 1405–1417 (2004).
Chen, F. et al. Broad distribution of hepatocyte proliferation in liver homeostasis and regeneration. Cell Stem Cell 26, 27–33 (2020).
Wang, B., Zhao, L., Fish, M., Logan, C. Y. & Nusse, R. Self-renewing diploid Axin2+ cells fuel homeostatic renewal of the liver. Nature 524, 180–185 (2015).
Lin, S. et al. Distributed hepatocytes expressing telomerase repopulate the liver in homeostasis and injury. Nature 556, 244–248 (2018).
Sun, T. et al. AXIN2+ pericentral hepatocytes have limited contributions to liver homeostasis and regeneration. Cell Stem Cell 26, 97–107 (2020).
Aloia, L. et al. Epigenetic remodelling licences adult cholangiocytes for organoid formation and liver regeneration. Nat. Cell Biol. 21, 1321–1333 (2019).
Moya, I. M. & Halder, G. Hippo–YAP/TAZ signalling in organ regeneration and regenerative medicine. Nat. Rev. Mol. Cell Biol. 20, 211–226 (2019).
Pepe-Mooney, B. J. et al. Single-cell analysis of the liver epithelium reveals dynamic heterogeneity and an essential role for YAP in homeostasis and regeneration. Cell Stem Cell 25, 23–38 (2019).
Yimlamai, D. et al. Hippo pathway activity influences liver cell fate. Cell 157, 1324–1338 (2014).
Planas-Paz, L. et al. YAP, but not RSPO-LGR4/5, signaling in biliary epithelial cells promotes a ductular reaction in response to liver injury. Cell Stem Cell 25, 39–53 (2019).
Michalopoulos, G. K. Liver regeneration after partial hepatectomy: critical analysis of mechanistic dilemmas. Am. J. Pathol. 176, 2–13 (2010).
Planas-Paz, L. et al. The RSPO-LGR4/5-ZNRF3/RNF43 module controls liver zonation and size. Nat. Cell Biol. 18, 467–479 (2016).
Chen, T., Oh, S., Gregory, S., Shen, X. & Diehl, A. M. Single-cell omics analysis reveals functional diversification of hepatocytes during liver regeneration. JCI Insight 5, e141024 (2020).
Glinka, A. et al. LGR4 and LGR5 are R-spondin receptors mediating Wnt/β-catenin and Wnt/PCP signalling. EMBO Rep. 12, 1055–1061 (2011).
Michalopoulos, G. K. & Bhushan, B. Liver regeneration: biological and pathological mechanisms and implications. Nat. Rev. Gastroenterol. Hepatol. 18, 40–55 (2021).
Paranjpe, S. et al. Combined systemic elimination of MET and epidermal growth factor receptor signaling completely abolishes liver regeneration and leads to liver decompensation. Hepatology 64, 1711–1724 (2016).
Tsagianni, A. et al. Combined systemic disruption of MET and epidermal growth factor receptor signaling causes liver failure in normal mice. Am. J. Pathol. 188, 2223–2235 (2018).
Fukano, S., Saitoh, Y., Uchida, K., Akiyoshi, T. & Takeda, K. I. Bile acid metabolism in partially hepatectomized rats. Steroids 45, 209–227 (1985).
Lesage, G. et al. Regrowth of the rat biliary tree after 70% partial hepatectomy is coupled to increased secretin-induced ductal secretion. Gastroenterology 111, 1633–1644 (1996).
Mitchell, J. R., Thorgeirsson, S. S., Potter, W. Z., Jollow, D. J. & Keiser, H. Acetaminophen-induced hepatic injury: protective role of glutathione in man and rationale for therapy. Clin. Pharmacol. Ther. 16, 676–684 (1974).
Oinonen, T. & Lindros, K. O. Zonation of hepatic cytochrome P-450 expression and regulation. Biochem. J. 329, 17–35 (1998).
Jaeschke, H. & Bajt, M. L. Intracellular signaling mechanisms of acetaminophen-induced liver cell death. Toxicol. Sci. 89, 31–41 (2006).
Bajt, M. L., Cover, C., Lemasters, J. J. & Jaeschke, H. Nuclear translocation of endonuclease G and apoptosis-inducing factor during acetaminophen-induced liver cell injury. Toxicol. Sci. 94, 217–225 (2006).
Ni, H. M. et al. Removal of acetaminophen protein adducts by autophagy protects against acetaminophen-induced liver injury in mice. J. Hepatol. 65, 354–362 (2016).
Lin, Z. et al. Adiponectin protects against acetaminophen-induced mitochondrial dysfunction and acute liver injury by promoting autophagy in mice. J. Hepatol. 61, 825–831 (2014).
Ni, H. M., Williams, J. A., Jaeschke, H. & Ding, W. X. Zonated induction of autophagy and mitochondrial spheroids limits acetaminophen-induced necrosis in the liver. Redox Biol. 1, 427–432 (2013).
Bhushan, B. et al. Dual role of epidermal growth factor receptor in liver injury and regeneration after acetaminophen overdose in mice. Toxicol. Sci. 155, 363–378 (2017).
Bhushan, B. et al. Pro-regenerative signaling after acetaminophen-induced acute liver injury in mice identified using a novel incremental dose model. Am. J. Pathol. 184, 3013–3025 (2014).
Bernal, W., Auzinger, G., Dhawan, A. & Wendon, J. Acute liver failure. Lancet 376, 190–201 (2010).
Bretherick, A. D. et al. Acute liver failure in Scotland between 1992 and 2009; incidence, aetiology and outcome. QJM 104, 945–956 (2011).
Muñoz-Espín, D. & Serrano, M. Cellular senescence: from physiology to pathology. Nat. Rev. Mol. Cell Biol. 15, 482 (2014).
Bird, T. G. et al. TGFbeta inhibition restores a regenerative response in acute liver injury by suppressing paracrine senescence. Sci. Transl Med. 10, eaan1230 (2018).
Bala, S., Marcos, M., Gattu, A., Catalano, D. & Szabo, G. Acute binge drinking increases serum endotoxin and bacterial DNA levels in healthy individuals. PLoS ONE 9, e96864 (2014).
Szabo, G. Gut-liver axis in alcoholic liver disease. Gastroenterology 148, 30–36 (2015).
Yang, R. et al. HMGB1 neutralization is associated with bacterial translocation during acetaminophen hepatotoxicity. BMC Gastroenterol. 14, 66 (2014).
Font-Burgada, J. et al. Hybrid periportal hepatocytes regenerate the injured liver without giving rise to cancer. Cell 162, 766–779 (2015).
Han, X. et al. Lineage tracing reveals the bipotency of SOX9+ hepatocytes during liver regeneration. Stem Cell Rep. 12, 624–638 (2019).
Ferreira-Gonzalez, S. et al. Paracrine cellular senescence exacerbates biliary injury and impairs regeneration. Nat. Commun. 9, 1020 (2018).
Roskams, T. A. et al. Nomenclature of the finer branches of the biliary tree: canals, ductules, and ductular reactions in human livers. Hepatology 39, 1739–1745 (2004).
Jors, S. et al. Lineage fate of ductular reactions in liver injury and carcinogenesis. J. Clin. Invest. 125, 2445–2457 (2015).
Takase, H. M. et al. FGF7 is a functional niche signal required for stimulation of adult liver progenitor cells that support liver regeneration. Genes Dev. 27, 169–181 (2013).
Rodrigo-Torres, D. et al. The biliary epithelium gives rise to liver progenitor cells. Hepatology 60, 1367–1377 (2014).
Speicher, T. et al. Knockdown and knockout of β1-integrin in hepatocytes impairs liver regeneration through inhibition of growth factor signalling. Nat. Commun. 5, 3862 (2014).
Wan, Y. et al. Substance P increases liver fibrosis by differential changes in senescence of cholangiocytes and hepatic stellate cells. Hepatology 66, 528–541 (2017). The authors provide evidence that liver fibrosis is reversible, and they help to elucidate some of the molecular mechanisms underlying fibrosis remodelling.
Michalopoulos, G. K., Barua, L. & Bowen, W. C. Transdifferentiation of rat hepatocytes into biliary cells after bile duct ligation and toxic biliary injury. Hepatology 41, 535–544 (2005).
Tarlow, B. D. et al. Bipotential adult liver progenitors are derived from chronically injured mature hepatocytes. Cell Stem Cell 15, 605–618 (2014).
Yovchev, M. I., Locker, J. & Oertel, M. Biliary fibrosis drives liver repopulation and phenotype transition of transplanted hepatocytes. J. Hepatol. 64, 1348–1357 (2016).
Yimlamai, D., Fowl, B. H. & Camargo, F. D. Emerging evidence on the role of the Hippo/YAP pathway in liver physiology and cancer. J. Hepatol. 63, 1491–1501 (2015).
Schaub, J. R. et al. De novo formation of the biliary system by TGFβ-mediated hepatocyte transdifferentiation. Nature 557, 247–251 (2018).
Huch, M. et al. Unlimited in vitro expansion of adult bi-potent pancreas progenitors through the Lgr5/R-spondin axis. EMBO J. 32, 2708–2721 (2013).
Shin, S., Upadhyay, N., Greenbaum, L. E. & Kaestner, K. H. Ablation of Foxl1-Cre-labeled hepatic progenitor cells and their descendants impairs recovery of mice from liver injury. Gastroenterology 148, 192–202 (2015).
Espanol-Suner, R. et al. Liver progenitor cells yield functional hepatocytes in response to chronic liver injury in mice. Gastroenterology 143, 1564–1575 (2012).
Suzuki, A., Sekiya, S., Buscher, D., Izpisua Belmonte, J. C. & Taniguchi, H. Tbx3 controls the fate of hepatic progenitor cells in liver development by suppressing p19ARF expression. Development 135, 1589–1595 (2008).
Lu, J. et al. Notch signaling coordinates progenitor cell-mediated biliary regeneration following partial hepatectomy. Sci. Rep. 6, 22754 (2016).
Boulter, L. et al. Macrophage-derived Wnt opposes Notch signaling to specify hepatic progenitor cell fate in chronic liver disease. Nat. Med. 18, 572–579 (2012).
Campana, L. & Iredale, J. P. Regression of liver fibrosis. Semin. Liver Dis. 37, 1–10 (2017).
Constandinou, C., Henderson, N. & Iredale, J. P. Modeling liver fibrosis in rodents. Methods Mol. Med. 117, 237–250 (2005).
Henderson, N. C. & Iredale, J. P. Liver fibrosis: cellular mechanisms of progression and resolution. Clin. Sci. 112, 265–280 (2007).
Pellicoro, A., Ramachandran, P., Iredale, J. P. & Fallowfield, J. A. Liver fibrosis and repair: immune regulation of wound healing in a solid organ. Nat. Rev. Immunol. 14, 181–194 (2014).
Karin, D., Koyama, Y., Brenner, D. & Kisseleva, T. The characteristics of activated portal fibroblasts/myofibroblasts in liver fibrosis. Differentiation 92, 84–92 (2016).
Kisseleva, T. & Brenner, D. A. Hepatic stellate cells and the reversal of fibrosis. J. Gastroenterol. Hepatol. 21, S84–S87 (2006).
Wang, P. et al. Promising therapy candidates for liver fibrosis. Front. Physiol. https://doi.org/10.3389/fphys.2016.00047 (2016).
Hahn, E., Wick, G., Pencev, D. & Timpl, R. Distribution of basement membrane proteins in normal and fibrotic human liver: collagen type IV, laminin, and fibronectin. Gut 21, 63–71 (1980).
Burt, A. D., Griffiths, M. R., Schuppan, D., Voss, B. & MacSween, R. N. M. Ultrastructural localization of extracellular matrix proteins in liver biopsies using ultracryomicrotomy and immuno-gold labelling. Histopathology 16, 53–58 (1990).
McGuire, R. F., Bissell, D. M., Boyles, J. & Roll, F. J. Role of extracellular matrix in regulating fenestrations of sinusoidal endothelial cells isolated from normal rat liver. Hepatology 15, 989–997 (1992).
Zadorozhna, M., Di Gioia, S., Conese, M. & Mangieri, D. Neovascularization is a key feature of liver fibrosis progression: anti-angiogenesis as an innovative way of liver fibrosis treatment. Mol. Biol. Rep. 47, 2279–2288 (2020).
Pellicoro, A., Ramachandran, P. & Iredale, J. P. Reversibility of liver fibrosis. Fibrogenesis Tissue Repair. 5, S26 (2012).
Dufour, J. F., DeLellis, R. & Kaplan, M. M. Regression of hepatic fibrosis in hepatitis C with long-term interferon treatment. Dig. Dis. Sci. 43, 2573–2576 (1998).
Dufour, J. F., DeLellis, R. & Kaplan, M. M. Reversibility of hepatic fibrosis in autoimmune hepatitis. Ann. Intern. Med. 127, 981–985 (1997).
Iredale, J. P. et al. Mechanisms of spontaneous resolution of rat liver fibrosis. Hepatic stellate cell apoptosis and reduced hepatic expression of metalloproteinase inhibitors. J. Clin. Invest. 102, 538–549 (1998).
Iredale, J. P. et al. Tissue inhibitor of metalloproteinase-1 messenger RNA expression is enhanced relative to interstitial collagenase messenger RNA in experimental liver injury and fibrosis. Hepatology 24, 176–184 (1996).
Issa, R. et al. Spontaneous recovery from micronodular cirrhosis: evidence for incomplete resolution associated with matrix cross-linking. Gastroenterology 126, 1795–1808 (2004).
Iredale, J. P. Tissue inhibitors of metalloproteinases in liver fibrosis. Int. J. Biochem. Cell Biol. 29, 43–54 (1997).
Tsuchida, T. & Friedman, S. L. Mechanisms of hepatic stellate cell activation. Nat. Rev. Gastroenterol. Hepatol. 14, 397–411 (2017).
Li, X. et al. Placental growth factor silencing ameliorates liver fibrosis and angiogenesis and inhibits activation of hepatic stellate cells in a murine model of chronic liver disease. J. Cell Mol. Med. 21, 2370–2385 (2017).
Xiang, D. M. et al. The HLF/IL-6/STAT3 feedforward circuit drives hepatic stellate cell activation to promote liver fibrosis. Gut 67, 1704–1715 (2018).
Furukawa, F. et al. p38 MAPK mediates fibrogenic signal through Smad3 phosphorylation in rat myofibroblasts. Hepatology 38, 879–889 (2003).
Troeger, J. S. et al. Deactivation of hepatic stellate cells during liver fibrosis resolution in mice. Gastroenterology 143, 1073–1083 (2012).
Kisseleva, T. et al. Myofibroblasts revert to an inactive phenotype during regression of liver fibrosis. Proc. Natl Acad. Sci. USA 109, 9448–9453 (2012). In this seminal article, Kisseleva and co-workers describe reversion to a quiescent phenotype as one of the mechanisms to switch off an HSC response during liver fibrosis.
Wright, M. C. et al. Gliotoxin stimulates the apoptosis of human and rat hepatic stellate cells and enhances the resolution of liver fibrosis in rats. Gastroenterology 121, 685–698 (2001).
Krenkel, O., Hundertmark, J., Ritz, T. P., Weiskirchen, R. & Tacke, F. Single cell RNA sequencing identifies subsets of hepatic stellate cells and myofibroblasts in liver fibrosis. Cells https://doi.org/10.3390/cells8050503 (2019).
Wilson, D. H. et al. Non-canonical Wnt signalling regulates scarring in biliary disease via the planar cell polarity receptors. Nat. Commun. 11, 445 (2020).
Stutchfield, B. M. et al. Quantifying changes in innate immune function following liver transplantation for chronic liver disease. HPB 21, 1322–1326 (2019).
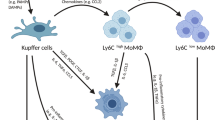
Duffield, J. S. et al. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J. Clin. Invest. 115, 56–65 (2005). In this article, the authors provide a comprehensive analysis of the role of CCR2 in the recruitment of macrophages in the injured liver, which is key to understand the functioning of therapies aiming at preventing extravasation of monocytes during acute and chronic liver disease.
Ramachandran, P. et al. Differential Ly-6C expression identifies the recruited macrophage phenotype, which orchestrates the regression of murine liver fibrosis. Proc. Natl Acad. Sci. USA 109, E3186–E3195 (2012). In this article, Ramachandran and colleagues identify prorestorative, infiltrating macrophages in models of chronic liver fibrosis for the first time, and provide a comprehensive characterization of their transcriptome and functions.
Scaffidi, P., Misteli, T. & Bianchi, M. E. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature 418, 191–195 (2002).
Campana, L., Bosurgi, L., Bianchi, M. E., Manfredi, A. A. & Rovere-Querini, P. Requirement of HMGB1 for stromal cell-derived factor-1/CXCL12-dependent migration of macrophages and dendritic cells. J. Leukoc. Biol. 86, 609–615 (2009).
Dumitriu, I. E., Bianchi, M. E., Bacci, M., Manfredi, A. A. & Rovere-Querini, P. The secretion of HMGB1 is required for the migration of maturing dendritic cells. J. Leukoc. Biol. 81, 84–91 (2007).
Schiraldi, M. et al. HMGB1 promotes recruitment of inflammatory cells to damaged tissues by forming a complex with CXCL12 and signaling via CXCR4. J. Exp. Med. 209, 551–563 (2012).
Yang, H. et al. Redox modification of cysteine residues regulates the cytokine activity of high mobility group box-1 (HMGB1). Mol. Med. 18, 250–259 (2012).
Yang, R. et al. High mobility group B1 impairs hepatocyte regeneration in acetaminophen hepatotoxicity. BMC Gastroenterol. 12, 45 (2012).
Lai, R. et al. Protective effect of Th22 cells and intrahepatic IL-22 in drug induced hepatocellular injury. J. Hepatol. 63, 148–155 (2015).
Zhou, M., Yang, H., Learned, R. M., Tian, H. & Ling, L. Non-cell-autonomous activation of IL-6/STAT3 signaling mediates FGF19-driven hepatocarcinogenesis. Nat. Commun. 8, 15433 (2017).
Campana, L. et al. The STAT3-IL-10-IL-6 pathway is a novel regulator of macrophage efferocytosis and phenotypic conversion in sterile liver injury. J. Immunol. 200, 1169–1187 (2018).
Bode, J. G., Ehlting, C. & Haussinger, D. The macrophage response towards LPS and its control through the p38(MAPK)-STAT3 axis. Cell Signal. 24, 1185–1194 (2012).
Lippai, D., Bala, S., Catalano, D., Kodys, K. & Szabo, G. Micro-RNA-155 deficiency prevents alcohol-induced serum endotoxin increase and small bowel inflammation in mice. Alcohol. Clin. Exp. Res. 38, 2217–2224 (2014).
Labeur, M. S. et al. Generation of tumor immunity by bone marrow-derived dendritic cells correlates with dendritic cell maturation stage. J. Immunol. 162, 168–175 (1999).
Hilkens, C. M., Kalinski, P., de Boer, M. & Kapsenberg, M. L. Human dendritic cells require exogenous interleukin-12-inducing factors to direct the development of naive T-helper cells toward the Th1 phenotype. Blood 90, 1920–1926 (1997).
Thakur, V., Pritchard, M. T., McMullen, M. R., Wang, Q. & Nagy, L. E. Chronic ethanol feeding increases activation of NADPH oxidase by lipopolysaccharide in rat Kupffer cells: role of increased reactive oxygen in LPS-stimulated ERK1/2 activation and TNF-alpha production. J. Leukoc. Biol. 79, 1348–1356 (2006).
Brenner, C., Galluzzi, L., Kepp, O. & Kroemer, G. Decoding cell death signals in liver inflammation. J. Hepatol. 59, 583–594 (2013).
Roychowdhury, S. et al. An early complement-dependent and TLR-4-independent phase in the pathogenesis of ethanol-induced liver injury in mice. Hepatology 49, 1326–1334 (2009).
Chen, M., Liu, J., Yang, W. & Ling, W. Lipopolysaccharide mediates hepatic stellate cell activation by regulating autophagy and retinoic acid signaling. Autophagy 13, 1813–1827 (2017).
Paik, Y. H. et al. Toll-like receptor 4 mediates inflammatory signaling by bacterial lipopolysaccharide in human hepatic stellate cells. Hepatology 37, 1043–1055 (2003).
Zhao, P. et al. HMGB1 release by H2O2-induced hepatocytes is regulated through calcium overload and 58-F interference. Cell Death Discov. 3, 17008 (2017).
Minsart, C. et al. New insights in acetaminophen toxicity: HMGB1 contributes by itself to amplify hepatocyte necrosis in vitro through the TLR4-TRIF-RIPK3 axis. Sci. Rep. 10, 5557 (2020).
Khambu, B. et al. HMGB1 promotes ductular reaction and tumorigenesis in autophagy-deficient livers. J. Clin. Invest. 128, 2419–2435 (2018).
Hernandez, C. et al. HMGB1 links chronic liver injury to progenitor responses and hepatocarcinogenesis. J. Clin. Invest. 128, 2436–2451 (2018).
Ge, X. et al. High mobility group box-1 drives fibrosis progression signaling via the receptor for advanced glycation end products in mice. Hepatology 68, 2380–2404 (2018).
Gluck, S. et al. Innate immune sensing of cytosolic chromatin fragments through cGAS promotes senescence. Nat. Cell Biol. 19, 1061–1070 (2017).
Takahashi, A. et al. Downregulation of cytoplasmic DNases is implicated in cytoplasmic DNA accumulation and SASP in senescent cells. Nat. Commun. 9, 1249 (2018).
Lin, L. et al. The STAT3 inhibitor NSC 74859 is effective in hepatocellular cancers with disrupted TGF-beta signaling. Oncogene 28, 961–972 (2009).
Wan, S. et al. Tumor-associated macrophages produce interleukin 6 and signal via STAT3 to promote expansion of human hepatocellular carcinoma stem cells. Gastroenterology 147, 1393–1404 (2014).
Shi, Z., Wakil, A. E. & Rockey, D. C. Strain-specific differences in mouse hepatic wound healing are mediated by divergent T helper cytokine responses. Proc. Natl Acad. Sci. USA 94, 10663–10668 (1997).
Wynn, T. A. et al. An IL-12-based vaccination method for preventing fibrosis induced by schistosome infection. Nature 376, 594–596 (1995).
Wynn, T. A. Fibrotic disease and the TH1/TH2 paradigm. Nat. Rev. Immunol. 4, 583–594 (2004).
Rolla, S. et al. The balance between IL-17 and IL-22 produced by liver-infiltrating T-helper cells critically controls NASH development in mice. Clin. Sci. 130, 193–203 (2016).
Wehr, A. et al. Chemokine receptor CXCR6-dependent hepatic NK T cell accumulation promotes inflammation and liver fibrosis. J. Immunol. 190, 5226–5236 (2013).
Dini, L., Pagliara, P. & Carla, E. C. Phagocytosis of apoptotic cells by liver: a morphological study. Microsc. Res. Tech. 57, 530–540 (2002).
Terpstra, V. & van Berkel, T. J. Scavenger receptors on liver Kupffer cells mediate the in vivo uptake of oxidatively damaged red blood cells in mice. Blood 95, 2157–2163 (2000).
Shi, J., Aisaki, K., Ikawa, Y. & Wake, K. Evidence of hepatocyte apoptosis in rat liver after the administration of carbon tetrachloride. Am. J. Pathol. 153, 515–525 (1998).
Lee, S. J., Park, S. Y., Jung, M. Y., Bae, S. M. & Kim, I. S. Mechanism for phosphatidylserine-dependent erythrophagocytosis in mouse liver. Blood 117, 5215–5223 (2011).
Uderhardt, S., Martins, A. J., Tsang, J. S., Lammermann, T. & Germain, R. N. Resident macrophages cloak tissue microlesions to prevent neutrophil-driven inflammatory damage. Cell 177, 541–555 (2019).
Fadok, V. A. et al. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J. Immunol. 148, 2207–2216 (1992).
Triantafyllou, E. et al. MerTK expressing hepatic macrophages promote the resolution of inflammation in acute liver failure. Gut 67, 333–347 (2018).
Proto, J. D. et al. Regulatory T cells promote macrophage efferocytosis during inflammation resolution. Immunity 49, 666–677 (2018).
Liew, P. X., Lee, W. Y. & Kubes, P. iNKT cells orchestrate a switch from inflammation to resolution of sterile liver injury. Immunity 47, 752–765 (2017).
Ding, B. S. et al. Divergent angiocrine signals from vascular niche balance liver regeneration and fibrosis. Nature 505, 97–102 (2014).
Kato, T. et al. Vascular endothelial growth factor receptor-1 signaling promotes liver repair through restoration of liver microvasculature after acetaminophen hepatotoxicity. Toxicol. Sci. 120, 218–229 (2011).
Bleriot, C. & Ginhoux, F. Understanding the heterogeneity of resident liver macrophages. Front. Immunol. 10, 2694 (2019).
Sierro, F. et al. A liver capsular network of monocyte-derived macrophages restricts hepatic dissemination of intraperitoneal bacteria by neutrophil recruitment. Immunity 47, 374–388 (2017).
Halpern, K. B. et al. Single-cell spatial reconstruction reveals global division of labour in the mammalian liver. Nature 542, 352–356 (2017).
Aizarani, N. et al. A human liver cell atlas reveals heterogeneity and epithelial progenitors. Nature https://doi.org/10.1038/s41586-019-1373-2 (2019).
Bonnardel, J. et al. Stellate cells, hepatocytes, and endothelial cells imprint the Kupffer cell identity on monocytes colonizing the liver macrophage niche. Immunity 51, 638–654 (2019).
MacParland, S. A. et al. Single cell RNA sequencing of human liver reveals distinct intrahepatic macrophage populations. Nat. Commun. 9, 4383 (2018). In this important article, MacParland and co-workers identify various populations of macrophages in human livers using a single-cell approach.
Beattie, L. et al. Bone marrow-derived and resident liver macrophages display unique transcriptomic signatures but similar biological functions. J. Hepatol. 65, 758–768 (2016).
Wardle, E. N. Bacteraemic and endotoxic shock. J. Clin. Pathol. 33, 888 (1980).
Canalese, J. et al. Reticuloendothelial system and hepatocytic function in fulminant hepatic failure. Gut 23, 265–269 (1982).
Holt, M. P., Cheng, L. & Ju, C. Identification and characterization of infiltrating macrophages in acetaminophen-induced liver injury. J. Leukoc. Biol. 84, 1410–1421 (2008). In this article, Holt and co-workers give a detailed description of macrophages infiltrating livers of paracetamol-overdosed mice, highlighting the deficit in phagocytosis of infiltrating macrophages.
Stutchfield, B. M. et al. CSF1 restores innate immunity after liver injury in mice and serum levels indicate outcomes of patients with acute liver failure. Gastroenterology 149, 1896–1909 (2015).
Ramachandran, P. et al. Resolving the fibrotic niche of human liver cirrhosis at single-cell level. Nature 575, 512–518 (2019).
Mossanen, J. C. et al. Chemokine (C-C motif) receptor 2-positive monocytes aggravate the early phase of acetaminophen-induced acute liver injury. Hepatology 64, 1667–1682 (2016).
Hokeness, K. L., Kuziel, W. A., Biron, C. A. & Salazar-Mather, T. P. Monocyte chemoattractant protein-1 and CCR2 interactions are required for IFN-alpha/beta-induced inflammatory responses and antiviral defense in liver. J. Immunol. 174, 1549–1556 (2005).
Saiman, Y. & Friedman, S. L. The role of chemokines in acute liver injury. Front. Physiol. 3, 213 (2012).
Karlmark, K. R. et al. Hepatic recruitment of the inflammatory Gr1+monocyte subset upon liver injury promotes hepatic fibrosis. Hepatology 50, 261–274 (2009).
Carlin, L. M., Auffray, C. & Geissmann, F. Measuring intravascular migration of mouse Ly6C(low) monocytes in vivo using intravital microscopy. Curr. Protoc. Immunol. https://doi.org/10.1002/0471142735.im1433s101 (2013).
Carlin, L. M. et al. Nr4a1-dependent Ly6Clow monocytes monitor endothelial cells and orchestrate their disposal. Cell 153, 362–375 (2013).
Wang, M. et al. Chronic alcohol ingestion modulates hepatic macrophage populations and functions in mice. J. Leukoc. Biol. 96, 657–665 (2014).
Bourdi, M. et al. Protection against acetaminophen-induced liver injury and lethality by interleukin 10: role of inducible nitric oxide synthase. Hepatology 35, 289–298 (2002).
Ryan, P. M. et al. Endogenous interleukin-4 regulates glutathione synthesis following acetaminophen-induced liver injury in mice. Chem. Res. Toxicol. 25, 83–93 (2012).
Dambach, D. M., Watson, L. M., Gray, K. R., Durham, S. K. & Laskin, D. L. Role of CCR2 in macrophage migration into the liver during acetaminophen-induced hepatotoxicity in the mouse. Hepatology 35, 1093–1103 (2002).
Seki, E. et al. CCR1 and CCR5 promote hepatic fibrosis in mice. J. Clin. Invest. 119, 1858–1870 (2009).
Friedman, S. et al. Efficacy and safety study of cenicriviroc for the treatment of non-alcoholic steatohepatitis in adult subjects with liver fibrosis: CENTAUR phase 2b study design. Contemp. Clin. Trials 47, 356–365 (2016).
Friedman, S. L. et al. A randomized, placebo-controlled trial of cenicriviroc for treatment of nonalcoholic steatohepatitis with fibrosis. Hepatology 67, 1754–1767 (2018).
Bird, T. G. et al. Bone marrow injection stimulates hepatic ductular reactions in the absence of injury via macrophage-mediated TWEAK signaling. Proc. Natl Acad. Sci. USA 110, 6542–6547 (2013).
Lorenzini, S. et al. Characterisation of a stereotypical cellular and extracellular adult liver progenitor cell niche in rodents and diseased human liver. Gut 59, 645–654 (2010).
Viebahn, C. S. et al. Invading macrophages play a major role in the liver progenitor cell response to chronic liver injury. J. Hepatol. 53, 500–507 (2010).
Van Hul, N. et al. Kupffer cells influence parenchymal invasion and phenotypic orientation, but not the proliferation, of liver progenitor cells in a murine model of liver injury. Am. J. Pathol. 179, 1839–1850 (2011).
Elsegood, C. L. et al. Kupffer cell-monocyte communication is essential for initiating murine liver progenitor cell-mediated liver regeneration. Hepatology 62, 1272–1284 (2015).
Carpino, G. et al. Macrophage activation in pediatric nonalcoholic fatty liver disease (NAFLD) correlates with hepatic progenitor cell response via Wnt3a pathway. PLoS ONE 11, e0157246 (2016).
Yamamoto, K. N. et al. Prediction of postoperative liver regeneration from clinical information using a data-led mathematical model. Sci. Rep. 6, 34214 (2016).
Olthoff, K. M. et al. Outcomes of adult living donor liver transplantation: comparison of the Adult-to-Adult Living Donor Liver Transplantation Cohort Study and the national experience. Liver Transplant. 17, 789–797 (2011).
Kasahara, M., Umeshita, K., Inomata, Y. & Uemoto, S., Japanese Liver Transplantation Society. Long-term outcomes of pediatric living donor liver transplantation in japan: an analysis of more than 2200 Cases listed in the registry of the Japanese Liver Transplantation Society. Am. J. Transplant. 13, 1830–1839 (2013).
van Lienden, K. P. et al. Portal vein embolization before liver resection: a systematic review. Cardiovasc. Intervent. Radiol. 36, 25–34 (2013).
am Esch, J. S. II et al. Portal application of autologous CD133+bone marrow cells to the liver: a novel concept to support hepatic regeneration. Stem Cell 23, 463–470 (2005).
Chenard-Neu, M. P. et al. Auxiliary liver transplantation: regeneration of the native liver and outcome in 30 patients with fulminant hepatic failure–a multicenter European study. Hepatology 23, 1119–1127 (1996).
Bismuth, H. et al. Auxiliary partial orthotopic liver transplantation for fulminant hepatitis. The Paul Brousse experience. Ann. Surg. 224, 712–726 (1996).
Tsolaki, E. et al. Hematopoietic stem cells and liver regeneration: differentially acting hematopoietic stem cell mobilization agents reverse induced chronic liver injury. Blood Cell Mol. Dis. 53, 124–132 (2014).
Wang, X. et al. Cell fusion is the principal source of bone-marrow-derived hepatocytes. Nature 422, 897–901 (2003).
Zhang, Y. et al. Therapeutic effect of hepatocyte growth factor-overexpressing bone marrow-derived mesenchymal stem cells on CCl4-induced hepatocirrhosis. Cell Death Dis. 9, 1186 (2018).
Ding, H. R. et al. Mesenchymal stem cells improve glycometabolism and liver regeneration in the treatment of post-hepatectomy liver failure. Front. Physiol. 10, 412 (2019).
Zhu, X., He, B., Zhou, X. & Ren, J. Effects of transplanted bone-marrow-derived mesenchymal stem cells in animal models of acute hepatitis. Cell Tissue Res. 351, 477–486 (2013).
Moore, J. K., Stutchfield, B. M. & Forbes, S. J. Systematic review: the effects of autologous stem cell therapy for patients with liver disease. Aliment. Pharmacol. Ther. 39, 673–685 (2014).
Newsome, P. N. et al. Granulocyte colony-stimulating factor and autologous CD133-positive stem-cell therapy in liver cirrhosis (REALISTIC): an open-label, randomised, controlled phase 2 trial. Lancet Gastroenterol. Hepatol. 3, 25–36 (2018).
Zhao, L., Chen, S., Shi, X., Cao, H. & Li, L. A pooled analysis of mesenchymal stem cell-based therapy for liver disease. Stem Cell Res. Ther. 9, 72 (2018).
Moore, J. K. et al. Phenotypic and functional characterization of macrophages with therapeutic potential generated from human cirrhotic monocytes in a cohort study. Cytotherapy 17, 1604–1616 (2015).
Moroni, F., Ammirati, E., Norata, G. D., Magnoni, M. & Camici, P. G. The role of monocytes and macrophages in human atherosclerosis, plaque neoangiogenesis, and atherothrombosis. Mediators Inflamm. 2019, 7434376 (2019). This is a first-in-human, phase I trial using autologous macrophages as therapy for cirrhosis.
Ma, P. F. et al. Cytotherapy with M1-polarized macrophages ameliorates liver fibrosis by modulating immune microenvironment in mice. J. Hepatol. 67, 770–779 (2017).
Mantovani, A. et al. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 25, 677–686 (2004).
Bhushan, B. & Apte, U. Liver regeneration after acetaminophen hepatotoxicity: mechanisms and therapeutic opportunities. Am. J. Pathol. 189, 719–729 (2019).
Krenkel, O., Mossanen, J. C. & Tacke, F. Immune mechanisms in acetaminophen-induced acute liver failure. Hepatobiliary Surg. Nutr. 3, 331–343 (2014).
Zigmond, E. et al. Infiltrating monocyte-derived macrophages and resident Kupffer cells display different ontogeny and functions in acute liver injury. J. Immunol. 193, 344–353 (2014).
Lewis, P. S. et al. Alternatively activated macrophages promote resolution of necrosis following acute liver injury. J. Hepatol. https://doi.org/10.1016/j.jhep.2020.02.031 (2020).
Heldin, C. H. & Moustakas, A. Signaling receptors for TGF-beta family members. Cold Spring Harb. Perspect Biol. https://doi.org/10.1101/cshperspect.a022053 (2016).
Fang, D., He, Y. & Luan, Z. Simvastatin augments activation of liver regeneration through attenuating transforming growth factor-beta1 induced-apoptosis in obstructive jaundice rats. Exp. Ther. Med. 14, 4839–4845 (2017).
Khalil, N., Bereznay, O., Sporn, M. & Greenberg, A. H. Macrophage production of transforming growth factor beta and fibroblast collagen synthesis in chronic pulmonary inflammation. J. Exp. Med. 170, 727–737 (1989).
Wynn, T. A. & Barron, L. Macrophages: master regulators of inflammation and fibrosis. Semin. Liver Dis. 30, 245–257 (2010).
Fabregat, I. & Caballero-Diaz, D. Transforming growth factor-beta-induced cell plasticity in liver fibrosis and hepatocarcinogenesis. Front. Oncol. 8, 357 (2018).
Pimpin, L. et al. Burden of liver disease in Europe: Epidemiology and analysis of risk factors to identify prevention policies. J. Hepatol. 69, 718–735 (2018).
Ikeda, H. et al. Bile ductular cell reaction with senescent hepatocytes in chronic viral hepatitis is lost during hepatocarcinogenesis. Pathol. Int. 59, 471–478 (2009).
Sancho-Bru, P. et al. Liver progenitor cell markers correlate with liver damage and predict short-term mortality in patients with alcoholic hepatitis. Hepatology 55, 1931–1941 (2012).
Gadd, V. L. et al. The portal inflammatory infiltrate and ductular reaction in human nonalcoholic fatty liver disease. Hepatology 59, 1393–1405 (2014).
Richardson, M. M. et al. Progressive fibrosis in nonalcoholic steatohepatitis: association with altered regeneration and a ductular reaction. Gastroenterology 133, 80–90 (2007).
Wood, M. J., Gadd, V. L., Powell, L. W., Ramm, G. A. & Clouston, A. D. Ductular reaction in hereditary hemochromatosis: the link between hepatocyte senescence and fibrosis progression. Hepatology 59, 848–857 (2014).
Sasaki, M., Ikeda, H., Haga, H., Manabe, T. & Nakanuma, Y. Frequent cellular senescence in small bile ducts in primary biliary cirrhosis: a possible role in bile duct loss. J. Pathol. 205, 451–459 (2005).
Carpino, G. et al. Hepatic stem/progenitor cell activation differs between primary sclerosing and primary biliary cholangitis. Am. J. Pathol. 188, 627–639 (2018).
Tabibian, J. H. et al. Characterization of cultured cholangiocytes isolated from livers of patients with primary sclerosing cholangitis. Lab. Invest. 94, 1126–1133 (2014).
Adam, R. et al. 2018 annual report of the European Liver Transplant Registry (ELTR) - 50-year evolution of liver transplantation. Transpl. Int. 31, 1293–1317 (2018).
Dhawan, A., Puppi, J., Hughes, R. D. & Mitry, R. R. Human hepatocyte transplantation: current experience and future challenges. Nat. Rev. Gastroenterol. Hepatol. 7, 288–298 (2010).
Iansante, V., Mitry, R. R., Filippi, C., Fitzpatrick, E. & Dhawan, A. Human hepatocyte transplantation for liver disease: current status and future perspectives. Pediatric Res. 83, 232–240 (2018).
Nasralla, D. et al. A randomized trial of normothermic preservation in liver transplantation. Nature 557, 50–56 (2018). This is the first randomized controlled trial using machine perfusion for normothermic preservation of donors’ livers before orthotopic liver transplantation.
Schlegel, A. et al. Outcomes of DCD liver transplantation using organs treated by hypothermic oxygenated perfusion before implantation. J. Hepatol. 70, 50–57 (2019).
Eshmuminov, D. et al. An integrated perfusion machine preserves injured human livers for 1 week. Nat. Biotechnol. https://doi.org/10.1038/s41587-019-0374-x (2020).
Schneeberger, S. Life of a liver awaiting transplantation. Nature 557, 40–41 (2018).
McAuley, D. F. et al. Clinical grade allogeneic human mesenchymal stem cells restore alveolar fluid clearance in human lungs rejected for transplantation. Am. J. Physiol. Lung Cell Mol. Physiol. 306, L809–L815 (2014).
Mordant, P. et al. Mesenchymal stem cell treatment is associated with decreased perfusate concentration of interleukin-8 during ex vivo perfusion of donor lungs after 18-hour preservation. J. Heart Lung Transplant. 35, 1245–1254 (2016).
Gregorini, M. et al. Perfusion of isolated rat kidney with mesenchymal stromal cells/extracellular vesicles prevents ischaemic injury. J. Cell Mol. Med. 21, 3381–3393 (2017).
Laing, R. W. et al. The delivery of multipotent adult progenitor cells to extended criteria human donor livers using normothermic machine perfusion. Front. Immunol. https://doi.org/10.3389/fimmu.2020.01226 (2020).
Acknowledgements
The authors thank R. Aird and B. Dwyer for helpful discussions regarding the draft manuscript.
Author information
Authors and Affiliations
Contributions
L.C. and H.E. wrote the first draft of the manuscript. S.F. and M.H. reviewed the first draft and added to the manuscript.
Corresponding author
Ethics declarations
Competing interests
S.F. has patents pending entitled “Macrophage-based therapy” in national territories of the USA, Europe, Japan, China and Australia. These patents have been derived from PCT/GB2017/052769 filed in 18 September 2017 and claim priority from UK application 1615923.8 filed on 19 September 2016. Both of the original patents have now been abandoned because the original UK patent and PCT patent are no longer live and have now been replaced by the national patents. L.C. is a co-founder and current employee of Resolution Therapeutic Ltd.
Additional information
Peer review information
Nature Reviews Molecular Cell Biology thanks Frank Tacke and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Glossary
- Cirrhosis
-
Terminal stage of liver fibrosis, when the scarring of the liver is hardly or not reversible. It is associated with high morbidity and mortality and may lead to the development of liver cancer.
- Hepatocellular carcinoma
-
Most common type of liver cancer, develops in people with chronic liver diseases of various causes. It is caused by an uncontrolled hyperproliferation of hepatocytes.
- Orthotopic liver transplantation
-
Surgical replacement of a non-functional liver with a healthy liver from a living or deceased donor. ‘Orthotopic’ refers to the donor liver being placed in the same anatomical position as the host liver.
- Necrosis
-
Premature and non-regulated form of cell death, which results in autolysis of the cell, with subsequent dissemination of the intracellular components in the extracellular space and inflammation.
- Apoptosis
-
A form of programmed cell death characterized by blebbing of the cellular membranes, condensation of chromatin and retention of membrane integrity, thereby preventing the triggering of inflammatory responses.
- Zonation
-
Definition of three distinct metabolic zones for hepatocytes along the sinusoids, depending on the oxygen gradient (higher near the hepatic artery and progressively lower closer to the central vein).
- Cellular senescence
-
A state of permanent cell cycle arrest in which cells exit the G1 phase and enter irreversibly the G0 phase. Senescent cells secrete factors in the microenvironment, triggering a senescence-associated secretory phenotype.
- Matrix metalloproteases
-
(MMPs). Zinc-dependent enzymes (endopeptidases) that hydrolyse peptide bonds in extracellular matrix molecules, thereby promoting their degradation. They are usually stored as proenzymes, and are converted into their active form when needed.
- Danger-associated molecular patterns
-
Molecules that are normally intracellular and that are passively released into the extracellular space when a cell dies following a pathway that does not preserve membrane integrity, such as necrosis.
- Phagocytosis
-
Engulfment of a cargo by a living cells. The cargo can be either a pathogen or a dead or dying cell. In mammalians, professional phagocytes such as macrophages perform high-rate phagocytosis in the case of infection or tissue damage.
- Pathogen-associated molecular patterns
-
Molecules that derive from pathogens and that allow the innate immune system to categorize which type of threat it has to fight thanks to the engagement of specific receptors on innate immune cells that recognize one specific type of molecule only.
- GMP–AMP synthase (cGAS)–stimulator of interferon genes (STING) pathway
-
Pathway of the innate immune system that detects the presence of cytosolic DNA (usually indicating a viral infection or tumorigenesis) and responds by triggering the expression of inflammatory genes, such as those encoding interferon-β, which drives antiviral responses.
- Fatty liver disease
-
A form of liver injury where the parenchymal cells are progressively replaced by adipocytes with subsequent loss of function of the liver.
- TH1 adaptive immune response
-
A branch of the adaptive immune response. T helper 1 (TH1) cells normally develop following antigenic stimulation and co-stimulation of the naive T cell in the presence of interferon-γ and are involved in the killing of infected or tumoural cells.
- TH2 adaptive immune response
-
A branch of the adaptive immune response. T helper 2 (TH2) cells normally develop following antigenic stimulation and co-stimulation of the naive T cell in the presence of IL-4 and IL-13, and they support type II immune responses, including the B cell response.
- Unconventional T cells
-
T cells expressing a γδ T cell receptor. They have a barrier function against invading pathogens, and they are active in local cancer immunosurveillance.
- TH17 adaptive immune responses
-
A branch of the adaptive immune response. T helper 17 (TH17) cells secrete cytokines belonging to the IL-17 family, particularly IL-17A, IL-17F, IL-22 and TNF. They are involved in host defence, attacking pathogens such as extracellular bacteria and fungi.
- Regulatory T cells
-
(Treg cells). Treg cells normally develop following antigenic stimulation and co-stimulation of the naive T cell in the presence of TGFβ and IL-2. They secrete cytokines such as IL-10, TGFβ and IL-35. They are involved in repression of T cell responses.
- TNF-related weak inducer of apoptosis
-
(TWEAK). Cytokine belonging to the TNF superfamily that acts in a pleiotropic fashion through interaction with its receptor, FN14. It has been implicated in processes such as cell proliferation, angiogenesis and control of inflammation.
- Haematopoietic stem cells
-
Stem cells that self-renew and give rise to other progenitor cells that in turn produce all of the cell lineages of the blood. They reside in the red bone marrow of most bones.
- Immunosuppression
-
In this instance, a therapeutic regime aimed at suppressing the host immune system to prevent rejection of the transplanted organ.
- Mesenchymal stromal cells
-
(MSCs). Stem/progenitor cells that can give rise to tissue of embryonic mesenchymal origin such as cartilage (chondrocytes), bone (osteoblasts), muscle (myocytes) and adipose tissue (adipocytes).
Rights and permissions
About this article
Cite this article
Campana, L., Esser, H., Huch, M. et al. Liver regeneration and inflammation: from fundamental science to clinical applications. Nat Rev Mol Cell Biol 22, 608–624 (2021). https://doi.org/10.1038/s41580-021-00373-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41580-021-00373-7
This article is cited by
-
Mitochondrial stress activates YAP/TAZ through RhoA oxidation to promote liver injury
Cell Death & Disease (2024)
-
Cellular and molecular mechanisms of skin wound healing
Nature Reviews Molecular Cell Biology (2024)
-
Splenectomy ameliorates liver cirrhosis by restoring the gut microbiota balance
Cellular and Molecular Life Sciences (2024)
-
A spatiotemporal atlas of cholestatic injury and repair in mice
Nature Genetics (2024)
-
Can endocan serve as a molecular “hepatostat” in liver regeneration?
Molecular Medicine (2023)