Abstract
Honey from cotton blossoms tends to crystallize rapidly resulting in a product with hard texture and low spreadability, even if it is processed via a controlled crystallization treatment that is often employed for production of creamed honey. The aim of this study was to develop a cotton honey-based spread (CHS) with acceptable physical and textural properties by adding fructose (1–6%) and water (up to 18% final moisture) into the initial cotton honey as well as controlling the temperature during crystallization, Tcryst (5–23 °C × 20 days) and a conditioning (tempering) step as a follow-up process, Tcond (20–30 °C × 10 days). Multi-instrumental analysis (rheometry, calorimetry, microscopy, colorimetry) and sensory (spreadability, mouthfeel) evaluation were used for characterization of textural properties of the CHS preparations. With increased fructose and water contents the CHS formulations exhibited significant (p < 0.05) lower Glucose/Water ratios (crystallinity index), steady shear and complex viscosities, hardness, spreadability work and melting enthalpy of sugar microcrystals, as well as improved organoleptic characteristics of this new spread product based on cotton honey. Moreover, with increasing Tcryst, only the lightness and the melting enthalpy decreased, while the damping factor increased. Instead, the Tcond did not affect any of the above parameters (p > 0.05). Strong correlations were identified between compositional, physicochemical attributes and sensory characteristics of the formulated product, implying that instrumental analyses of CHS along with sensory tests can be a valuable holistic approach for understanding the macrostructure, evaluating the physical properties and quality attributes, and predicting the consumer preference of the new spread product.
Similar content being viewed by others
Introduction
Honey is a natural, complex food produced by bees (Apis melifera), either from the nectars of flowers or honeydew, composed mainly of sugars (60–85%) [1], with fructose (32–44%) and glucose (23–38%) being the most predominant carbohydrate components [2,3,4,5]. Fructose is highly soluble in a honey matrix, remaining in solution for a long time and being responsible for the hygroscopicity of the product [6], whereas glucose, which is less soluble, is responsible for crystallization of some honeys [7]. Water is the second most important component of honey; the moisture content ranges between 13 and 25%, being optimal at about 18% [5]. According to European Council Directive 110/2001/EC [8], the water content of native honey products must not exceed 20%, with the exception of the heather honey which must not be higher than 23%. Water content as well as water activity affect both the quality and stability of honey during storage, since they have a strong impact on physicochemical, organoleptic and microbiological characteristics of honey [6, 9, 10].
Crystallization of honey, commonly called granulation, is an undesirable physical phenomenon, affecting handling, processing and consumer acceptance of the product. Mechanisms that control crystallization of carbohydrates have been studied in many food systems, mostly in those composed of concentrated sucrose solutions, such as confectionery products [11, 12]. Crystallization consists of two consecutive events, nucleation and crystal growth controlled both by thermodynamics and kinetics [11,12,13]; thermodynamics have a major impact on nucleation rate, whereas kinetics significantly influence the rate of crystal growth. More specifically, in primary homogeneous crystallization process, nucleation occurs when enough sugar molecules start to form clusters that reach a critical size in order to overcome the energy barrier and thereby lead to crystal lattice formation which minimizes their free energy [11, 12]. The Gibbs free energy change for the formation of a crystal nucleus with the critical size is governed by the interfacial energy between the nucleus and the liquid medium, with the level of supersaturation acting as driving force; crystals can form in supersaturated solutions when the concentration of sugars increases above the solubility concentration favoring the sugar-sugar molecular interactions [12, 13]. Additionally, the rate of nucleation is governed by the Gibbs free energy associated with the diffusion of sugar molecules across the interface of the nucleus-amorphous (liquid) matrix and thus is also governed by the viscosity of the medium. Following the nucleation stage, sugar molecules diffuse and affix at the surface of crystals resulting in crystal growth, while water molecules and other dissolved substances are being removed from the growing crystal surface. Since honey is a supersaturated solution, the excess of sugars (mainly glucose) tends to go out of solution, forming stable nuclei (nucleation) which is followed by crystal growth [14, 15]. The final size of the formed crystals depends on the composition and the botanical origin of honey.
Some varieties of honey do not crystallize readily, while others are more prone to this physical process. The factors that influence the rate of crystallization are many, such as glucose and water content, storage temperature and presence of ‘nucleation seeds’ acting as foreign nucleation sites, also called primers, such as pollen, wax, dust or primary glucose microcrystals (primary heterogeneous crystallization); in heterogenous nucleation which is the predominant form in foods, the foreign nuclei decrease the energy and level of supersaturation required for sugar nucleation [12]. Honey composition is also a determinant of the nucleation process, i.e., the higher the glucose and the lower the water content of honey, the faster the crystallization process [5, 6, 9, 10, 16] since nucleation rates are increased when higher supersaturation levels are reached due to elevation of glucose concentration [11, 12]. Furthermore, the nucleation rate and crystal growth, which are both diffusion-controlled processes, can be enhanced by increasing curing temperature due to decrease of medium viscosity [12, 13]. On the other hand, in confectionery products, in which the content of total solids is constant, a temperature increase can result in reduction of supersaturation level due to increase of sugar solubility. These two competing phenomena of decreasing supersaturation and increasing of molecular mobility leads to an optimum temperature at which sugar crystallization rate is maximum [17]. Therefore, the highest rate of honey crystallization has been observed by many authors at storage temperature of ~ 14 °C [1, 5, 7, 18, 19]. At higher temperatures, above 18 °C, honey crystallization slows down considerably, as the extent of glucose supersaturation decreases, while when the temperature exceeds 30 °C the glucose crystals begin to melt, and the honey regains its fluidity. On the other hand, crystallization does not occur rapidly at temperatures below 10 °C, because the viscosity of honey greatly increases, so the diffusion coefficient decreases, and crystal formation is slower [17, 20, 21].
Several indices have been proposed as crystallization rate predictors of honey [6, 7, 10, 16, 22,23,24]. These crystallization rate indices are the ratios of G/W, F/G and (G-W)/F, where G, F and W are glucose, fructose and water content in honey, respectively. The G/W ratio seems to be the most reliable indicator in this respect, i.e., when G/W is less than 1.7, very slow granulation occurs, whereas when this ratio is greater than 2.0 the phenomenon is fast [7, 16, 25]. Dyce [26] introduced a method of controlled, secondary, crystallization in honey by adding a finely granulated honey as a primary crystallization nuclei (starter) fraction into the liquid honey at 5–10% level that after storage at 14 °C results in a completely crystallized product having a large number of small crystals which are not ‘perceived’ as dispersed particles in the human palate. The product obtained by this technique is called creamed (or set) honey and characterized by a rather smooth and creamy texture, with mild flavor, lighter color and easily spreadable like butter at room temperature [22, 27,28,29].
Honey from cotton blossoms is a honey with light color, mild aroma and very sweet taste, accounting for about 20% of the annual harvest of honey in Greece. Alissandrakis et al. [30] have found that cotton honey obtained from different locations in Greece contains more than 35 phenolic compounds with high antioxidant capacity and other bioactivities. Some of these compounds, such as benzenepropanol, homovanillyl alcohol, (E)- and (Z)-p-methoxy-cinnamic acid, 2-methyl-p-phthalaldehyde, coniferaldehyde, p-coumaric acid, ferulic acid, scopoletin and scoparone have been proposed by these researchers to be used as biomarkers for cotton honey, since they are present only in cotton honey compared to other honey products from nine different floral origins; scopoletin and scoparone are simple coumarins well known for their significant pharmacological properties. However, cotton honey, because of its sugar composition (high glucose content), crystallizes fairly rapidly within 1–2 months after harvesting, acquiring a rather hard and very coarse texture. For this reason, despite its high bioactivity, this honey is usually used as bee feed, and its promotion on the market for human consumption becomes impossible unless it is mixed with other types of honey of different botanical origin, which are not as prone to granulation; however, the latter option leads to a honey product in which the cotton honey is highly diluted. On the other hand, preliminary experiments conducted before the present study in our laboratory, to enhance the marketability of cotton honey by controlled crystallization for producing a creamed honey product, showed that the textural attributes were still far different from the typical sensorial features of other creamed honey products, still exhibiting hard texture and low spreadability characteristics. Thus, the aim of the present study was to produce a finely crystallized new spread product based on cotton honey (CHS), with high stability upon storage and acceptability, exhibiting the typical macroscopic physical properties of creamed honey by controlling several compositional and process parameters. For this purpose, the effects of addition of fructose and water into cotton honey at relatively low levels, along with the adoption of certain temperature protocols (for both the crystallization and the conditioning stages) on the textural characteristics and macro-structure of the final product were studied. For the first time in the present investigation a multi-instrumental analysis (rheometry, calorimetry, microscopy, colorimetry) and a sensory (spreadability, mouthfeel) evaluation approach were employed to evaluate the physical properties of such formulated honey-based spread products.
Materials and Methods
Experimental Design
A response surface methodology (RSM) experimental design, as proposed by Draper and John [31], was employed to study the effects of 1 qualitative, water addition (WA), and 3 quantitative variables, fructose addition level (FA), crystallization temperature (Tcryst) and conditioning temperature (Tcond) on the physicochemical and sensory properties of CHS preparations (Table 1). The quantitative variables were examined at five experimental levels –α, −1, 0, + 1, + α, where α = 2n/4 and n was the number of variables; thus, fructose added at 0, 1, 3, 5, and 6% (w/w) level, crystallization temperature was at 5, 9, 14, 20 and 23 °C and conditioning temperature was set at 20, 22, 25, 28 and 30 °C. For the qualitative variable, the coded level ‘0’ referred to product formulations prepared without addition of extra water into the initial cotton honey sample, while the level ‘1’ was assigned to samples containing a supplementary amount of water (~ 2%), without however the total water content of the final product to exceed 18%.
Preparation of Samples
Cotton honey used in this study was gifted by a local beekeeper from Neoi Epivates (Thessaloniki, Greece). The preparation of CHS samples was carried out according to Dyce method for creamed honey production [26]. Cotton honey was firstly heated at 40 °C for 3 days until there were no detectable sugar crystals, as viewed by a cross polarized light microscope (OLYMPUS Bx51, Olympus Optical Co Ltd., Tokyo, Japan). Then, pure fructose in powder form (Fytro, JOTIS S.A., Athens) was added to some honey samples at 1–6% (w/w) level and the mixture was heated at 40 °C for 2 additional days for elimination of any undissolved fructose crystals; i.e., absence of sugar crystals was confirmed by the microscope. When these preparations were cooled down to room temperature, the additional water was incorporated to some samples and then, a commercial creamed honey (Georgakas, Arnaia, Chalkidiki, Greece) was introduced as a crystallization starter into all cotton honey-based preparations at 10% (w/w) level by mixing them with a mixer (KENWOOD, KM 282, United Kingdom) for 15 min at the lowest speed. Small portions of the dispersed samples (20–23 g) were then kept in glass jars (40 ml) with screw caps in incubators (Sanyo Incubator, MIR-154, SanyoElectric Co. Ltd, Ora-Gun, Gunma, Japan) at 5–23 °C for 20 days to induce crystallization, followed by a conditioning period for 10 days at 20–30 °C and finally, stored at 20 °C for 3.5 months (storage period); the commercial creamed honey was also examined as ‘control product’ as well. The above final storage temperature was chosen because 20 °C is the temperature that could be used at retail food stores for preservation of the CHS product.
Composition
Glucose, fructose and sucrose contents were determined by High Performance Liquid Chromatography (HPLC) (Agilent Technologies 1200, Tokyo, Japan) according to the harmonized method prescribed by the International Honey Commission [32]. Samples (1 g/ml) were dissolved in a methanol (Chem-Lab, Belgium)/water (25:75 v/v) mixture and filtered through a nylon filter 0.45 μm (BGB, USA) before the injection (10 μl). The sugars were eluted isocratically from a Zorbax Carbohydrate Analysis Column (4.6 mm ID × 150 mm × 5 μm) using as mobile phase an acetonitrile (Sigma-Aldrich, USA)/water (80:20 v/v) mixture at 30 °C with a flow rate of 1.3 ml/min and detected with a refractive index detector (RID).
Moisture content of CHS preparations was measured using a hand-held honey moisture refractometer (ATAGO, HHR-2 N, Japan) at 20 °C, elaborating a precision level of 0.1%. The moisture and sugar contents were determined at the end of the conditioning period (0 days of storage time) of the CHS samples; any sugar crystals of the samples were eliminated by heating at 40 °C before both tests.
Analyses of Physicochemical Parameters
All physicochemical characteristics of the CHS samples were examined at three different time intervals of the storage period (0, 45 and 105 days) in triplicates, except for the textural parameters which were evaluated in five replicates; sampling of replicates for each analysis was carried out using different containers (jars).
Water Activity
Water activity (aw) of CHS samples was measured at 20 °C using an Aqualab 3TE water activity meter (Decagon Devices, Inc., Pullman, Washington, USA).
Color
The color of samples was determined in the CIE 1976 L*, a*, b* scale using a Chroma Meter CR-410 (Konica Minolta Inc, USA). The L*, which ranges from 0 to 100, describes the lightness, the a* value expresses the intensity of red color in the positive range and the green in the negative range, whereas the b* value indicates the intensity of yellow color in the positive range and the blue in the negative range.
Degree of Crystallization
The degree of crystallization (Dcryst) was measured using the cross polarized light microscope (OLYMPUS Bx51) at 10 × magnification. The crystallization degree of CHS samples was calculated based on measurements in 10 randomly chosen areas of the viewed sections using a computer-aided program (Image-Pro Plus Version 5.0, USA) and expressed as percentage of total area of the field covered by sugar crystals.
Differential Scanning Calorimetry (DSC)
Thermal analysis was performed by differential scanning calorimetry (DSC) using a PL DSC-Gold calorimeter (Polymer Labs. Ltd, Epsom, UK). Samples of 20–30 mg of CHS preparations were hermetically sealed in aluminum pans and an empty pan was used as a reference. The onset (To) and peak (Tm) melting temperatures as well as the melting enthalpy (ΔH) of crystals of granulated CHSs were calculated from the thermal scans, within the temperature range of 10 to 100 °C, at a heating rate of 10 °C/min.
Rheology
The rheological properties of samples were examined by a rotational Physica MCR 300 rheometer (Physica Messtechnic CmbH, Stuttgart, Germany) using a plate-plate geometry with 50 mm diameter and 1 mm gap between the two plates. The temperature was regulated by a Paar Physica circulating bath and a controlled peltier system (TEZ 150P/MCR) with an accuracy of ± 0.1 °C. The data of the rheological measurements were analyzed with the supporting rheometer software US200 V2.21.
Two types of measurement were performed at 20 °C: (a) flow behavior by measuring steady shear viscosity (η) over a range of shear rates (\(\dot{\gamma }\)) of 0.01–50 s−1 and (b) oscillatory measurements to obtain the storage (G`) and loss (G``) moduli, the damping factor (tanδ = G``/ G`) and the complex viscosity (η*) at 0.1% strain level and a range of angular frequencies (ω) of 3–300 rad·s−1. For statistical analysis of the rheological data, values of η at 2 s−1 shear rate and G`, G``, η* and tanδ at 20 rad s−1 angular frequency were used.
Texture Analysis
Large deformation mechanical properties of CHS samples were examined by a spreadability test using a Texture Analyser (TA-XT2i, Stable Micro systems, Godalming, Surrey, UK) with the TTC Spreadability Rig (HDP/SR*). The test was performed at a crosshead speed of 3 mm/s and the parameters of hardness (Fmax) and work of spreadability (SW) were calculated from the peak force and the area of the force–displacement curve, respectively.
Sensory Evaluation
The 16 CHS samples were tested after 45 days of storage using Quantitative Descriptive Analysis (QDA) by a trained panel of 12 assessors who evaluated the degree of spreadability and mouthfeel perception, giving a score from 1 to 10 using selected standards, two for each parameter; a score of ‘1’expressing the highest spreadability (the easiest to spread) and the coarsest texture (large crystals detectable by palate) of the new spread products, while with ‘10’ the lowest spreadability (the most difficult to spread) and creamed-smooth texture (small crystals undetectable by palate), respectively for the two parameters. The assessors also scored the general acceptability of honey samples from ‘1’ (dislike extremely) to ‘10’ (like extremely) according to their own preference. The selected standards for the parameter of spreadability were food products purchased from the local market that largely differed in this attribute. More specifically, ketchup (scored 1) and cow’s milk butter (scored 10) were used as standards for the highest and lower spreadability, respectively; butter was preserved at refrigerator temperature during the whole session in a screw cap vial placed in a beaker with crushed ice in order to maintain its low spreadability. The standards for the mouthfeel assessment were a multifloral honey sample with large crystals perceptible by palate (scored 1, coarse texture) purchased from a local beekeeper and stored at room temperature for over six months to naturally crystallize, and a honey sample with small and not perceptible crystals identifying the creamy texture (scored 10); the latter standard was the commercial creamed honey that was also used as ‘control product’ for comparison purposes in the present study. In addition to the given standards, instructions about how to assess the mouthfeel (by rubbing the sample with the tongue on the palate for about 10 s) and spreadability (by spreading the sample with a plastic knife on a rusk, applying four consecutive movements of alternating direction) were also provided to the panelists. The sensory analysis of 16 samples which were marked with different three-digit blinding codes was performed in four different sessions in which four different CHS products were examined per session. The four formulated cotton honey spreads and the four standards were served concurrently to the panelists and asked to be examined in the same order; panelists were asked to rinse their mouth by water between the samples. Testing was performed in a sensory laboratory room, set up with individual booths for each panelist under fluorescent lighting (cool white, 6500 K color temperature) equivalent to daylight.
Before the actual sensory analysis of the CHS samples two sessions were performed that included a prescreening of 20 candidate panelists using a ranking test and a training session for QDA testing. During the first session, the candidate assessors were familiarized with the two examined sensory parameters (spreadability and mouthfeel). A consensus vocabulary was thus developed and a multi-samples sensory ranking test for each parameter was performed; i.e., the candidates had to classify four different (naturally and controlled) crystallized honey samples according to the perceived intensity of each attribute and the individuals that reversed more than one adjacent pair of samples were excluded from the sensory panel. During the second training session the selected 12 assessors were practiced in performing the actual QDA test; i.e., assessors were first familiarized with the grading scale (1 to 10), the selected standards and the test procedure, and then rated the mouthfeel and spreadability characteristics of two samples differing in these attributes.
Statistical Analysis
Further to the four independent variables used at the initial experimental design, a new independent variable, the storage time (S) at three levels (0, 45 and 105 days) was introduced to the regression model in order to study the impact of storage on properties of the CHS product as well (aging effects). Moreover, a second regression model was developed at which the two independent variables of fructose and water addition were replaced by a new variable, the crystallization rate index values (G/W) of the 16 samples, as calculated from the compositional analysis of the modified honey preparations (Table 1). For describing the effect of the five (S, WA, FA, Tcryst, Tcond) or four (S, G/W, Tcryst, Tcond) independent variables on the quality attributes of the CHS preparations, multiple regression analysis was performed using the statistical software Minitab (Release 15) to fit linear (first-order) mathematical models to the data of the response variables (physicochemical and sensory parameters of the CHSs). Analysis of variance (ANOVA) was performed (p-value < 0.05) to identify the significance of different effects on the response variables; moreover, the Pearson's correlation analysis was performed to identify any significant relationships between them.
Results and Discussion
Compositional and Color Parameters of the Spread Products
The cotton honey that was used as base raw material for the creaming study, before addition of the starter had 41.9% fructose, 37.3% glucose and 16.3% water (w/w); the calculated crystallization indices G/W, (G-W)/F and F/G for this honey were 2.29, 0.50 and 1.12, respectively. As expected, this sample tends to crystallize rapidly (in ~ 1 month), since it has high glucose concentration (> 35%), and exhibits high G/W ratio (> 2.1), high G-W/F ratio (> 0.4) and a fairly low F/G ratio (~ 1.1) [5, 6, 16, 24, 25].
The composition and crystallization indices of the 16 different CHS samples are summarized in Table 1. The fructose and glucose content varied within the range of 40.3–47.1% and 29.8–35.8%, respectively, due to the different levels of added fructose. For samples prepared without addition of water, the moisture content was 15.4–16.0%, whereas when small amounts of water were added before crystallization the final water content was raised to 17.2—17.8%. These compositional interventions resulted in G/W, (G-W)/F and F/G values in broad ranges of 1.69–2.27, 0.26–0.49 and 1.13–1.56, respectively. More specifically, the addition of fructose decreased considerably the G/W and (G-W)/F indices and increased the F/G ratio, compared to sample 9, which was not supplemented with fructose; these compositional changes would not favor the rapid formation and growth of sugar crystals. Similar behavior is expected for the samples formulated with addition of water. Various authors have also reported decreased crystallization rates for honeys having high fructose [6, 16, 22, 24] and water [16, 23, 25] contents.
The color of honey is one of the most important quality parameters for consumer acceptability and is related to the content of phenols, minerals, pollen and hydroxymethylfurfural (HMF) [33]. Apart from these constituents, however, the color of honey becomes lighter upon crystallization; the lightness of the crystallized honey is influenced by the size and shape of the formed crystals due to light reflection [7, 34]. The L* of CHS samples after 45 days of storage varied between 37.6–57.9, whereas the respective L* value for the control sample (commercial creamed honey) was 58.3 (Table 2). Regression analysis showed that both fructose and water addition, as well as crystallization temperature effected significantly (p < 0.01) the parameter L* (Table 3). With increase of fructose and water level there was a decrease in the L*, indicative of darker products (Fig. 1a), most likely due to decreased crystallization (i.e., the light is not reflected by large numbers of crystals and the final products have a darker color) [22]. Furthermore, the highest L* values were obtained at the temperature range of 9–14 °C (Fig. 1a); i.e., close to the temperature where the highest crystallization rate is occurred. At higher temperatures crystallization is not favored, leading to decreased L* values. The parameter L* also decreased (p < 0.05, Table 3) after long storage (105 days) at 20 °C (Fig. 1a); moreover, regression analysis revealed that upon storage the a* increased, implying augmentation of the redness, possibly due to formation of Maillard reaction products which are yellow–brown pigmented polymers.
The aw of liquid honey is lower than 0.6, thus inhibiting microbial growth and preventing fermentation by osmophilic yeasts that can lead to sour taste development [6, 35]. In the current work, the aw values of CHS varied between 0.534 and 0.604 at the end of storage, whereas the aw of the commercial creamed honey was 0.580. In accordance with previous reports [6, 35], significant (p < 0.001) positive (r > 0.87) correlations were found between the moisture content of the CHS samples and their aw values at the three storage time intervals examined. Moreover, the water activity of CHS preparations increased (Table 3) up to 45 days of storage, and then it seemed to reach a plateau value (Fig. 1b). Other researchers have also reported that aw increases during storage when crystallization of the product occurs, a process which leads to release of free water molecules and thus a higher aw [10, 24]. It is also noteworthy that aw was significantly affected (p < 0.001) by fructose addition, exhibiting a negative regression coefficient value (Table 3); i.e., high levels of fructose (6%) largely decrease the aw of the CHS, presumably due to substantial reduction of crystallization as more soluble sugars remain in the honey matrix and thus enhance the water binding properties (Fig. 1b).
A second regression model was also applied to evaluate the response variables (properties) where the two independent variables of water and fructose addition were replaced by the ratio of G/W (Table 4), since the latter has been reported as the most reliable crystallization index [16, 23]. Fructose supplementation and water addition resulted in reduction of the G/W index (Table 1) and therefore, a strong positive correlation (p < 0.001) between this index and L* values of honey-based preparations was noted (Table 5). Interestingly, a broad range of physical properties are strongly interrelated (p < 0.001) with the G/W index (Table 4).
Extent of Crystallization of the Spread Products
For the extent of crystallization, the percentage of covered area by the formed crystals in micrographs (named degree of crystallization, Dcryst) and the melting enthalpy of crystals (ΔH), estimated by cross polarized microscopy and differential scanning calorimetry (DSC), respectively, were monitored as crystallization indices. Crossed polarized micrographs (Fig. 2) revealed no traces of crystals in the initial cotton honey used as raw material after its liquefaction (Fig. 2b); this process was required before the controlled crystallization was started since the cotton honey initially obtained from the honey producer was naturally crystallized (Fig. 2a). The naturally crystallized cotton honey exhibited much larger crystals compared to all CHS samples that were crystallized following the various controlled composition-temperature–time regimes (Fig. 2d, e and f). Differences in morphology of the crystals rather than in size were observed between the commercial creamed honey (Fig. 2c) used as crystallization starter material (control) and those formulated cotton honey-based products with added water and fructose at 5% level (Fig. 2f); the control sample exhibited more elongated (thinner) crystals. Nevertheless, the degree of crystallization for the supplemented with water and fructose sample after 105 days of storage (30%) was similar to that of control (32%). The CHS product prepared without any compositional intervention had the highest Dcryst value, 55%, among all tested samples and also appeared to have higher amount of large crystals (Fig. 2d) compared to that with added water and 5% fructose (Fig. 2f). The CHS sample, in which only fructose was added at 5% level, showed an intermediate crystallization degree, 37% and crystal pattern (Fig. 2e). Dettori et al. [22] did not observe any differences in both crystal size and morphology among three samples of creamed honey obtained with different crystallization rates (fast, medium and slow).
Representative cross polarized micrographs of honey crystals of a naturally crystallized cotton honey, b liquefied cotton honey before processing for cotton honey-based spread making, c control (commercial creamed honey), d cotton honey-based spread without addition of water and fructose (sample 9) and e cotton honey-based spread without addition of water and with addition 5% fructose (sample 2) f cotton honey-based spread with addition of water and 5% fructose (sample 5); all micrographs of cotton honey-based spread samples were obtained at the end of their storage (105 days)
The degree of crystallization, Dcryst, of the 16 different samples, as determined by microscopy, varied between 21.8–54.8% after 45 days of storage (Table 2). Regression analysis showed that the Dcryst of products was significantly affected only by fructose addition (p < 0.001) (Table 3); i.e., with increase of fructose content the extent of crystallization was reduced. It seems that samples characterized by slower crystallization behavior exhibited lower degree of crystallization. As a result, a significant (p < 0.01) positive correlation between Dcryst values and G/W crystallization was noted (Table 5).
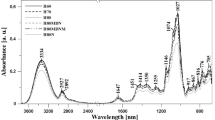
The endothermic peaks in the thermograms (Fig. 3) of CHS products represent the melting of glucose crystals and the respective ΔH values are related to the total amount of crystallized sugars (glucose), without giving any information about their size [19]. Other parameters derived from the thermograms are the To and Tm, representing the initiation and the peak melting temperature of the glucose crystals, respectively. The ΔΗ values for crystal melting of the formulated honey spread products at 45th day of storage ranged between 11.8–26.9 mJ/mg, whereas To and Tm were between 30.8–33.6 °C and 33.4–58.1 °C, respectively (Table 2). Factors that influenced significantly (p < 0.001) the ΔΗ parameter were the addition of fructose and water, the crystallization temperature, and the storage time, whereas the Tm was affected by the storage time and the fructose supplementation (Table 3). As expected, the ΔΗ decreased with increasing levels of the variables that do not favor the crystallization process, i.e., increase of moisture and fructose levels in the formulated honey preparations (Fig. 4a, Table 3) results in decrease of glucose content (Table 1) and thereby leads to decrease of glucose supersaturation which is the driving force for crystallization. This trend was confirmed by the significant (p < 0.001) positive regression (Table 4) and correlation (Table 5) coefficients between ΔH and G/W index. The increase of ΔH values with increasing G/W ratio in CHS preparations was also apparent in the respective contour plots of Fig. 5a. Similarly, low values of the G/W ratio in creamed honey have been reported to yield low crystallization rate and a relatively small number of crystals [9, 22, 23]. Additionally, low crystallization temperatures (5–9 °C) resulted in the highest ΔH values, while with a further increase in temperature there was a gradual decrease of the amount of formed crystals (Fig. 4a). It appears that above this temperature range further elevation of temperature decreases the supersaturation extent of glucose due to enhanced sugar solubility which becomes the predominant factor compared to the increase of molecular mobility with increasing temperature; as a result, there is a decline in the driving force for crystallization. Therefore, sample 8 that was a cotton sample with added water and 5% fructose, crystallized at 20 °C, exhibited lower ΔΗ than the unsupplemented cotton honey, sample 9, which was crystallized at 14 °C (Fig. 3a); the first sample had quite similar ΔΗ value with the commercial creamed honey (control), implying that the amount of formed glucose crystals in CHS can be regulated by controlling the composition and crystallization temperature. Furthermore, the crystal melting enthalpy increased up to 45 days of storage, whereas upon longer storage this parameter did not show any further noticeable change (Figs. 3b and 4a).
Contour plots illustrating the effect of crystallization index G/W (glucose/water)—storage time (hold values: Tcryst = 14 °C, Tcond = 25 °C) on melting enthalpy of crystals (ΔΗ) (a) and steady shear viscosity (η) (b) and crystallization index G/W—crystallization temperature (Hold values: Tcond = 25 °C, S = 52.5 days) on damping factor (tanδ) (c) and spreadability (d) of cotton honey-based spread preparations; Tcryst: crystallization temperature, Tcond: conditioning temperature, S: storage time
Similar to our findings, Dettori et al. [22] have observed that honey varieties which tend to crystallize slowly exhibit smaller ΔΗ than honey varieties which are more prone to crystallization. In addition, Lupano [19] and Venir et al. [7], who examined the effect of crystallization temperature on both size and shape of crystals, they found that the rate and the degree of crystallization were smaller when honeys were stored at temperatures above 20 °C. Moreover, Tomaszewska-Gras et al. [36] reported a negative linear correlation between moisture content and ΔΗ of crystallized honey samples.
Τhe peak melting temperature increased (p < 0.001) with storage time and decreased with increasing level of added fructose (Table 3, Fig. 4b). Finally, it is worth pointing out the significant positive linear correlations observed between the Dcryst, and ΔΗ (p < 0.01) and Tm (p < 0.05) (Table 6). This implies that the higher the extent of crystallization, the more energy is required to melt the formed crystals and the more thermostable the crystals are. Significant (p < 0.01) positive correlations were also found between L* values and the two parameters expressing the degree of crystallization, Dcryst and ΔΗ (Table 6).
Rheological Behavior of the Spread Products
The values of rheological parameters (measurements at 20 °C) obtained from flow curves and mechanical spectra of CHS preparations stored for 45 days varied within the ranges of 19.3–616.0 Pa·s, 14.7–372.7 Pa·s, 13.5–2420.5 Pa, 306.7–11,110.7 Pa and 3.2–23.7, for η, η*, G`, G``, and tanδ, respectively (Table 2). The regression analysis showed that the fructose and water addition affected significantly (p < 0.01) all the rheological parameters (Table 3); all these parameters were negatively influenced by the above independent variables, except tanδ which registered a positive regression coefficient. An increase in tanδ value with water and fructose addition indicated a less aggregated aqueous dispersion system. In accordance with these findings, the regression coefficients of the G/W variable showed a strong (p < 0.001) positive and negative effect of this crystallization index on the η and tanδ parameters, respectively (Table 4). Therefore, the differences in the values of rheological parameters among the CHS preparations could be attributed mostly to their variations in water content and sugar composition which largely govern the amount of glucose crystals formed and thereby the rheological responses. This is also confirmed by the significant positive and negative correlations found between the η and tanδ, respectively, and the two parameters expressing the extent of crystallization, Dcryst (p < 0.01) and ΔH (p < 0.001) (Table 6); moreover, significant (p < 0.05) positive correlations were found between other rheological parameters (η* and G΄΄) and both Dcryst and ΔH.
Figure 6a illustrates the mechanical spectra of three representative CHS preparations and the control sample. It is evident that sample 9, having the original composition of cotton honey (base raw material), showed a strong pseudoplastic behavior as indicated by the abrupt decrease of η* with increasing angular frequency; this type of flow profile could be attributed to the presence of higher amount of crystal particles, in agreement with the high Dcryst and ΔΗ values of this sample (Table 2). Samples with added fructose at 5% (sample 2) exhibited a mild shear thinning behavior similar to that of control (Fig. 6a), being consistent with their lower crystallization extent (Table 2). Instead, sample 8 that was supplemented with both water and a high level (5%) of added fructose seemed to behave nearly as Newtonian fluid (Fig. 6a), in agreement with its even lower Dcryst and ΔΗ values (Table 2). For the latter sample, the G`` values were largely greater than those of G` all over the frequency range examined, and both moduli exhibited a great frequency dependency, confirming the Newtonian flow character of this sample (Fig. 6a). Overall, the low extent of crystallization of this CHS preparation resulted in a typical Newtonian rheological response often exerted by liquid honey samples, as reported by many authors [5, 33, 37, 38].
Previous studies showed that the viscosity of honey largely decreases with increasing water content [9, 21, 33, 39, 40]. Moreover, Laos et al. [24] found that sugar composition is also an important determinant for the rheological behavior of honey upon crystallization. This is consistent with our observation that the addition of fructose in cotton honey, which decreased the G/W ratio (Table 1), resulted in less viscous CHS product compared to those with unmodified composition (Table 2). Hence, significant correlations (Table 5) between G/W ratio and the rheological parameters were found, which were positive for the η (p < 0.001), η*, and G΄΄ (p < 0.05), and negative for the tanδ (p < 0.001). Additionally, the relevant contour plots elaborate the increase of η (Fig. 5b) and decrease of tanδ (Fig. 5c) values with increasing G/W crystallization index. It is also worth noting that the tanδ showed a strong (p < 0.01) positive linear relation with crystallization temperature (Tables 3 and 4, Fig. 5c), indicating a weakening of the mixed liquid – sugar crystal network structure with increasing curing temperature, possibly due to approaching at the To of crystals in the spreads.
The Fmax and SW parameters, which express the force and work required to spread the CHS samples, varied in the ranges of 8.5–48.8 N and 13.2–133.7 N·mm, respectively, following 45 days of storage, while the control creamed honey exhibited values of 48.6 Ν for hardness and 90.6 Ν·mm for the spreadability work (Table 2). Figure 6b illustrates representative curves derived from the large deformation spreadability test of four CHS preparations. Sample 3, at which a low level of fructose (1%) was incorporated, showed the highest Fmax and SW values among all samples, including the control, while the hardness largely decreased when the fructose level was raised to 6% (sample10); nevertheless, the lowest values for Fmax and SW among all samples were found for sample 8 where a high level of added fructose (5%) was combined with water inclusion into this product formulation (Fig. 6b and Tables 1 and 2).
The findings from large deformation mechanical test clearly agree with the results from colorimetric, calorimetric, microscopic and rheological measurements, i.e., compositional changes that enhance the tendency of crystallization resulted in lighter color as well as higher amounts and thermostability of sugar crystal aggregations in the CHS products which in turn increased their difficultly to flow as well as the forces and work required for product spreading (Tables 3, 4 and 5). Therefore, both Fmax and SW parameters were positively correlated (p < 0.05) with L*, η, Dcryst, ΔH and Tm response variables and negatively with the tanδ parameter (Table 6). These findings clearly reveal that all methods of instrumental analysis employed in the present work can be a reliable and useful toolbox for monitoring quality attributes of the new CHS product.
Effect of Physicochemical Parameters on Sensory Attributes of the Spread Products
Mouthfeel, spreadability and overall acceptability of the CHS products were evaluated by a sensory analysis panel (Table 7). The highest score for mouthfeel (4.08) was given for sample 14 which was a sample with added water and fructose at 3% level indicating that it was perceived by accessors as the sample with the smoothest texture and undetectable crystals in the palate (Tables 1 and 7); however, this sample did not significantly (p > 0.05) differ from the other samples, with exception of sample 9. As expected, the latter preparation (sample 9) that had the original cotton honey sample composition (no added water and fructose) received the lowest score among all the CHS preparations for mouthfeel perception (1.83) and the highest value for spreadability (7.00); these scores mean that this sample was identified as a product with the coarsest texture containing grainy particles (i.e., large sugar crystals) and the greatest difficulty to spread it. In contrast, the lowest score for spreadability (2.33), expressing the lowest resistance of product spreading, was obtained by sample 8 which was formulated with addition of both water and a high fructose level (5%).
Regression analysis indicated that all the examined independent variables (FA, WA, Dcryst, Dcond and G/W) did not relate (p > 0.05) with the mouthfeel parameter (data not shown). On the other hand, the regression analysis showed that with addition of water and fructose there was strong (p < 0.01, R2 > 0.86) negative and positive effects on spreadability and overall acceptability of the CHS products, respectively (Table 3). The reverse trends were found for the effect of G/W crystallization index on these sensorial parameters, as revealed by the respective regression coefficient values (Table 5). Thus, the overall product acceptability significantly (p < 0.001) increased with decreasing the tendency for crystallization (i.e., decreasing G/W ratio) in the formulated cotton honey-based spreads, while the difficulty to spread the CHS products (spreadability) increased (p < 0.001) with increasing G/W ratio (Table 5, Fig. 5d). A strong positive correlation (r > 0.92 and p < 0.001) was displayed between the sensorial perception of spreadability and the two mechanical parameters of hardness and spreadability work, as determined by the texture analyser (Table 5). Similarly, the spreadability attribute, evaluated by the sensory analysis panel, was significantly correlated (p < 0.05) with all the other physical properties (L*, Dcryst, ΔH, Tm, η, η*, G΄΄, tanδ) derived from instrumental analysis; all correlations were positive with exception of the tanδ parameter. In contrast, the mouthfeel parameter showed a weak negative (r = −0.5), but significant (p < 0.05), correlation with only two rheological parameters, η* and G΄΄, indicating that creamed honey samples perceived by assessors as products with harsher texture had higher resistance to flow.
The overall acceptability of the new spread product was negatively correlated with most of the physical properties assessed by instrumental analyses (L*, Dcryst, ΔH, Tm, η, Fmax and SW) and positively with the tanδ (Table 5). These results showed that consumers seem to prefer formulated spread products with darker color, small amount of sugar crystals, less viscous and those that they can easily spread. Moreover, the significant (p < 0.001) negative correlations between overall acceptability and both spreadability scores from sensory panel and the crystallization index imply high consumer preference for CHS products with low tendency to crystallize and low resistance to spreading. Overall, the strong correlations between sensorial attributes and compositional—physicochemical parameters of the CHS preparations revealed that composition analysis and multi-instrumental quality assessment can be a useful toolbox for characterization of macroscopic physical properties and prediction of consumer acceptability of this new product.
Conclusions
This study explores the use of a multi-instrumental analysis (rheology, texture analysis, calorimetry, miscoscopy, colorimetry) in combination with sensory (spreadability, mouthfeel) evaluation for characterization of macroscopic physical properties of a cotton honey-based spread (CHS) made by controlled crystallization. Therefore, several physicochemical and sensory properties were monitored upon storage of the new spread product made with different composition and temperature–time regimes for crystallization and conditioning (tempering). Mostly, sample composition of the formulated spread before the controlled crystallization process was found to largely govern the physicochemical and sensory properties of the final products; augmenting the fructose content and inclusion of water into cotton honey, decreased the glucose/water ratio, a well-known crystallization index, thus resulting in significant improvement of quality attributes and consumer acceptability of the new spread product. Hence, supplementation of cotton honey with fructose up to 6% level and/or addition of water to reach a final moisture content about 17.5% significantly decreased the following physical parameters of the CHS products: color lightness, water activity, extent of crystallization (amount and melting enthalpy of sugar crystals), thermostability of the sugar crystals, steady shear viscosity, loss modulus and, instrumental force and work required for spreading the product as well as sensorial perception of difficulty to spread (spreadability); on the other hand, damping factor and overall sensorial acceptability significantly increased by the addition of fructose and water inclusion into cotton honey submitted to controlled crystallization. The crystallization temperature also affected a few of the quality parameters, whereas the conditioning temperature did not seem to have any significant impact; i.e., the color lightness of CHS products and the melting enthalpy of the formed sugar crystals significantly decreased with increasing crystallization temperature above 9 °C. Overall, formulations with a decreased glucose/water ratio gave less viscous and softer spread products displaying less resistance to spreading and a lower water activity, color lightness and extent of crystallization; such spreads also appeared more appealing to consumers. Interestingly, significant positive linear correlations were found between the extent of crystallization, measured by microscopy and calorimetry (melting enthalpy), of the spread products with color lightness and crystal melting temperature, as well as with rheological parameters estimated by both rheometry (steady shear and complex viscosity, and loss modulus) and large deformation mechanical testing (force and work of spreadability). The damping factor of CHS products and overall sensorial acceptability were also found to be negatively correlated with all the above physical parameters, while the spreadability (difficulty to spread the product), as perceived by the sensory panelists, showed a significant positive correlation with them. Generally, the multi-instrumental methodology (colorimetry, rheology, calorimetry, microscopy and large deformation mechanical testing) employed in the present study was verified to be a reliable analytical framework for characterization of macroscopic physical properties of the cotton honey-based spread product and a useful predicting tool for product preference by the consumers. This study also demonstrated the potential use of cotton honey as main ingredient for production of spread products suitable for human consumption and being rich in bioactive compounds. In this context, it would be reasonable to expect an improvement in the marketability of cotton honey.
References
A.A. Machado De-Melo, L.B.D. Almeida-Muradian, M.T. Sancho, A. Pascual-Maté, Composition and properties of Apis melifera honey: A review. J. Apic. Res. 57, 5–37 (2017). https://doi.org/10.1080/00218839.2017.1338444
M.M. Cavia, M.A. Fernandez-Muino, E. Gömez-Alonso, M.J. Montes-Pérez, J.F. Huidobro, M.T. Sancho, Evolution of fructose and glucose in honey over one year: influence of induced granulation. Food Chem. 78, 157–161 (2002). https://doi.org/10.1016/S0308-8146(01)00393-4
M.A. Kamal, P. Klein, Determination of sugars in honey by liquid chromatography. Saudi J. Biol. Sci. 18, 17–21 (2011). https://doi.org/10.1016/j.sjbs.2010.09.003
J. Wang, Q. X. Li, in Advances in Food and Nutrition Research, vol. 62, ed. by S. L. Taylor (Academic, 2011)
J.W. White Jr, in Advances in Food Research, vol 24, ed. C.O. Chichester (Academic Press, 1978)
R.A. Gleiter, H. Horn, H.D. Isengard, Influence of type and state of crystallization on the water activity of honey. Food Chem. 96, 441–445 (2006). https://doi.org/10.1016/j.foodchem.2005.03.051
E. Venir, M. Spaziani, E. Maltini, Crystallization in “Tarassaco” Italian honey studied by DSC. Food Chem. 122, 410–415 (2010). https://doi.org/10.1016/j.foodchem.2009.04.012
Council Directive (EC) 2001/11 of 20 December 2001 relating to honey. OJ L10/47-52
S. Yanniotis, S. Skaltsi, S. Karaburnioti, Effect of moisture on the viscosity of honey at different temperatures. J. Food Eng. 72, 372–377 (2006). https://doi.org/10.1016/j.jfoodeng.2004.12.017
M.C. Zamora, J. Chirife, Determination of water activity change due to crystallization in honeys from Argentina. Food Control 17, 59–64 (2006). https://doi.org/10.1016/j.foodcont.2004.09.003
R.W. Hartel, A.V. Shastry, Sugar crystallization in food products. Crit. Rev. Food Sci. 30, 49–112 (1991). https://doi.org/10.1080/10408399109527541
R.W. Hartel, R. Ergun, S. Vogel, Phase/state transitions of confectionery sweeteners: thermodynamic and kinetic aspects. Compr. Rev. Food Sci. Food Saf. 10, 17–32 (2011). https://doi.org/10.1111/j.1541-4337.2010.00136.x
A. Saleki-Gerhardt, G. Zografi, Non-isothermal and isothermal crystallization of sucrose from the amorphous state. Pharm. Res. 11, 1166–1173 (1994). https://doi.org/10.1023/A:1018945117471
H. Kiani, D.W. Sun, Water crystallization and its importance to freezing in foods: a review. Trends Food Sci. Tech. 22, 407–426 (2011). https://doi.org/10.1016/j.tifs.2011.04.011
E. Lopez-Quiroga, R. Wang, O. Gouseti, P.J. Fryer, S. Bakalis, Crystallisation in concentrated systems: A modelling approach. Food Bioprod. Process 100, 525–534 (2016). https://doi.org/10.1016/j.fbp.2016.07.007
I. Manikis, A. Thrasivoulou, The relation of physicochemical characteristics of honey and the crystallization sensitive parameters. Apiacta 36, 106–122 (2001)
L. Slade, H. Levine, Beyond water activity: Recent advances based on an alternative approach to the assessment of food quality and safety. Crit. Rev. Food Sci. 30, 115–360 (1991). https://doi.org/10.1080/10408399109527543
H.I. Assil, R. Sterling, P. Sporns, Crystal control in processed liquid honey. J. Food Sci. 56, 1034–1037 (1991). https://doi.org/10.1111/j.1365-2621.1991.tb14635.x
C.E. Lupano, DSC study of honey granulation stored at various temperatures. Food Res. Int. 30, 683–688 (1997). https://doi.org/10.1016/S0963-9969(98)00030-1
N.A. Al-Habsi, F.J. Davis, K. Niranjan, Development of novel methods to determine crystalline glucose content of honey based on DSC, HPLC, and viscosity measurements, and their use to examine the setting propensity of honey. J. Food Sci. 78, E845–E852 (2013). https://doi.org/10.1111/1750-3841.12103
P.A. Sopade, P. Halley, B. Bhandari, B. D’Arcy, C. Doebler, N. Caffin, Application of the Williams-Landel-Ferry model to the viscosity-temperature relationship of Australian honeys. J. Food Eng. 56, 67–75 (2003). https://doi.org/10.1016/S0260-8774(02)00149-8
A. Dettori, S. Tappi, L. Piana, M. Dalla Rosa, P. Rocculi, Kinetic of induced honey crystallization and related evolution of structural and physical properties. LWT-Food Sci. Technol. 95, 333–338 (2018). https://doi.org/10.1016/j.lwt.2018.04.092
I. Dobre, L.A. Georgescu, P. Alexe, O. Escuredo, M.C. Seijo, Rheological behavior of different honey types from Romania. Food Res. Int. 49, 126–132 (2012). https://doi.org/10.1016/j.foodres.2012.08.009
K. Laos, E. Kirs, R. Pall, K. Martverk, The crystallization behaviour of Estonian honeys. Agron. Res. 9, 427–432 (2011)
E.A. Tosi, E. Ré, H. Lucero, L. Bulacio, Effect of honey high-temperature short-time heating on parameters related to quality, crystallization phenomena and fungal inhibition. LWT-Food Sci. Technol. 37, 669–678 (2004). https://doi.org/10.1016/j.lwt.2004.02.005
E.J. Dyce, in: Honey: A Comprehensive Survey, ed. E.E. Crane (Oxford University Press, New York, 1975)
A.M. Abd Elhamid, H.F. Abou-Shaara, Producing clover and cotton creamed honey under cooling conditions and potential use as feeding to honey bee colonies. J. Apic. Res. 31, 135–142 (2016). https://doi.org/10.17519/apiculture.2016.04.31.1.59
Y.W. Chen, C.H. Lin, F.Y. Wu, H.H. Chen, Rheological properties of crystallized honey prepared by a new type of nuclei. J. Food Process Eng. 32, 512–527 (2009). https://doi.org/10.1111/j.1745-4530.2007.00227.x
S. Karasu, O.S. Toker, M.T. Yilmaz, S. Karaman, E. Dertli, Thermal loop test to determine structural changes and thermal stability of creamed honey: Rheological characterization. J. Food Eng. 150, 90–98 (2015). https://doi.org/10.1016/j.jfoodeng.2014.10.004
E. Alissandrakis, A.C. Kibaris, P.A. Tarantilis, P.C. Harizanis, M. Polissiou, Flavour compounds of Greek cotton honey. J. Sci. Food Agr. 85, 1444–1452 (2005). https://doi.org/10.1002/jsfa.2124
N.R. Draper, J.A. John, Response-surface designs for quantitative and qualitative factors. Technometrics 30, 423–428 (1988). https://doi.org/10.1080/00401706.1988.10488437
International Honey Commission (IHC), Harmonized methods of the international honey commission (2009), https://www.ihc-platform.net/ihcmethods2009.pdf
A. Lazaridou, C.G. Biliaderis, N. Bacandritsos, A.G. Sabatini, Composition, thermal and rheological behaviour of selected Greek honeys. J Food Eng 64, 9–21 (2004). https://doi.org/10.1016/j.jfoodeng.2003.09.007
A.I. Neguerela, C. Perez-Arquillue, Color measurement of rosemary honey in the solid state by reflectance spectroscopy with black background. J. AOAC Int. 83, 669–674 (2000). https://doi.org/10.1093/jaoac/83.3.669
J. Chirife, M.C. Zamora, A. Motto, The correlation between water activity and % moisture in honey: fundamental aspects and application to Argentine honeys. J. Food Eng. 72, 287–292 (2006). https://doi.org/10.1016/j.jfoodeng.2004.12.009
J. Tomaszewska-Gras, S. Bakier, K. Goderska, K. Mansfeld, Differential scanning calorimetry for determining the thermodynamic properties of selected honeys. J. Apic. Sci. 59, 109–118 (2015). https://doi.org/10.1515/jas-2015-0012
B. Bhandari, B. D’ Arcy, C. Kelly, Rheology and crystallization kinetics of honey: present status. Int. J. Food Prop. 2, 217–226 (1999). https://doi.org/10.1080/10942919909524606
C.C. Da Costa, R.G. Pereira, The influence of propolis on the rheological behaviour of pure honey. Food Chem. 76, 417–421 (2002). https://doi.org/10.1016/S0308-8146(01)00298-9
Z. Ren, X. Bian, L. Lin, Y. Bai, W. Wang, Viscosity and melt fragility in honey-water mixtures. J. Food Eng. 100, 705–710 (2010). https://doi.org/10.1016/j.jfoodeng.2010.06.004
H. Tavakolipour, A.K. Ashtari, Rhelogy of selected Persian honeys. Int. J. Food Eng. 6, 1–12 (2010). https://doi.org/10.2202/1556-3758.1799
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that there is no conflict of interest.
Compliance with Ethics Requirements
This article does not contain any studies with human participants or animals performed by any of the author.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sereti, V., Lazaridou, A., Tananaki, C. et al. Development of a Cotton Honey-Based Spread by Controlling Compositional and Processing Parameters. Food Biophysics 16, 365–380 (2021). https://doi.org/10.1007/s11483-021-09677-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11483-021-09677-9