Abstract
Purpose
In the present perspective, emergence of resistant strains of Leishmania donovani and severe side effects resulting from the use of conventional anti-leishmanial therapies present an urgent need for developing novel agents against this parasite. We have explored the effectiveness of secondary plant metabolites as alternative choices in the treatment for visceral leishmaniasis (vl).
Methods
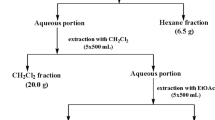
The plant Parthenium hysterophorus L. (Asteraceae) was collected from the West Bengal State University Campus, Barasat, West Bengal, India. The leaves of this plant were extracted by different solvents, such as ethyl acetate, water, petroleum ether and hexane. Gas chromatography–mass spectrometry (GC–MS) analysis was also carried out for the identification of compounds in the hexane soluble fraction (PHFd) with substantial anti-leishmanial activities. The antipromastigote activity and cytotoxicity of this fraction were evaluated by the tetrazolium MTT assay. Other biochemical and physiological parameters were studied by microscopic observation and flow cytometric analyses.
Results
PHFd showed considerable activity against L. donovani promastigotes (IC50: 20 µg/ml). The PHFd also inhibited in vitro growth of L. major LV39 promastigotes dose dependently with an IC50 of 40 µg/ml. The GC–MS studies of this particular fraction revealed the presence of four major compounds with different retention times (RT) of 26.08, 33.11, 36.41, and 41.20 min. In this study, we also established that PHFd could induce DNA damage and subsequent apoptosis of L. donovani promastigotes with a concomitant increase in generations of reactive oxygen species (ROS) in a time-dependent manner. This fraction was also found to be effective in nitric oxide-mediated inhibition of intracellular amastigotes (IC50:12.5 µg/ml) without any noticeable cytotoxicity towards murine splenocytes in vitro.
Conclusion
This study provides the basis for additional phytochemical and pharmacological studies on the antiprotozoal applications of P. hysterophorus.








Similar content being viewed by others
References
World Health Organization, fact-sheets on leishmaniasis (Updated: 02.03.2020)
Mishra BB, Singh RK, Srivastava A, Tripathi VJ, Tiwari VK (2009) Fighting against leishmaniasis: search of alkaloids as future true potential anti-leishmanial agents. Min Rev Med Chem 9(1):107–123. https://doi.org/10.2174/138955709787001758
Hazra S, Ghosh S, Das SM, Sharma S, Das M, Saudagar P, Prajapati VK, Dubey VK, Sundar S, Hazra B (2013) Evaluation of a diospyrin derivative as antileishmanial agent and potential modulator of ornithine dearboxylase of L.donovani. Exp Parasitol 135:407–413. https://doi.org/10.1016/j.exppara.2013.07.021
Pandey BD, Pandey K, Kaneko O, Yanagi T, Hirayama K (2009) Relapse of visceral leishmaniasis after miltefosine treatment in a nepalese patient. Am J Trop Med Hyg 80(4):580–582. https://doi.org/10.4269/ajtmh.2009.80.580
Sundar S, Pandey K, Thakur CP, Jha TK, Das VN, Verma N, Lal CS, Verma D, Alam S, Das P (2014) Efficacy and safety of amphotericin B emulsion versus liposomal formulation in indian patients with visceral leishmaniasis: a randomized, open-label study. PLoS Negl Trop Dis 8(9):e3169. https://doi.org/10.1371/journal.pntd.0003169
Sundar S, Singh A, Rai M, Prajapati VK, Singh AK, Ostyn B, Boelaert M, Dujardin JC, Chakraborty J (2012) Efficacy of miltefosine in the treatment of visceral leishmaniasis in India after a decade of use. Clin Infect Dis 55(4):543–550. https://doi.org/10.1093/cid/cis474
Seifert K, Perez-Victoria FJ, Stettler M, Sanchez-Canete MP, Castanys S, Gamarro F, Simon LC (2007) Inactivation of the miltefosine transporter LdMT, causes miltefosine resistance that is conferred to the amastigote stage of L. donovani and persists in vivo. Int J Antimicrob Agents 30(3):229–235. https://doi.org/10.1016/j.ijantimicag.2007.05.007
Chun CT, Hongjie Z (2019) Plant natural products for human health. Int J Mol Sci 20(4):830. https://doi.org/10.3390/ijms20040830
Ramji N, Ramji N, Iyer R, Chandrasekaran S (2002) Phenolic antibacterials from Piper betle in the prevention of halitosis. J Ethnopharmacol 83(1):149–152. https://doi.org/10.1016/s0378-8741(02)00194-0
Kumar S, Pandey S, Pandey AK (2014) In vitro antibacterial, antioxidant, and cytotoxic activities of Parthenium hysterophorus and characterization of extracts by LC-MS analysis. Biomed Res Int 495154:1–10. https://doi.org/10.1155/2014/495154
Dipankar CR, Munan S (2013) Toxicology, phytochemistry, bioactive compounds and pharmacology of Parthenium hysterophorus. J Med Plants Stud 1(3):126–141
Talemos S, Abreham A, Fisseha M, Balcha A (2013) Distribution status and the impact of parthenium weed (Parthenium hysterophorus L.) at Gedeo Zone (Southern Ethiopia). Afr J Agric Res 8(4):386–397. https://doi.org/10.5897/AJAR12.1646
Kalsi PS, Mittal V, Singh IP, Chhabra BR (1995) Pseudoguaianolides from Parthenium hysterophorus. Fitoterapia 66(2):191
Baliga MS, Jagetia GC, Ulloor JN, Baliga MP, Venkatesh P, Reddy R, Rao KVNM, Baliga BS, Devi S, Raju SK, Veeresh V, Reddy TK, Bairy KL (2004) The evaluation of the acute toxicity and long-term safety of hydroalcoholic extract of Sapthatparna (Alstonia scholaris) in mice and rats. Toxicol Lett 151:317–326. https://doi.org/10.1016/j.toxlet.2004.01.015
Fazal H, Ahmad N, Ullah I, Inayat H, Khan L, Abbas BH (2011) Antibacterial Potential in Parthenium hysterophorus, Stevia rebaudiana and Ginkgo biloba. Pak J Bot 43(2):1307–1313
Malarkodi E, Manoharan A (2013) Antifungal activity of Parthenium hysterophorus L. J Chem Pharm Res 5(1):137–139
Mallick S, Dey S, Mandal S, Dutta A, Mukherjee D, Biswas G, Chatterjee S, Mallick S, Lai TK, Acharya K, Pal C (2015) A novel triterpene from Astraeus hygrometricus induces reactive oxygen species leading to death in L.donovani. Future Microbiol 10(5):763–789. https://doi.org/10.2217/fmb.14.149
Shimasaki H (1994) Assay of fluorescent lipid peroxidation products. Meth Enzymol 233:338–346. https://doi.org/10.1016/s0076-6879(94)33039-5
Mukherjee D, Singh CB, Dey S, Mandal S, Ghosh J, Mallick S, Hussain A, Swapana N, Ross SA, Pal C (2015) Induction of apoptosis by zerumbone isolated from Zingiber zerumbet (L.) Smith in protozoan parasite L.donovani due to oxidative stress. Braj J Infect Dis 20(1):48–55. https://doi.org/10.1016/j.bjid.2015.10.002
Mallick S, Dutta A, Dey S, Ghosh J, Mukherjee D, Sultana S, Mandal S, Paloi S, Khatua S, Acharya K, Pal C (2014) Selective inhibition of L.donovani by active extracts of wild mushrooms used by the tribal population of India: an in vitro exploration for new leads against parasitic protozoans. Exp Parasitol 138:9–17. https://doi.org/10.1016/j.exppara.2014.01.002
Kamau SW, Hurtado M, Müller UU, Grimm F, Nunez R (2000) Flow cytometric assessment of allopurinol susceptibility in Leishmania infantum promastigote. Cytometry 40:353–360. https://doi.org/10.1002/1097-0320(20000801)40:4<353::aid-cyto11>3.0.co;2-0
De Macedo-Silva ST, Julio A, de Souza W, Fernandes C (2013) In vitro activity of the antifungal azoles itraconazole and posaconazole against Leishmania amazonensis. PLoS ONE 8(12):e83247. https://doi.org/10.1371/journal.pone.0083247
Sheela D, Uthayakumari F (2013) Gc-ms analysis of bioactive constituents from coastal sand dune taxon—Sesuvium portulacastrum (L.). Bio Disc 4(1):47–53
Sen N, Das BB, Ganguly A, Mukherjee T, Tripathi G, Bandyopadhyay S, Rakhit S, Sen T, Majumder HK (2004) Camptothecin induced mitochondrial dysfunction leading to programmed cell death in unicellular hemoflagellate L.donovani. Cell Death Differ 11(8):924–936. https://doi.org/10.1038/sj.cdd.4401435
Liew FY, Li Y, Millott S (1990) Tumor necrosis factor-alpha synergizes with IFN-gamma in mediating killing of Leishmania major through the induction of nitric oxide. J Immunol 145(12):4306–4310
Acknowledgements
We acknowledge the Vice Chancellor, West Bengal State University for providing the research infrastructure for this work. We also thank the DST-FIST, Govt. of India [Ref: SR/FST/LS1-001/2014] and DBT-BOOST, Govt. of West Bengal [Ref: 49 (11)/BT (Estt)/1P-4/2013 (Part-1)] for providing funds for the Flowcytometry facility in the Department of Zoology, WBSU, Barasat. We also appreciate the Director, CU BD Centre of Excellence for Nano biotechnology and DBT-IPLS, University of Calcutta, for use of the SEM, and confocal microscopy facilities. The flow cytometric data were analysed by Flowing Software version 2.5 (Perttu Terho, Centre for Biotechnology, Turku, Finland: www.flowingsoftware.com).
Author information
Authors and Affiliations
Contributions
JG, SC, SD and DM performed the experiments, analysed the data, and wrote the draft manuscript. SM, AD, TD, and BS implemented some additional experiments. NG and SB. helped in designing experiments. CP designed the experiments, analysed the data, and wrote the final manuscript. The manuscript has been read and approved by all the authors.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ghosh, J., Chakraborty, S., Dey, S. et al. Potential Anti-leishmanial Activity of a Semi-purified Fraction Isolated from the Leaves of Parthenium hysterophorus. Acta Parasit. 66, 1480–1489 (2021). https://doi.org/10.1007/s11686-021-00416-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11686-021-00416-1