Abstract
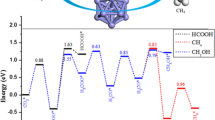
Electrocatalytic CO2 reduction reaction (CO2RR) to obtain C2 products has drawn widespread attentions. Copper-based materials are the most reported catalysts for CO2 reduction to C2 products. Design of high-efficiency pseudo-copper catalysts according to the key characteristics of copper (Cu) is an important strategy to understand the reaction mechanism of C2 products. In this work, density function theory (DFT) calculations are used to predict nickel-zinc (NiZn) alloy catalysts with the criteria similar structure and intermediate adsorption property to Cu catalyst. The calculated tops of 3d states of NiZn3(001) catalysts are the same as Cu(100), which is the key parameter affecting the adsorption of intermediate products. As a result, NiZn3(001) exhibits similar adsorption properties with Cu(100) on the crucial intermediates *CO2, *CO and *H. Moreover, we further studied CO formation, CO hydrogenation and C-C coupling process on Ni-Zn alloys. The free energy profile of C2 products formation shows that the energy barrier of C2 products formation on NiZn3(001) is even lower than Cu(100). These results indicate that NiZn3 alloy as pseudo-copper catalyst can exhibit a higher catalytic activity and selectivity of C2 products during CO2RR. This work proposes a feasible pseudo-copper catalyst and provides guidance to design high-efficiency catalysts for CO2RR to C2 or multi-carbon products.

Similar content being viewed by others
References
Z. Cui, W. Du, C. Xiao, Q. Li, R. Sa, C. Sun, and Z. Ma, Enhancing hydrogen evolution of MoS2 Basal planes by combining single-boron catalyst and compressive strain, Front. Phys. 15(6), 63502 (2020)
K. Chen, H. Li, Y. Xu, K. Liu, H. Li, X. Xu, X. Qiu, and M. Liu, Untying thioether bond structures enabled by “voltage-scissors” for stable room temperature sodium-sulfur batteries, Nanoscale 11(13), 5967 (2019)
X. Li, Y. B. Zhao, F. Fan, L. Levina, M. Liu, R. Quintero-Bermudez, X. Gong, L. N. Quan, J. Fan, Z. Yang, S. Hoogland, O. Voznyy, Z. H. Lu, and E. H. Sargent, Bright colloidal quantum dot light-emitting diodes enabled by efficient chlorination, Nat. Photon. 12(3), 159 (2018)
Y. Wei, G. Xing, K. Liu, G. Li, P. Dang, S. Liang, M. Liu, Z. Cheng, D. Jin, and J. Lin, New strategy for designing orangish-red-emitting phosphor via oxygen-vacancy-induced electronic localization, Light Sci. Appl. 8(1), 15 (2019)
K. Chen, W. Fan, C. Huang, and X. Qiu, Enhanced stability and catalytic activity of bismuth nanoparticles by modified with porous silica, J. Phys. Chem. Solids 110, 9 (2017)
Q. Li, S. Qiu, and B. Jia, Theoretical investigation of CoTa2O6/graphene heterojunctions for oxygen evolution reaction, Front. Phys. 16(1), 13503 (2021)
Z. Q. Wang, T. Y. Lü, H. Q. Wang, Y. P. Feng, and J. C. Zheng, Review of borophene and its potential applications, Front. Phys. 14(3), 33403 (2019)
Y. H. Lui, B. Zhang, and S. Hu, Rational design of photoelectrodes for photoelectrochemical water splitting and CO2 reduction, Front. Phys. 14(5), 53402 (2019)
J. Fu, K. Jiang, X. Qiu, J. Yu, and M. Liu, Product selectivity of photocatalytic CO2 reduction reactions, Mater. Today 32, 222 (2020)
J. Fu, K. Liu, K. Jiang, H. Li, P. An, W. Li, N. Zhang, H. Li, X. Xu, H. Zhou, D. Tang, X. Wang, X. Qiu, and M. Liu, Graphitic carbon nitride with dopant induced charge localization for enhanced photoreduction of CO2 to CH4, Adv. Sci. 6(18), 1900796 (2019)
J. Fu, S. Wang, Z. Wang, K. Liu, H. Li, H. Liu, J. Hu, X. Xu, H. Li, and M. Liu, Graphitic carbon nitride based single-atom photocatalysts, Front. Phys. 15(3), 33201 (2020)
R. Kas, R. Kortlever, H. Yilmaz, M. T. M. Koper, and G. Mul, Manipulating the hydrocarbon selectivity of copper nanoparticles in CO2 electroreduction by process conditions, ChemElectroChem 2(3), 354 (2015)
M. Zhong, K. Tran, Y. Min, C. Wang, Z. Wang, C. T. Dinh, P. De Luna, Z. Yu, A. S. Rasouli, P. Brodersen, S. Sun, O. Voznyy, C. S. Tan, M. Askerka, F. Che, M. Liu, A. Seifitokaldani, Y. Pang, S. C. Lo, A. Ip, Z. Ulissi, and E. H. Sargent, Accelerated discovery of CO2 electrocatalysts using active machine learning, Nature 581(7807), 178 (2020)
R. Reske, M. Duca, M. Oezaslan, K. J. P. Schouten, M. T. M. Koper, and P. Strassert, Controlling catalytic selectivities during CO2 electroreduction on thin Cu metal overlayers, J. Phys. Chem. Lett. 4(15), 2410 (2013)
F. Calle-Vallejo and M. T. Koper, Theoretical considerations on the electroreduction of CO to C2 species on Cu(100) electrodes, Angew. Chem. Int. Ed. 52(28), 7282 (2013)
X. Wang, Z. Wang, F. P. García de Arquer, C. T. Dinh, A. Ozden, Y. C. Li, D. H. Nam, J. Li, Y. S. Liu, J. Wicks, Z. Chen, M. Chi, B. Chen, Y. Wang, J. Tam, J. Y. Howe, A. Proppe, P. Todorović, F. Li, T. T. Zhuang, C. M. Gabardo, A. R. Kirmani, C. McCallum, S. F. Hung, Y. Lum, M. Luo, Y. Min, A. Xu, C. P. O’Brien, B. Stephen, B. Sun, A. H. Ip, L. J. Richter, S. O. Kelley, D. Sinton, and E. H. Sargent, Efficient electrically powered CO2-to-ethanol via suppression of deoxygenation, Nat. Energy 5(6), 478 (2020)
P. An, L. Wei, H. Li, B. Yang, K. Liu, J. Fu, H. Li, H. Liu, J. Hu, Y. R. Lu, H. Pan, T. S. Chan, N. Zhang, and M. Liu, Enhancing CO2 reduction by suppressing hydrogen evolution with polytetrafluoroethylene protected copper nanoneedles, J. Mater. Chem. A 8(31), 15936 (2020)
H. Zhou, K. Liu, H. Li, M. Cao, J. Fu, X. Gao, J. Hu, W. Li, H. Pan, J. Zhan, Q. Li, X. Qiu, and M. Liu, Recent advances in different-dimension electrocatalysts for carbon dioxide reduction, J. Colloid Interface Sci. 550, 17 (2019)
Y. Zhou, F. Che, M. Liu, C. Zou, Z. Liang, P. De Luna, H. Yuan, J. Li, Z. Wang, H. Xie, H. Li, P. Chen, E. Bladt, R. Quintero-Bermudez, T. K. Sham, S. Bals, J. Hofkens, D. Sinton, G. Chen, and E. H. Sargent, Dopant-induced electron localization drives CO2 reduction to C2 hydrocarbons, Nat. Chem. 10(9), 974 (2018)
S. Nitopi, E. Bertheussen, S. B. Scott, X. Liu, A. K. Engstfeld, S. Horch, B. Seger, I. E. L. Stephens, K. Chan, C. Hahn, J. K. Norskov, T. F. Jaramillo, and I. Chorkendorff, Progress and perspectives of electrochemical CO2 reduction on copper in aqueous electrolyte, Chem. Rev. 119(12), 7610 (2019)
Y. Y. Birdja, E. Pérez-Gallent, M. C. Figueiredo, A. J. Göttle, F. Calle-Vallejo, and M. T. M. Koper, Advances and challenges in understanding the electrocatalytic conversion of carbon dioxide to fuels, Nat. Energy 4(9), 732 (2019)
W. Luo, X. Nie, M. J. Janik, and A. Asthagiri, Facet dependence of CO2 reduction paths on Cu electrodes, ACS Catal. 6(1), 219 (2016)
H. Li, F. Calle-Vallejo, M. J. Kolb, Y. Kwon, Y. Li, and M. T. Koper, Why (1 0 0) terraces break and make bonds: Oxidation of dimethyl ether on platinum single-crystal electrodes, J. Am. Chem. Soc. 135(38), 14329 (2013)
M. T. Koper, Structure sensitivity and nanoscale effects in electrocatalysis, Nanoscale 3(5), 2054 (2011)
X. G. Zhang, S. Feng, C. Zhan, D. Y. Wu, Y. Zhao, and Z. Q. Tian, Electroreduction reaction mechanism of carbon dioxide to C2 products via Cu/Au bimetallic catalysis: A theoretical prediction, J. Phys. Chem. Lett. 11(16), 6593 (2020)
Z. X. Chen, K. M. Neyman, A. B. Gordienko, and N. Rösch, Surface structure and stability of PdZn and PtZn alloys: Density-functional slab model studies, Phys. Rev. B 68(7), 075417 (2003)
D. Kim, J. Resasco, Y. Yu, A. M. Asiri, and P. Yang, Synergistic geometric and electronic effects for electrochemical reduction of carbon dioxide using gold-copper bimetallic nanoparticles, Nat. Commun. 5(1), 4948 (2014)
A. Nilsson, L. G. M. Pettersson, B. Hammer, T. Bligaard, C. H. Christensen, and J. K. Nørskov, The electronic structure effect in heterogeneous catalysis, Catal. Lett. 100(3–4), 111 (2005)
J. K. Norskov, F. Abild-Pedersen, F. Studt, and T. Bligaard, Density functional theory in surface chemistry and catalysis, Proc. Natl. Acad. Sci. USA 108(3), 937 (2011)
M. Luo, Z. Wang, Y. C. Li, J. Li, F. Li, Y. Lum, D. H. Nam, B. Chen, J. Wicks, A. Xu, T. Zhuang, W. R. Leow, X. Wang, C. T. Dinh, Y. Wang, Y. Wang, D. Sinton, and E. H. Sargent, Hydroxide promotes carbon dioxide electroreduction to ethanol on copper via tuning of adsorbed hydrogen, Nat. Commun. 10(1), 5814 (2019)
A. Bagger, W. Ju, A. S. Varela, P. Strasser, and J. Rossmeisl, Electrochemical CO2 reduction: A classification problem, ChemPhysChem 18(22), 3266 (2017)
Y. Zheng, A. Vasileff, X. Zhou, Y. Jiao, M. Jaroniec, and S. Z. Qiao, Understanding the roadmap for electrochemical reduction of CO2 to multi-carbon oxygenates and hydrocarbons on copper-based catalysts, J. Am. Chem. Soc. 141(19), 7646 (2019)
Z. Zhao and G. Lu, Computational screening of nearsurface alloys for CO2 electroreduction, ACS Catal. 8(5), 3885 (2018)
S. Lee, G. Park, and J. Lee, Importance of Ag-Cu biphasic boundaries for selective electrochemical reduction of CO2 to ethanol, ACS Catal. 7(12), 8594 (2017)
X. Lv, L. Shang, S. Zhou, S. Li, Y. Wang, Z. Wang, T. K. Sham, C. Peng, and G. Zheng, Electron-deficient Cu sites on Cu3Ag1 catalyst promoting CO2 electroreduction to alcohols, Adv. Energy Mater. 10(37), 2001987 (2020)
D. Ren, B. S. H. Ang, and B. S. Yeo, Tuning the selectivity of carbon dioxide electroreduction toward ethanol on oxide-derived CuxZn catalysts, ACS Catal. 6(12), 8239 (2016)
H. S. Jeon, J. Timoshenko, F. Scholten, I. Sinev, A. Herzog, F. T. Haase, and B. R. Cuenya, Operando insight into the correlation between the structure and composition of CuZn nanoparticles and their selectivity for the electrochemical CO2 reduction, J. Am. Chem. Soc. 141(50), 19879 (2019)
A. R. Paris and A. B. Bocarsly, Ni-Al films on glassy carbon electrodes generate an array of oxygenated organics from CO2, ACS Catal. 7(10), 6815 (2017)
A. R. Paris and A. B. Bocarsly, Mechanistic insights into C2 and C3 product generation using Ni3Al and Ni3Ga electrocatalysts for CO2 reduction, Faraday Discuss. 215, 192 (2019)
D. A. Torelli, S. A. Francis, J. C. Crompton, A. Javier, J. R. Thompson, B. S. Brunschwig, M. P. Soriaga, and N. S. Lewis, Nickel-gallium-catalyzed electrochemical reduction of CO2 to highly reduced products at low overpotentials, ACS Catal. 6(3), 2100 (2016)
R. Kortlever, I. Peters, C. Balemans, R. Kas, Y. Kwon, G. Mul, and M. T. Koper, Palladium-gold catalyst for the electrochemical reduction of CO2 to C1-C5 hydrocarbons, Chem. Commun. (Camb.) 52(67), 10229 (2016)
K. J. P. Schouten, E. Pérez Gallent, and M. T. M. Koper, Structure sensitivity of the electrochemical reduction of carbon monoxide on copper single crystals, ACS Catal. 3(6), 1292 (2013)
H. A. Hansen, C. Shi, A. C. Lausche, A. A. Peterson, and J. K. Norskov, Bifunctional alloys for the electroreduction of CO2 and CO, Phys. Chem. Chem. Phys. 18(13), 9194 (2016)
M. J. Cheng, E. L. Clark, H. H. Pham, A. T. Bell, and M. Head-Gordon, Quantum mechanical screening of singleatom bimetallic alloys for the selective reduction of CO2 to C1 hydrocarbons, ACS Catal. 6(11), 7769 (2016)
A. Vasileff, C. Xu, Y. Jiao, Y. Zheng, and S. Z. Qiao, Surface and interface engineering in copper-based bimetallic materials for selective CO2 electroreduction, Chem 4(8), 1809 (2018)
M. Karamad, V. Tripkovic, and J. Rossmeisl, Intermetallic alloys as CO electroreduction catalysts — Role of isolated active sites, ACS Catal. 4(7), 2268 (2014)
Y. Cai and X. Luo, First-principles investigation of carbon dioxide adsorption on MN4 doped graphene, AIP Adv. 10(12), 125013 (2020)
A. C. Hegde, K. Venkatakrishna, and N. Eliaz, Electrodeposition of Zn-Ni, Zn-Fe and Zn-Ni-Fe alloys, Surf. Coat. Tech. 205(7), 2031 (2010)
G. Kresse and J. Hafner, Ab initio molecular-dynamics simulation of the liquid-metal-amorphous-semiconductor transition in germanium, Phys. Rev. B 49(20), 14251 (1994)
G. Kresse and J. Furthmüller, Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set, Phys. Rev. B 54(16), 11169 (1996)
G. Kresses and J. Hafner, Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set, Comput. Mater. Sci. 6(1), 15 (1996)
J. P. Perdew, J. A. Chevary, S. H. Vosko, K. A. Jackson, M. R. Pederson, D. J. Singh, and C. Fiolhais, Atoms, molecules, solids, and surfaces: Applications of the generalized gradient approximation for exchange and correlation, Phys. Rev. B 46(11), 6671 (1992)
K. Liu, J. Fu, L. Zhu, X. Zhang, H. Li, H. Liu, J. Hu, and M. Liu, Single-atom transition metals supported on black phosphorene for electrochemical nitrogen reduction, Nanoscale 12(8), 4903 (2020)
J. K. Nörskov, T. Bligaard, J. Rossmeisl, and C. H. Christensen, Towards the computational design of solid catalysts, Nat. Chem. 1(1), 37 (2009)
J. Li, Z. Wang, C. McCallum, Y. Xu, F. Li, Y. Wang, C. M. Gabardo, C. T. Dinh, T. T. Zhuang, L. Wang, J. Y. Howe, Y. Ren, E. H. Sargent, and D. Sinton, Constraining CO coverage on copper promotes high-efficiency ethylene electroproduction, Nat. Catal. 2(12), 1124 (2019)
T. K. Todorova, M. W. Schreiber, and M. Fontecave, Mechanistic understanding of CO2 reduction reaction (CO2RR) toward multicarbon products by heterogeneous copper-based catalysts, ACS Catal. 10(3), 1754 (2020)
D. D. Zhu, J. L. Liu, and S. Z. Qiao, Recent advances in inorganic heterogeneous electrocatalysts for reduction of carbon dioxide, Adv. Mater. 28(18), 3423 (2016)
S. Hanselman, M. T. M. Koper, and F. Calle-Vallejo, Computational comparison of late transition metal (100) surfaces for the electrocatalytic reduction of CO to C2 species, ACS Energy Lett. 3(5), 1062 (2018)
Acknowledgements
The authors gratefully thank the National Natural Science Foundation of China (Grant Nos. 21872174, 22002189, and U1932148), the International Science and Technology Cooperation Program (Grant Nos. 2017YFE0127800 and 2018YFE0203402), the Hunan Provincial Science and Technology Program (No. 2017XK2026), the Hunan Provincial Natural Science Foundation (Grant Nos. 2020JJ2041 and 2020JJ5691), the Hunan Provincial Science and Technology Plan Project (No. 2017TP1001), the Shenzhen Science and Technology Innovation Project (No. JCYJ20180307151313532), the Key R&D Program of Hunan Province (No. 2020WK2002).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Zhang, XD., Liu, K., Fu, JW. et al. Pseudo-copper Ni-Zn alloy catalysts for carbon dioxide reduction to C2 products. Front. Phys. 16, 63500 (2021). https://doi.org/10.1007/s11467-021-1079-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11467-021-1079-4