Abstract
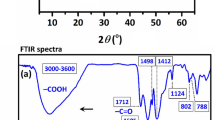
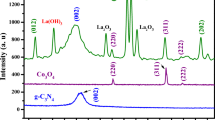
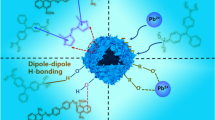
Layered double hydroxides are traditional positively charged inorganic materials generally considered as efficient and low-cost adsorbents for the removal of anionic organic molecules. In this study, we prepared a series of g-C3N4@NiCo LDH composites by loading 10–30 wt% of g-C3N4 onto the LDH through the electrostatic self-assembly method. The bare LDH and g-C3N4 loaded LDH composites were characterized by XRD, SEM-EDS, Zeta, DLS, and FTIR techniques. Results revealed that extra peak corresponds to g-C3N4 originating in the XRD patterns, distorted morphology of LDH, reduction in positive surface zeta potential, and enhancement in hydrodynamic size after loading of g-C3N4 affirmed the successful formation of the composite. The adsorption performance of as-modified LDH was evaluated by removing the most commonly used salicylic acid and methylene blue as anionic and cationic model pollutant, respectively, from aqueous solution. The adsorption mechanism for both the pollutants by as-synthesized samples follows Langmuir isotherm. The results demonstrated that the bare LDH exhibited maximum adsorption efficiency of 75.16 mg/g and only 3.66 mg/g for salicylic acid and methylene blue, respectively. With 30 wt% loading of g-C3N4, the adsorption capacity for methylene blue increased to 25.16 mg/g almost 6–7 times higher than that of bare LDH. On the other hand, the opposite effect on adsorptive removal of salicylic acid was observed with increase in the wt% loading of g-C3N4. With 30 wt% loading of g-C3N4, the adsorption capacity for salicylic acid decreased to 38.37 mg/g, almost half that of bare LDH. A possible mechanism has been proposed. The kinetics for adsorption of salicylic acid onto bare LDH obeys the second-order model aside from the methylene blue adsorption which follows first-order kinetics. On the other hand, the kinetics of adsorption for both the pollutants onto (10–30) CN- LDH composites follows second order kinetics.
Similar content being viewed by others
References
D. E. Helbling, Curr. Opin. Biotechnol., 33, 142 (2015).
M. B. Ahmed, J. L. Zhou, H. H. Ngo and W. Guo, Sci. Total Environ, 532, 112 (2015).
M. Zubair, M. Daud, G. McKay, F. Shehzad and M. A. Al-Harthi, Appl. Clay Sci., 143, 279 (2017).
I. Levchuk, J. J. R. Márquez and M. Sillanpää, Chemosphere, 192, 90 (2018).
N. Baliarsingh, K. M. Parida and G. C. Pradhan, Ind. Eng. Chem. Res., 53, 3834 (2014).
A. Talaiekhozani, M. R. Talaei and S. Rezania, J. Environ. Chem. Eng., 5, 1828 (2017).
R.-r. Shan, L.-g. Yan, Y.-m. Yang, K. Yang, S.-j. Yu, H.-q. Yu, B.-c. Zhu and B. Du, J. Ind. Eng. Chem., 21, 561 (2015).
C. Fonseca Couto, L. C. Lange and M. C. Santos Amaral, J. Water Process Eng., 26, 156 (2018).
L. Mohapatra, K. Parida and M. Satpathy, J. Phys. Chem. C., 116, 7350 (2012).
G. Zhang, X. Zhang, Y. Meng, G. Pan, Z. Ni and S. Xia, Chem. Eng. J., 392, 123684 (2020).
G. Arrabito, A. Bonasera, G. Prestopino, A. Orsini, A. Mattoccia, E. Martinelli, B. Pignataro and P. G. Medaglia, Crystals, 9, 361 (2019).
A. Baruah, S. Mondal, L. Sahoo and U. K. Gautam, J. Solid State Chem., 280, 120963 (2019).
M. Zubair, N. Jarrah, M. S. Manzar, M. Al-Harthi, M. Daud, N. D. Mu’azu and S. A. Haladu, J. Mol. Liq., 230, 344 (2017).
P. Chakraborty and R. Nagarajan, Appl. Clay Sci., 118, 308 (2015).
Y. Zheng, B. Cheng, W. You, J. Yu and W. Ho, J. Hazard. Mater., 369, 214 (2019).
Y.-l. Long, J.-g. Yu, F.-p. Jiao and W. j. Yang, Trans. Nonferrous Met. Soc. China English Ed., 26, 2701 (2016).
D. Huang, C. Liu, C. Zhang, R. Deng, R. Wang, W. Xue, H. Luo, G. Zeng, Q. Zhang and X. Guo, Bioresour. Technol., 276, 127 (2019).
D. Bin Jiang, C. Jing, Y. Yuan, L. Feng, X. Liu, F. Dong, B. Dong and Y. X. Zhang, J. Colloid Interface Sci., 540, 398 (2019).
B. Zhang, Z. Dong, D. Sun, T. Wu and Y. Li, J. Ind. Eng. Chem., 49, 208 (2017).
Y. Yang, B. Mao, G. Gong, D. Li, Y. Liu, W. Cao, L. Xing, J. Zeng, W. Shi and S. Yuan, Int. J. Hydrogen Energy, 44, 15882 (2019).
B. Ou, J. Wang, Y. Wu, S. Zhao and Z. Wang, Chem. Eng. J., 380, 122600 (2020).
Z. Sun, H. Wang, Z. Wu and L. Wang, Catal. Today, 300, 160 (2018).
X. Chen, Y. Li and L. Li, Appl. Surf. Sci., 508, 1 (2020).
X. Chen, W. Zhang, L. Zhang, L. Feng, J. Wen, J. Yang, C. Zhang, J. Jiang and H. Wang, Appl. Surf. Sci., 481, 1335 (2019).
Z. Ezzeddine, I. Batonneau-Gener, Y. Pouilloux and H. Hamad, J. Mol. Liq., 223, 763 (2016).
P. M. K. Reddy, P. Verma and C. Subrahmanyam, J. Taiwan Inst. Chem. Eng., 58, 500 (2016).
T. Wang, X. Liu, C. Ma, Y. Liu, H. Dong, W. Ma, Z. Liu, M. Wei, C. Li and Y. Yan, J. Taiwan Inst. Chem. Eng., 93, 298 (2018).
S. Megala, M. Sathish, S. Harish, M. Navaneethan, S. Sohila, B. Liang and R. Ramesh, Appl. Surf. Sci., 509, 144656 (2020).
S. Tonda, S. Kumar, M. Bhardwaj, P. Yadav and S. Ogale, ACS Appl. Mater. Interfaces, 10, 2667 (2018).
S. Nayak, L. Mohapatra and K. Parida, J. Mater. Chem. A, 3, 18622 (2015).
T. Li, G.H. Li, L.H. Li, L. Liu, Y. Xu, H.Y. Ding and T. Zhang, Korean J. Chem. Eng. ACS Appl. Mater. Interfaces, 8, 2562 (2016).
H. Hu, J. Liu, Z. Xu, L. Zhang, B. Cheng and W. Ho, Appl. Surf. Sci., 478, 981 (2019).
S. Xia, F. Liu, Z. Ni, J. Xue and P. Qian, J. Colloid Interface Sci., 405, 195 (2013).
Z. Peng, Y. Xin, X. Dong and Z. Quan, Ceram. Int., 40, 2115 (2014).
Y. Ao, D. Wang, P. Wang, C. Wang, J. Hou and J. Qian, Mater. Res. Bull., 80, 23 (2016).
P. Wang, D. H. L. Ng, M. Zhou and J. Li, Appl. Clay Sci., 178, 105131 (2019).
B. N. Mahato, T. Krithiga and M. A. M. Thangam, Surf. Interfaces, 23, 100636 (2021).
H. Ouassif, E.M. Moujahid, R. Lahkale, R. Sadik, F.Z. Bouragba, E. M. Sabbar and M. Diouri, Surf. Intefaces, 18, 100401 (2020).
L. Lu, J. Li, D. H. L. Ng, P. Yang, P. Song and M. Zuo, J. Ind. Eng. Chem., 46, 315 (2017).
I. Langmuir, J. Am. Chem. Soc., 40, 1361 (1918).
S. Chilukoti and T. Thangavel, Inorg. Chem. Commun., 100, 107 (2019).
B. Li, Y. Zhang, X. Zhou, Z. Liu, Q. Liu and X. Li, J. Alloys Compd., 673, 265 (2016).
Y. Zhou, J. Li, Y. Yang, B. Luo, X. Zhang, E. Fong, W. Chu and K. Huang, J. Alloys Compd., 788, 1029 (2019).
S. Li, Y. Yang, S. Huang, Z. He, C. Li, D. Li, B. Ke, C. Lai and Q. Peng, Appl. Clay Sci., 188, 105414 (2020).
K. V. Kumar and K. Porkodi, Chem. Eng. J., 148, 20 (2009).
S. A. A. Moaty, A. A. Farghali, M. Moussa and R. Khaled, J. Taiwan Inst. Chem. Eng., 71, 441 (2017).
Z. Zhou, S. Ouyang, P. Li, L. Shan, R. Ma and P. Zhang, Appl. Clay Sci., 188, 105500 (2020).
P. Sirajudheen, P. Karthikeyan, K. Ramkumar and S. Meenakshi, J. Mol. Liq., 318, 114200 (2020).
Acknowledgement
The authors thank SAI labs, Thapar institute of engineering and technology for XRD, SEM-EDS analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors do not have any conflict of interest in the publication of the manuscript.
Supporting Information
11814_2021_784_MOESM1_ESM.pdf
Impact of g-C3N4 loading on NiCo LDH for adsorptive removal of anionic and cationic organic pollutants from aqueous solution
Rights and permissions
About this article
Cite this article
Kaur, H., Singh, S. & Pal, B. Impact of g-C3N4 loading on NiCo LDH for adsorptive removal of anionic and cationic organic pollutants from aqueous solution. Korean J. Chem. Eng. 38, 1248–1259 (2021). https://doi.org/10.1007/s11814-021-0784-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11814-021-0784-6