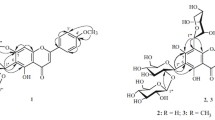
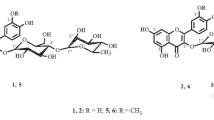
Six new compounds including four flavonoids (1–3, 5) and two iridoids (4 and 6) were found in three Veronica species growing in eastern Siberia. Their structures were studied by UV and NMR spectroscopy and mass spectrometry. 6-Hydroxyluteolin-7-O-(6″-O-isoferuloyl)-β-D-glucopyranoside (veroniside A, 1) was isolated from V. daurica Steven; 6-hydroxyluteolin-7-O-(6″-O-isovanilloyl)-β-D-glucopyranoside (veroniside B, 2) and scutellarein-7-O-(6″-O-vanilloyl)-β-D-glucopyranoside (veroniside C, 3), from V. spicata subsp. incana (L.) Walters (V. incana L.); and 6-O-sinapoyl catalpol (veroniside D, 4), scutellarein-7-O-(6″-O-protocatechoyl)glucoside (veroniside E, 5), and 6-O-isoferuloyl asystasioside E (veroniside F, 6), from V. longifolia L.
Similar content being viewed by others
References
B. Salehi, M. S. Shetty, N. V. Anil Kumar, J. Zivkovic, D. Calina, A. O. Docea, S. Emamzadeh-Yazdi, C. S. Kilic, T. Goloshvili, S. Nicola, G. Pignata, F. Sharopov, M. Del Mar Contreras, W. C. Cho, N. Martins, and J. Sharifi-Rad, Molecules, 24, 2454 (2019).
P. A. Kosachev, D. Albach, D. N. Shaulo, and A. I. Shmakov, Turczaninowia, 16, 8 (2013).
S. M. Batorova, G. P. Yakovlev, and T. A. Aseeva, Guide to Medicinal Plants of Traditional Tibetan Medicine, Nauka, Novosibirsk, 2003, 291 pp.
J. Suomi, H. Siren, K. Hartonen, and M.-L. Riekkola, J. Chromatogr. A, 868, 73 (2000).
S. R. Jensen, C. H. Gotfredsen, U. S. Harput, and I. Saracoglu, J. Nat. Prod., 73, 1593 (2010).
D. C. Albach, R. J. Grayer, So. R. Jensen, F. Ozgokce, and N. C. Veitch, Phytochemistry, 64, 1295 (2003).
K. Bock and C. Pedersen, Adv. Carbohydr. Chem. Biochem., 41, 27 (1983).
H. Sudo, T. Ide, H. Otsuka, E. Hirata, A. Takushi, and Y. Takeda, Phytochemistry, 46, 1231 (1997).
I. Saracoglu, F. N. Oztunca, A. Nagatsu, and U. S. Harput, Pharm. Biol., 49, 1150 (2011).
H. Inouye, Y. Takeda, and H. Nishimura, Phytochemistry, 13, 2219 (1974).
J. H. Kwak, H. J. Kim, K. H. Lee, S. C. Kang, and O. P. Zee, Arch. Pharm. Res., 32, 207 (2009).
K. Machida, M. Ogawa, and M. Kikuchi, Chem. Pharm. Bull., 46, 1056 (1998).
D. de Beer, E. Joubert, C. J. Malherbe, and J. D. Brand, J. Chromatogr. A, 1218, 6179 (2011).
A. Cakir, A. Mavi, C. Kazaz, A. Yildirim, and O. I. Kufrevioglu, Turk. J. Chem., 30, 483 (2006).
V. M. Malikov and M. P. Yuldashev, Chem. Nat. Compd., 38, 358 (2002).
H. Y. Al Ati, G. A. Fawzy, A. A. El Gamal, A. T. Khalil, K. El Din El Tahir, M. S. Abdel-Kader, and A.-H. Gilani, Pak. J. Pharm. Sci., 28, 1533 (2015).
I. Saracoglu, M. Varel, U. S. Harput, and A. Nagatsu, Phytochemistry, 65, 2379 (2004).
Y. Meng, A. J. Krzysiak, M. J. Durako, J. I. Kunzelman, and J. L. C. Wright, Phytochemistry, 69, 2603 (2008).
I. Calis, M. F. Lahloub, E. Rogenmoser, and O. Sticher, Phytochemistry, 23, 2313 (1984).
D. N. Olennikov and N. I. Kashchenko, Chem. Nat. Compd., 55, 256 (2019).
D. N. Olennikov and N. I. Kashchenko, Chem. Nat. Compd., 50, 589 (2014).
D. C. Albach, R. J. Grayer, G. C. Kite, and S. R. Jensen, Biochem. Syst. Ecol., 33, 1167 (2005).
A. A. Ahmed, T. J. Mabry, and S. A. Matlin, Phytochemistry, 28, 1751 (1989).
J. Kawabata, K. Mizuhata, E. Sato, T. Nishioka, Y. Aoyama, and T. Kasai, Biosci., Biotechnol. Biochem., 67, 445 (2003).
R. J. Grayer-Barkmeijer, Biochem. Syst. Ecol., 6, 131 (1978).
H. Olsen, K. Aaby, and G. I. A. Borge, J. Agric. Food Chem., 57, 2816 (2009).
Z. F. Peng, D. Strack, A. Baumert, R. Subramaniam, N. K. Goh, T. F. Chia, S. N. Tan, and L. S. Chia, Phytochemistry, 62, 219 (2003).
K. Machida, M. Ogawa, and M. Kikuchi, Chem. Pharm. Bull., 46, 1056 (1998).
H. Demuth, S. R. Jensen, and B. J. Nielsen, Phytochemistry, 28, 3361 (1989).
D. N. Olennikov, N. K. Chirikova, A. G. Vasilieva, and I. A. Fedorov, Antioxidants, 9, 526 (2020).
D. N. Olennikov and N. K. Chirikova, Chem. Nat. Compd., 55, 1032 (2019).
D. N. Olennikov, Plants, 9, 1555 (2020).
D. N. Olennikov, N. K. Chirikova, N. I. Kashchenko, T. G. Gornostai, I. Y. Selyutina, and I. N. Zilfikarov, Int. J. Mol. Sci., 18, 2579 (2017).
M. Akabane, A. Yamamoto, S. Aizawa, A. Taga, and S. Kodama, Anal. Sci., 30, 739 (2014).
D. N. Olennikov, A. I. Gadimli, J. I. Isaev, N. I. Kashchenko, A. S. Prokopyev, T. N. Katayeva, N. K. Chirikova, and C. Vennos, Metabolites, 9, 271 (2019).
Acknowledgment
The work was supported by the Ministry of Education and Science of the Russian Federation (Projects Nos. FSRG-2020-0019 and 121030100227-7) and the RFBR (Project No. 19-09-00361).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Prirodnykh Soedinenii, No. 3, May–June, 2021, pp. 374–380.
Rights and permissions
About this article
Cite this article
Olennikov, D.N., Chirikova, N.K. New Acylated Flavone-O-Glycosides and Iridoids from the Genus Veronica. Chem Nat Compd 57, 436–444 (2021). https://doi.org/10.1007/s10600-021-03382-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-021-03382-2