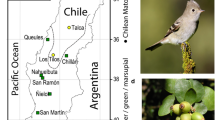
Abstract
Fruiting phenology is a critical aspect of plant fitness, as it is directly linked to the next-generation offspring delivery. Both abiotic and biotic factors presumably exert natural selection on plant phenology. Despite the role of climate in shaping fruiting phenology is well established, whether frugivores exert phenotypic selection on fruiting phenology has not yet been tested. We estimated the regime and magnitude of frugivore-mediated selection on fruiting phenology in three distant (> 500 km) populations of the Blue Passionflower (Passiflora caerulea) along one year. We measured phenological fruit traits (fruiting onset, fruiting peak, length of the fruiting season) and fruit crop size, and used animal fruit removal as a fitness component. We found highly variable fruiting phenologies between populations, yet phenological stages in lower latitudes were longer than in higher latitudes. One population showed a positive relationship between fruiting onset and fruiting peak among individuals, indicating that fruiting later in the season delayed the fruiting peak. Frugivores favored large fruit crop sizes in the three populations and early fruiting onsets in two populations. In two populations, frugivores selected favorable combinations of fruit crop size and fruiting peak (favoring plants with large crops and early fruiting peaks), as well as favorable combinations of fruiting peak and the length of the fruiting season (favoring plants with early fruiting peaks and extended fruiting seasons). Some degree of similarity in selection patterns among populations suggests that, despite strong geographic variation in climate and animal assemblage composition, some level of functional redundancy occurs in terms of phenotypic trait selection. Overall, our results show that fruiting phenology may be a highly variable life-history trait of plant populations, and support the idea that biotic interactors, conditional on heritable traits and selection pressures sustained over time, could potentially shape phenological fruiting characteristics.






Similar content being viewed by others
References
Aizen MA, Vázquez DP (2006) Flowering phenologies of hummingbird plants from the temperate forest of southern South America: is there evidence of competitive displacement? Ecography 29(3):357–366
Albrecht J, Bohle V, Berens DG, Jaroszewicz B, Selva N, Farwig N (2015) Variation in neighbourhood context shapes frugivore-mediated facilitation and competition among co-dispersed plant species. J Ecol 103(2):526–536
Alcántara JM, Rey PJ, Valera F, Sánchez-Lafuente AM, Gutiérrez JE (1997) Habitat alteration and plant intra-specific competition for seed dispersers. An example with Olea europaea var. sylvestris. Oikos 79(2):291–300
Arnold SJ (1986) Limits on stabilizing, disruptive, and correlational selection set by the opportunity for selection. Am Nat 128(1):143–146
Austen EJ, Weis AE (2015) What drives selection on flowering time? An experimental manipulation of the inherent correlation between genotype and environment. Evolution 69(8):2018–2033
Bandeira CT, Fortes SKG, Toebe M, Saifert L, Giacobbo CL, Welter LJ (2016) Sample size for estimate the average of Passiflora caerulea fruits traits. Ciência Rural 46(10):1729–1736
Beavon MA, Kelly D (2015) Dispersal of banana passionfruit (Passiflora tripartita var. mollissima) by exotic mammals in New Zealand facilitates plant invasiveness. N Z J Ecol 39(1):43–49
Benkman CW (2013) Biotic interaction strength and the intensity of selection. Ecol Lett 16(8):1054–1060
Blendinger PG (2017) Functional equivalence in seed dispersal effectiveness of Podocarpus parlatorei in Andean fruit-eating bird assemblages. Front Ecol Evol 5:57
Burns KC (2002) Seed dispersal facilitation and geographic consistency in bird–fruit abundance patterns. Global Ecol Biogeogr 11(3):253–259
Burns KC (2005) Is there limiting similarity in the phenology of fleshy fruits? J Veg Sci 16(6):617–624
Cardozo G, Beltzer A, Collins P (2008) Variación primavero-estival de la diversidad y abundancia de la comunidad de aves en la Reserva Ecológica de la Ciudad Universitaria UNL “El Pozo.” Miscelánea 17(2):367–386
Chapurlat E, Ågren J, Sletvold N (2015) Spatial variation in pollinator-mediated selection on phenology, floral display and spur length in the orchid Gymnadenia conopsea. New Phytol 208(4):1264–1275
Cleland EE, Chuine I, Menzel A, Mooney HA, Schwartz MD (2007) Shifting plant phenology in response to global change. Trends Ecol Evol 22(7):357–365
Conner JK, Hartl DL (2004) A primer of ecological genetics. Sinauer, Sunderland
Corlett RT (2013) Where are the subtropics? Biotropica 45(3):273–275
Crow JF (1958) Some possibilities for measuring selection intensities in man. Human Biol 61(5/6):763–775
de Souza VA, Byrne DH, Taylor JF (1998) Heritability, genetic and phenotypic correlations, and predicted selection response of quantitative traits in peach: II. An analysis of several fruit traits. J Am Soc Hortic Sci 123(4):604–611
Deginani NB (2001) Las especies argentinas del género Passiflora (Passifloraceae). Darwiniana 39(1/2):43–129
Delucchi G, Julianello AA, Correa RF (1993) Los espacios verdes y el arbolado urbano en el área de La Plata. Museo 1:72–82
Dossett M, Lee J, Finn CE (2008) Inheritance of phenological, vegetative, and fruit chemistry traits in black raspberry. J Am Soc Hortic Sci 133(3):408–417
Elzinga JA, Atlan A, Biere A, Gigord L, Weis AE, Bernasconi G (2007) Time after time: flowering phenology and biotic interactions. Trends Ecol Evol 22(8):432–439
Eriksson O, Ehrlén J (1991) Phenological variation in fruit characteristics in vertebrate-dispersed plants. Oecologia 86(4):463–470
Eynard C, Calviño A, Ashworth L (2017) Cultivo de plantas nativas. Universidad Nacional de Córdoba, Córdoba
Fenner M (1998) The phenology of growth and reproduction in plants. Perspectives Plant Ecol 1(1):78–91
Forget PM, Wenny D (2005) How to elucidate seed fate? A review of methods used to study seed removal and secondary seed dispersal. In: Forget PM, Lambert JE, Hulme PE, Vander Wall SB (eds) Seed fate predation, dispersal and seedling establishment. CAB International Publishing, Wallingford, pp 379–393
Forrest J, Miller-Rushing AJ (2010) Toward a synthetic understanding of the role of phenology in ecology and evolution. Philos T R Soc B 365:3101–3112
García MA, Hoc PS (1997) Floral biology and reproductive system of Passiflora caerulea (Passifloraceae). Beitr Biol Pflanzen 70(1):1–20
García ME, Della Ceca LS, Micheletti MI, Piacentini RDN, Ordano MA, Reyes NJ, Buedo S, González JA (2018) Satellite and ground atmospheric particulate matter detection over Tucumán city, Argentina, space-time distribution, climatic and seasonal variability. AIMS Environ Sci 5(3):173–194
García M, Benítez-Vieyra S, Sérsic AN, Pauw A, Cocucci AA, Traveset A, Sazatornil F, Paiaro V (2020) Is variation in flower shape and length among native and non-native populations of Nicotiana glauca a product of pollinator-mediated selection? Evol Ecol 34(6):893–913
Gómez JM (1993) Phenotypic selection on flowering synchrony in a high mountain plant, Hormathophylla spinosa (Cruciferae). J Ecol 81(4):605–613
Gómez JM (2004) Bigger is not always better: conflicting selective pressures on seed size in Quercus ilex. Evolution 58(1):71–80
González-Varo JP, Arroyo JM, Jordano P (2019) The timing of frugivore-mediated seed dispersal effectiveness. Mol Ecol 28(2):219–231
Gordo O, Sanz JJ (2010) Impact of climate change on plant phenology in Mediterranean ecosystems. Glob Change Biol 16(3):1082–1106
Grau A, Kortsarz AM (2012) Guía de Arbolado de Tucumán. Universidad Nacional de Tucumán, Tucumán
Gremillion KJ (1989) The development of a mutualistic relationship between humans and maypops (Passiflora incarnata L.) in the southeastern United States. Journal of Ethnobiology 9(2):135–155
Guimarães PR Jr, Galetti M, Jordano P (2008) Seed dispersal anachronisms: rethinking the fruits extinct megafauna ate. PLoS ONE 3(3):e1745
Hall MC, Willis JH (2006) Divergent selection on flowering time contributes to local adaptation in Mimulus guttatus populations. Evolution 60(12):2466–2477
Harrison RD, Rønsted N, Xu L, Rasplus JY, Cruaud A (2012) Evolution of fruit traits in Ficus subgenus Sycomorus (Moraceae): to what extent do frugivores determine seed dispersal mode? PLoS ONE 7(6):e38432
Herrera CM (1981) Fruit variation and competition for dispersers in natural populations of Smilax aspera. Oikos 36(1):51–58
Herrera CM (1985) Determinants of plant-animal coevolution: the case of mutualistic dispersal of seeds by vertebrates. Oikos 44(1):132–141
Herrera CM (1998) Long-term dynamics of Mediterranean frugivorous birds and fleshy fruits: a 12-year study. Ecol Monogr 68(4):511–538
Howe HF, Estabrook GF (1977) On intraspecific competition for avian dispersers in tropical trees. Am Nat 111(981):817–832
Izhaki I (2002) The role of fruit traits in determining fruit removal in East Mediterranean ecosystems. In: Levey DJ, Silva WR, Galetti M (eds) Seed dispersal and frugivory: ecology, evolution, and conservation. CAB International Publishing, Wallingford, pp 161–175
Jammalamadaka SR, Sarma YR (1988) A correlation coefficient for angular variables. In: Matusita K (ed) Statistical theory and data analysis II. North Holland Publishers, Amsterdam, pp 349–364
Janson CH, Stiles EW, White DW (1986) Selection on plant fruiting traits by brown capuchin monkeys: a multivariate approach. In: Estrada A, Fleming TH (eds) Frugivores and seed dispersal. Springer, Dordrecht, pp 83–92
Jordano P (1994) Spatial and temporal variation in the avian-frugivore assemblage of Prunus mahaleb: patterns and consequences. Oikos 71:479–491
Jordano P (1995) Frugivore-mediated selection on fruit and seed size: birds and St Lucie’s cherry Prunus mahaleb. Ecology 76(8):2627–2639
Jordano P, Herrera CM (1995) Shuffling the offspring: uncoupling and spatial discordance of multiple stages in vertebrate seed dispersal. Ecoscience 2(3):230–237
Killip EP (1938) The American species of Passifloraceae. Field Mus Nat Hist Bot Ser 19:1–613
Lacey EP, Roach DA, Herr D, Kincaid S, Perrott R (2003) Multigenerational effects of flowering and fruiting phenology in Plantago lanceolata. Ecology 84(9):2462–2475
Lande R, Arnold SJ (1983) The measurement of selection on correlated characters. Evolution 37(6):1210–1226
Landler L, Ruxton GD, Malkemper EP (2019) The Hermans-Rasson test as a powerful alternative to the Rayleigh test for circular statistics in biology. BMC Ecol 19(1):1–8
Levey DJ (1987) Seed size and fruit-handling techniques of avian frugivores. Am Nat 129(4):471–485
Mardia KV (1976) Linear-circular correlation coefficients and rhythmometry. Biometrika 63(2):403–405
Mardia KV, Jupp PE (2009) Directional statistics, vol 494. Wiley, Chichester
Martínez I, García D, Obeso JR (2007) Allometric allocation in fruit and seed packaging conditions the conflict among selective pressures on seed size. Evol Ecol 21(4):517–533
Mello MAR, Leiner NO, Guimarães PR, Jordano P (2005) Size-based fruit selection of Calophyllum brasiliense (Clusiaceae) by bats of the genus Artibeus (Phyllostomidae) in a Restinga area, southeastern Brazil. Acta Chiropt 7(1):179–182
Mendoza I (2020) Estadística circular aplicada a la Ecología. Ecosistemas 29:1995
Minetti JL (2005) El clima del noroeste argentino. Laboratorio Climatológico Sudamericano. Fundación Carl C. zon Caldenius, Tucumán
Moorad JA, Wade MJ (2013) Selection gradients, the opportunity for selection, and the coefficient of determination. Am Nat 181(3):291–300
Morellato LPC, Alberti LF, Hudson IL (2010) Applications of circular statistics in plant phenology: a case studies approach. In: Hudson IL, Keatley M (eds) Phenological research: methods for environmental and climate change analysis. Springer, Dordrecht, pp 357–371
Morrissey MB, Sakrejda K (2013) Unification of regression-based methods for the analysis of natural selection. Evolution 67(7):2094–2100
Morrissey M, Sakrejda K (2014) gsg: calculation of selection coefficients. R package version 2.0. Available from: https://CRAN.R-project.org/package=gsg. Accessed Dec 2020
Muluken D, Wassu M, Endale G (2016) Variability, heritability and genetic advance in Ethiopian okra [Abelmoschus esculentus (L.) Monech] collections for tender fruit yield and other agro-morphological traits. J Appl Life Sci Int 4:1–12
Munguía-Rosas MA, Ollerton J, Parra-Tabla V, De Nova JA (2011) Meta-analysis of phenotypic selection on flowering phenology suggests that early flowering plants are favoured. Ecol Lett 14(5):511–521
Naoe S, Masaki T, Sakai S (2018) Effects of temporal variation in community-level fruit abundance on seed dispersal by birds across woody species. Am J Bot 105(11):1792–1801
Nikolić D (2006) Components of variability and heritability of phenological phases in interspecies progenies of F1 generation in grapevine. Genetika 38(1):49–58
Nychka D, Furrer R, Paige J, Sain S (2017) fields: tools for spatial data. R package version 9.6. Available from http://www.image.ucar.edu/~nychka/Fields. Accessed Dec 2020
Ordano M, Blendinger PG, Lomáscolo SB, Chacoff NP, Sánchez MS, Núñez Montellano MG, Jiménez J, Ruggera AR, Valoy M (2017) The role of trait combination in the conspicuousness of fruit display among bird-dispersed plants. Funct Ecol 31(9):1718–1727
Ortiz-Pulido R, Rico-Gray V (2000) The effect of spatio-temporal variation in understanding the fruit crop size hypothesis. Oikos 91(3):523–527
Palacio FX (2019) Hummingbirds (Trochilidae) as frugivores: a review and the first records from Argentina. Ornitología Neotropical 30:99–102
Palacio FX, Ordano M (2018) The strength and drivers of bird-mediated selection on fruit crop size: a meta-analysis. Front Ecol Evol 6:18
Palacio FX, Girini JM, Ordano M (2017a) Linking the hierarchical decision-making process of fruit choice and the phenotypic selection strength on fruit traits by birds. J Plant Ecol 10(4):713–720
Palacio FX, Valoy M, Bernacki F, Sánchez MS, Núñez-Montellano MG, Varela O, Ordano M (2017b) Bird fruit consumption results from the interaction between fruit-handling behaviour and fruit crop size. Ethol Ecol Evol 29(1):24–37
Palacio FX, Benitez-Vieyra S, Ordano M (2019) Measuring natural selection on multivariate phenotypic traits: a protocol for verifiable and reproducible analyses of natural selection. Isr J Ecol Evol. https://doi.org/10.1163/22244662-20191064
Palacio FX, Siepielski AM, Lacoretz MV, Ordano M (2020) Selection on fruit traits is mediated by the interplay between frugivorous birds, fruit flies, parasitoid wasps, and seed-dispersing ants. J Evol Biol 33(7):874–886
Parra-Tabla V, Vargas CF (2004) Phenology and phenotypic natural selection on the flowering time of a deceit-pollinated tropical orchid Myrmecophila Christinae. Ann Bot-London 94(2):243–250
Pires MM, Galetti M, Donatti CI, Pizo MA, Dirzo R, Guimarães PR (2014) Reconstructing past ecological networks: the reconfiguration of seed-dispersal interactions after megafaunal extinction. Oecologia 175(4):1247–1256
Primack RB, Kang H (1989) Measuring fitness and natural selection in wild plant populations. Annu Rev Ecol Syst 20(1):367–396
R Core Team (2020) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available from: https://www.R-project.org/
Rao GR, Korwar GR, Shanker AK, Ramakrishna YS (2008) Genetic associations, variability and diversity in seed characters, growth, reproductive phenology and yield in Jatropha curcas (L.) accessions. Trees 22(5):697–709
Rathcke B, Lacey EP (1985) Phenological patterns of terrestrial plants. Annu Rev Ecol Syst 16(1):179–214
Rumeu B, Álvarez-Villanueva M, Arroyo JM, González-Varo JP (2019) Interspecific competition for frugivores: population-level seed dispersal in contrasting fruiting communities. Oecologia 190(3):605–617
Russo SE (2003) Responses of dispersal agents to tree and fruit traits in Virola calophylla (Myristicaceae): implications for selection. Oecologia 136:80–87
Saastamoinen M, Bocedi G, Cote J, Legrand D, Guillaume F, Wheat CW, Fronhofer EA, Garcia C, Henry R, Husby A, Baguette M, Bonte D, Coulon A, Kokko H, Matthysen E, Niitepõld K, Nonaka E, Stevens VM, Travis JMJ, Donohue K, Bullock JM, Delgado M (2018) Genetics of dispersal. Biol Rev 93(1):574–599
Sandring S, Ågren J (2009) Pollinator-mediated selection on floral display and flowering time in the perennial herb Arabidopsis lyrata. Evolution 63(5):1292–1300
Schluter D (1988) Estimating the form of natural selection on a quantitative trait. Evolution 42(5):849–861
Schupp EW, Jordano P, Gómez JM (2017) A general framework for effectiveness concepts in mutualisms. Ecol Lett 20(5):577–590
Schupp EW, Zwolak R, Jones LR et al (2019) Intrinsic and extrinsic drivers of intraspecific variation in seed dispersal are diverse and pervasive. AoB Plants 11(6):plz067
Siepielski AM, Benkman CW (2007) Extreme environmental variation sharpens selection that drives the evolution of a mutualism. Proc R Soc B 274(1620):1799–1805
Snell RS, Beckman NG, Fricke E, Loiselle BA, Carvalho CS, Jones LR, Lichti NI, Lustenhouwer N, Schreiber SJ, Strickland C, Sullivan LL, Cavazos BR, Giladi I, Hastings A, Holbrook KM, Jongejans E, Kogan O, Montaño-Centellas F, Rudolph J, Rogers HS, Zwolak R, Schupp EW (2019) Consequences of intraspecific variation in seed dispersal for plant demography, communities, evolution and global change. AoB Plants 11(4):plz016
Snow DW (1965) A possible selective factor in the evolution of fruiting seasons in tropical forest. Oikos 274–281
Snow DW (1971) Evolutionary aspects of fruit-eating by birds. Ibis 113(2):194–202
Sobral M, Larrinaga AR, Guitián J (2010) Do seed-dispersing birds exert selection on optimal plant trait combinations? Correlated phenotypic selection on the fruit and seed size of hawthorn (Crataegus monogyna). Evol Ecol 24(6):1277–1290
Sobral M, Guitián J, Guitián P, Larrinaga AR (2013) Selective pressure along a latitudinal gradient affects subindividual variation in plants. PLoS ONE 8(9):e74356
Tancred SJ, Zeppa AG, Cooper M, Stringer JK (1995) Heritability and patterns of inheritance of the ripening date of apples. HortSci 30(2):325–328
Thompson JN (2005) The geographic mosaic of coevolution. University of Chicago Press, Chicago
Thompson JN, Willson MF (1979) Evolution of temperate fruit/bird interactions: phenological strategies. Evolution 33(3):973–982
Tigano A, Friesen VL (2016) Genomics of local adaptation with gene flow. Molec Ecol 25(10):2144–2164
Ting S, Hartley S, Burns KC (2008) Global patterns in fruiting seasons. Global Ecol Biogeogr 17(5):648–657
Torres C, Dambolena JS, Zunino MP, Galetto L (2012) Nectar characteristics and pollinators for three native co-occurring insect pollinated Passiflora (Passifloraceae) from central Argentina. Int J Plant Reprod Biol 4(2):121–126
Trunschke J, Sletvold N, Ågren J (2017) Interaction intensity and pollinator-mediated selection. New Phytol 214(3):1381–1389
Tsagris M, Athineou G, Sajib A, Amson E, Waldstein MJ (2020) Directional: directional statistics. R package version 4.1. Available from: https://CRAN.R-project.org/package=Directional. Accessed Dec 2020
van Schaik CP, Terborgh JW, Wright SJ (1993) The phenology of tropical forests: adaptive significance and consequences for primary consumers. Annu Rev Ecol Syst 24(1):353–377
Varela GM, Cocucci AA, Sersic AN (2016) Función de la corona en Passiflora caerulea (Passifloraceae) como atrayente de sus vectores de polinización. Bol Soc Argent Bot 51(1):99–110
Wang BC, Smith TB (2002) Closing the seed dispersal loop. Trends Ecol Evol 17(8):379–386
Weis AE, Nardone E, Fox GA (2014) The strength of assortative mating for flowering date and its basis in individual variation in flowering schedule. J Evol Biol 27(10):2138–2151
Wheelwright NT (1985) Competition for dispersers, and the timing of flowering and fruiting in a guild of tropical trees. Oikos 44(3):465–477
Wheelwright NT (1993) Fruit size in a tropical tree species: variation, preference by birds, and heritability. Vegetatio 107(1):163–174
Williams PA, Karl BJ, Bannister P, Lee WG (2000) Small mammals as potential seed dispersers in New Zealand. Austral Ecol 25(5):523–532
Wolkovich EM, Cook BI, Allen JM et al (2012) Warming experiments underpredict plant phenological responses to climate change. Nature 485(7399):494–497
Wood SN (2017) Generalized additive models: an introduction with R. Chapman and Hall/CRC, Boca Raton
Yamada M, Yamane H, Ukai Y (1995) Genetic analysis of fruit ripening time in Japanese persimmon. J Am Soc Hortic Sci 120(6):886–890
Acknowledgements
We thank Rafael Jiménez and Rodrigo Moreno Ten for providing field assistance. Bárbara Malagisi, Carmela Marín, Cecilia Morgan, Elián Guerrero, Gabriela F. Ruellán, Gustavo Herrera, Laura Haag, Paloma Borghello, and Renato A. García provided information on plant locations. Adam M. Siepielski provided insightful comments on the idea of the manuscript. This study was funded by Fondo para la Investigación Científica y Tecnológica (Project PICT-2017-0081 given to F.X.P). Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) and Fundación Miguel Lillo (Project Z-0048-1 given to M.O.) also provided partial funding. Two anonymous reviewers improved early versions of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
About this article
Cite this article
Palacio, F.X., Cataudela, J.F., Montalti, D. et al. Do frugivores exert selection on fruiting phenology? Potential scenarios across three plant populations of a Neotropical vine, Passiflora caerulea. Evol Ecol 35, 555–574 (2021). https://doi.org/10.1007/s10682-021-10121-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10682-021-10121-0