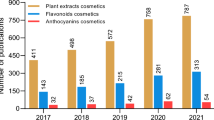
Abstract
Ligninolytic enzymes are potential candidates in whitening cosmetics because of their high melanin decolorization potential. Lignin peroxidase (LiP) owns a special place in dermatology and cosmetology industries among ligninolytic enzymes due to its comparatively high redox potential than other enzymes. The unpurified LiPs after fungal fermentation are commonly studied in cosmetics formulations due to difficulties in enzyme purification. The deconstruction potential of LiP could be attributed to the versatility of substrates, including both non-phenolic and phenolic compounds and xenobiotics. The exploration of ligninolytic enzymes for potential biotechnological applications has commonly been reported over the years. This review aimed to summarize the futuristic applications and current functionalities of ligninolytic enzymes in modern cosmetics formulations as a skin-lightening agent through melanin decolorization applications. Studies suggest high throughput applications of LiP in the cosmetic sector, where it has the potential to replace hydroquinone and other skin-lightening agents whose safety has resulted in controversies. Moreover, pigmentation disorders such as hyperpigmentation could be treated in a sustainable manner using such enzyme-based approaches.
Graphic Abstract


Copyright© 2019 Elsevier B.V

Copyright© 2019 Elsevier B.V

Copyright© 2017 The Author(s). Licensee InTech

Copyright © 2019 The Authors. Published by Elsevier B.V
Similar content being viewed by others
References
Bilal M, Iqbal H (2020) New insights on unique features and role of nanostructured materials in cosmetics. Cosmetics 7:24
Bilal M, Mehmood S, Iqbal H (2020) The beast of beauty: environmental and health concerns of toxic components in cosmetics. Cosmetics 7:13
Bilal M, Iqbal HM (2019) An insight into toxicity and human-health-related adverse consequences of cosmeceuticals—a review. Sci Total Environ 670:555–568
Khan IA, Abourashed EA (2011) Leung’s encyclopedia of common natural ingredients: used in food, drugs and cosmetics. Wiley, New York
Lee S, Youm D (2020) The effects of manufacturing and sales companies and manufacturing companies’ reputation and product types on the perceived product quality: focusing on cosmetic products. J Digit Converg 18:71–81
Sautebin L (2008) Understanding the adverse effects of cosmetics. Drug Saf 31:433–436
Sahota A (ed) (2014) Sustainability: how the cosmetics industry is greening up. Wiley, West Sussex
Dahal RH, Shim DS, Kim J (2017) Development of actinobacterial resources for functional cosmetics. J Cosmet Dermatol 16:243–252
Ergun SO, Urek RO (2017) Production of ligninolytic enzymes by solid state fermentation using Pleurotus ostreatus. Ann Agrar Sci 15:273–277
Chowdhary P, Shukla G, Raj G, Ferreira LFR, Bharagava RN (2019) Microbial manganese peroxidase: a ligninolytic enzyme and its ample opportunities in research. SN Appl Sci 1:1–12
Asgher M, Urooj Y, Qamar SA, Khalid N (2020) Improved exopolysaccharide production from Bacillus licheniformis MS3: optimization and structural/functional characterization. Int J Biol Macromol 151:984–992
Bilal M, Iqbal HM (2020) Recent advancements in the life cycle analysis of lignocellulosic biomass. Curr Sustain Renew Energy Rep 7:100–107
Bilal M, Iqbal HMN (2021) Ligninolysis Potential of Ligninolytic Enzymes: A Green and Sustainable Approach to Bio-transform Lignocellulosic Biomass into High-Value Entities. In: Pathak P, Srivastava RR (eds) Alternative energy resources The Handbook of Environmental Chemistry, vol 99. Springer, Cham, pp 151–171
Mehmood T, Nadeem F, Qamar SA, Bilal M, Iqbal HMN (2021) Bioconversion of agro-industrial waste into value-added compounds. Sustainable bioconversion of waste to value added products, advances in science, technology & innovation. Springer Nature, Dordrecht
Madeira JV Jr, Contesini FJ, Calzado F, Rubio MV, Zubieta MP, Lopes DB, de Melo RR (2017) Agro-industrial residues and microbial enzymes: an overview on the eco-friendly bioconversion into high value-added products. Biotechnol Microbial Enzymes 2017:475–511
De Corato U, De Bari I, Viola E, Pugliese M (2018) Assessing the main opportunities of integrated biorefining from agro-bioenergy co/by-products and agroindustrial residues into high-value added products associated to some emerging markets: a review. Renew Sustain Energy Rev 88:326–346
Singh AK, Bilal M, Iqbal HM, Raj A (2021) Lignin peroxidase in focus for catalytic elimination of contaminants: a critical review on recent progress and perspectives. Int J Biol Macromol 177:58–82
Arevalo-Gallegos A, Ahmad Z, Asgher M, Parra-Saldivar R, Iqbal HM (2017) Lignocellulose: a sustainable material to produce value-added products with a zero-waste approach: a review. Int J Biol Macromol 99:308–318
Bilal M, Asgher M, Iqbal HM, Hu H, Zhang X (2017) Biotransformation of lignocellulosic materials into value-added products: a review. Int J Biol Macromol 98:447–458
Asgher M, Afzal M, Qamar SA, Khalid N (2020) Optimization of biosurfactant production from chemically mutated strain of Bacillus subtilis using waste automobile oil as low-cost substrate. Environ Sustain 3:405–413
Asgher M, Arshad S, Qamar SA, Khalid N (2020) Improved biosurfactant production from Aspergillus niger through chemical mutagenesis: characterization and RSM optimization. SN Appl Sci 2:1–11
Asgher M, Rani A, Khalid N, Qamar SA, Bilal M (2021) Bioconversion of sugarcane molasses waste to high-value exopolysaccharides by engineered Bacillus licheniformis. Case Stud Chem Environ Eng 2021:100084
Bilal M, Iqbal HM (2019) Sustainable bioconversion of food waste into high-value products by immobilized enzymes to meet bio-economy challenges and opportunities: a review. Food Res Int 123:226–240
Bilal M, Asgher M (2016) Enhanced catalytic potentiality of Ganoderma lucidum IBL-05 manganese peroxidase immobilized on sol-gel matrix. J Mol Catal B 128:82–93
Qamar SA, Asgher M, Bilal M (2020) Immobilization of alkaline protease from Bacillus brevis using Ca-alginate entrapment strategy for improved catalytic stability, silver recovery, and dehairing potentialities. Catal Lett 150:3572–3583
Asgher M, Asad MJ, Legge RL (2006) Enhanced lignin peroxidase synthesis by Phanerochaete chrysosporium in solid state bioprocessing of a lignocellulosic substrate. World J Microbiol Biotechnol 22:449–453
Asgher M, Qamar SA, Bilal M, Iqbal HM (2020) Bio-based active food packaging materials: sustainable alternative to conventional petrochemical-based packaging materials. Food Res. Intl. 2020:109625
Asgher M, Wahab A, Bilal M, Iqbal HMN (2016) Lignocellulose degradation and production of lignin modifying enzymes by Schizophyllum commune IBL-06 in solid-state fermentation. Biocatal Agric Biotechnol 6:195–201
Rehman S, Bhatti HN, Bilal M, Asgher M (2019) Optimization of process variables for enhanced production of extracellular lipase by Pleurotus ostreatus IBL-02 in solid-state fermentation. Pak J Pharm Sci 2019:32
Jović J, Hao J, Kocić-Tanackov S, Mojović L (2020) Improvement of lignocellulosic biomass conversion by optimization of fungal ligninolytic enzyme activity and molasses stillage supplementation. Biomass Convers 2020:1–17
Ruiz-Duenas FJ, Fernández E, Martínez MJ, Martínez AT (2011) Pleurotus ostreatus heme peroxidases: an in silico analysis from the genome sequence to the enzyme molecular structure. CR Biol 334:795–805
Das A, Mondal C, Roy S (2015) Pretreatment methods of ligno-cellulosic biomass: a review. J Eng Sci Technol 2015:8
Yoo CG, Meng X, Pu Y, Ragauskas AJ (2020) The critical role of lignin in lignocellulosic biomass conversion and recent pretreatment strategies: a comprehensive review. Bioresour Technol 301:122784
Ijaz A, Anwar Z, Zafar Y, Hussain I, Muhammad A, Irshad M, Mehmood S (2011) Optimization of cellulase enzyme production from corn cobs using Alternaria alternata by solid state fermentation. J Cell Mol Biol 9:51–56
Asgher M, Iqbal HMN, Irshad M (2012) Characterization of purified and xerogel immobilized novel lignin peroxidase produced from Trametes versicolor IBL-04 using solid state medium of corncobs. BMC Biotechnol 12:1–8
Munir N, Asgher M, Tahir IM, Riaz M, Bilal M, Shah SA (2015) Utilization of agro-wastes for production of ligninolytic enzymes in liquid state fermentation by Phanerochaete chrysosporium-IBL-03. IJCBS 7:9–14
Aslam S, Asgher M (2011) Partial purification and characterization of ligninolytic enzymes produced by Pleurotus ostreatus during solid state fermentation. Afr J Biotechnol 10:17875–17883
Ghany TMA, Bakri MM, Al-Rajhi AM, Al Abboud MA, Alawlaqi MM, Shater ARM (2020) Impact of copper and its nanoparticles on growth, ultrastructure, and laccase production of Aspergillus niger using corn cobs wastes. BioResources 15:3289–3306
Yuliana T, Putri NZ, Komara DZ, Mardawati E, Lanti I, Rahimah S (2020) Study of Ganoderma lucidum in laccase production using corncob and paddies straw substrates on submerged fermentation system. Pak J Biol Sci 23:1060–1065
Andriani A, Sukorini A, Perwitasari U (2019) Enhancement of laccase production in a new isolated Trametes hirsuta LBF-AA017 by lignocellulosic materials and its application for removal of chemical dyes. IOP conference series: earth and environmental science. IOP Publishing, New York, p 012015
Gupta A, Jana AK (2019) Production of laccase by repeated batch semi-solid fermentation using wheat straw as substrate and support for fungal growth. Bioproc Biosyst Eng 42:499–512
Shradhdha S, Murty DS (2020) Production of lignolytic and cellulolytic enzymes by using basidiomycetes fungi in the solid state fermentation of different agro-residues. Res J Biotechnol 15:9
Elisashvili V, Kachlishvili E, Asatiani MD (2018) Efficient production of lignin-modifying enzymes and phenolics removal in submerged fermentation of olive mill by-products by white-rot basidiomycetes. Int Biodeterior Biodegrad 134:39–47
Khan MU, Ahring BK (2019) Lignin degradation under anaerobic digestion: Influence of lignin modifications: a review. Biomass Bioenergy 128:105325
Bilal M, Iqbal HM (2020) Ligninolytic enzymes mediated ligninolysis: an untapped biocatalytic potential to deconstruct lignocellulosic molecules in a sustainable manner. Catal Lett 150:524–543
Wong DW (2009) Structure and action mechanism of ligninolytic enzymes. Appl Biochem Biotechnol 157:174–209
Asgher M, Nasir I, Khalid N, Qamar SA (2020) Development of biocomposites based on bacterial cellulose reinforced delignified rice husk-PVA plasticized with glycerol. J Polym Res 27:1–11
Khalid N, Asgher M, Qamar SA (2020) Evolving trend of Boletus versicolor IBL-04 by chemical mutagenesis to overproduce laccase: process optimization, 3-step purification, and characterization. Ind Crop Prod 155:112771
Gasser CA, Hommes G, Schäffer A, Corvini PFX (2012) Multi-catalysis reactions: new prospects and challenges of biotechnology to valorize lignin. Appl Microbiol Biotechnol 95:1115–1134
Giardina P, Faraco V, Pezzella C, Piscitelli A, Vanhulle S, Sannia G (2010) Laccases: a never-ending story. Cell Mol Life Sci 67:369–385
Mayer AM, Staples RC (2002) Laccase: new functions for an old enzyme. Phytochemistry 60:551–565
Sharma KK, Kuhad RC (2008) Laccase: enzyme revisited and function redefined. Indian J Microbiol 48:309
Amara S, Perrot T, Navarro D, Deroy A, Benkhelfallah A, Chalak A, Record E (2018) Enzyme activities of two recombinant heme-containing peroxidases, TvDyP1 and TvVP2, identified from the secretome of Trametes versicolor. Appl Environ Microbiol 2018:84
Mori T, Wang J, Tanaka Y, Nagai K, Kawagishi H, Hirai H (2017) Bioremediation of the neonicotinoid insecticide clothianidin by the white-rot fungus Phanerochaete sordida. J Hazard Mater 321:586–590
Wang J, Tanaka Y, Ohno H, Jia J, Mori T, Xiao T, Hirai H (2019) Biotransformation and detoxification of the neonicotinoid insecticides nitenpyram and dinotefuran by Phanerochaete sordida YK-624. Environ Pollut 252:856–862
Meehnian H, Jana AK, Jana MM (2017) Pretreatment of cotton stalks by synergistic interaction of Daedalea flavida and Phlebia radiata in co-culture for improvement in delignification and saccharification. Int Biodeterior Biodegrad 117:68–77
Mäkinen MA, Risulainen N, Mattila H, Lundell TK (2018) Transcription of lignocellulose-decomposition associated genes, enzyme activities and production of ethanol upon bioconversion of waste substrate by Phlebia radiata. Appl Microbiol Biotechnol 102:5657–5672
Sadaqat B, Khatoon N, Malik AY, Jamal A, Farooq U, Ali MI, Huang Z (2020) Enzymatic decolorization of melanin by lignin peroxidase from Phanerochaete chrysosporium. Sci Rep 10:1–10
Seo H, Kim KJ, Kim YH (2018) In silico-designed lignin peroxidase from Phanerochaete chrysosporium shows enhanced acid stability for depolymerization of lignin. Biotechnol Biofuels 11:1–13
Falade AO, Eyisi OA, Mabinya LV, Nwodo UU, Okoh AI (2017) Peroxidase production and ligninolytic potentials of fresh water bacteria Raoultella ornithinolytica and Ensifer adhaerens. Biotechnol Rep 16:12–17
Paz A, Costa-Trigo I, de Souza Oliveira RP, Domínguez JM (2020) Ligninolytic enzymes of endospore-forming Bacillus aryabhattai BA03. Curr Microbiol 77:702–709
de Cassia Pereira J, Giese EC, de Souza Moretti MM, dos Santos Gomes AC, Perrone OM, Boscolo M, Martins DAB (2017) Effect of metal ions, chemical agents and organic compounds on lignocellulolytic enzymes activities. Enzyme Inhib Activators 29:139–164
Cho UM, Choi DH, Yoo DS, Park SJ, Hwang HS (2019) Inhibitory effect of ficin derived from fig latex on inflammation and melanin production in skin cells. Biotechnol Bioproc Eng 24:288–297
Huang WS, Wang YW, Hung KC, Hsieh PS, Fu KY, Dai LG, Dai NT (2018) High correlation between skin color based on CIELAB color space, epidermal melanocyte ratio, and melanocyte melanin content. PeerJ 6:e4815
Mori K, Ando I, Kukita A (2001) Generalized hyperpigmentation of the skin due to vitamin B12 deficiency. J Dermatol 28:282–285
Ito S, Wakamatsu K, Sarna T (2018) Photodegradation of eumelanin and pheomelanin and its pathophysiological implications. Photochem Photobiol 94:409–420
Smith DF, Casadevall A (2019) The role of melanin in fungal pathogenesis for animal hosts. Fungal Physiol Immunopathog 2019:1–30
Nosanchuk JD, Casadevall A (2006) Impact of melanin on microbial virulence and clinical resistance to antimicrobial compounds. Antimicrob Agen Chemother 50:3519–3528
Geib E, Gressler M, Viediernikova I, Hillmann F, Jacobsen ID, Nietzsche S, Brock M (2016) A non-canonical melanin biosynthesis pathway protects Aspergillus terreus conidia from environmental stress. Cell Chem Biol 23:587–597
De Marchi F, Galeotti G, Simenas M, Tornau EE, Pezzella A, MacLeod J, Rosei F (2018) Room-temperature surface-assisted reactivity of a melanin precursor: silver metal–organic coordination versus covalent dimerization on gold. Nanoscale 10:16721–16729
Cordero RJ, Casadevall A (2020) Melanin. Curr Biol 30:R142–R143
Smit N, Vicanova J, Pavel S (2009) The hunt for natural skin whitening agents. Int J Mol Sci 10:5326–5349
Jacobsen JA, Fullagar JL, Miller MT, Cohen SM (2011) Identifying chelators for metalloprotein inhibitors using a fragment-based approach. J Med Chem 54:591–602
Ullah S, Park C, Ikram M, Kang D, Lee S, Yang J, Moon HR (2019) Tyrosinase inhibition and anti-melanin generation effect of cinnamamide analogues. Bioorg Chem 87:43–55
Hua Y, Ma C, Wei T, Zhang L, Shen J (2020) Collagen/chitosan complexes: preparation, antioxidant activity, tyrosinase inhibition activity, and melanin synthesis. Int J Mol Sci 21:313
Bin BH, Kim ST, Bhin J, Lee TR, Cho EG (2016) The development of sugar-based anti-melanogenic agents. Int J Mol Sci 17:583
Chaowattanapanit S, Silpa-Archa N, Kohli I, Lim HW, Hamzavi I (2017) Postinflammatory hyperpigmentation: a comprehensive overview: treatment options and prevention. J Am Acad Dermatol 77:607–621
Migas P, Krauze-Baranowska M (2015) The significance of arbutin and its derivatives in therapy and cosmetics. Phytochem Lett 13:35–40
Tabolacci C, Rossi S, Lentini A, Provenzano B, Turcano L, Facchiano F, Beninati S (2013) Aloin enhances cisplatin antineoplastic activity in B16–F10 melanoma cells by transglutaminase-induced differentiation. Amino Acids 44:293–300
Jacobus Berlitz S, De Villa D, Maschmann Inácio LA, Davies S, Zatta KC, Guterres SS, Külkamp-Guerreiro IC (2019) Azelaic acid-loaded nanoemulsion with hyaluronic acid–a new strategy to treat hyperpigmentary skin disorders. Drug Dev Ind Pharm 45:642–650
Singh BK, Park SH, Lee HB, Goo YA, Kim HS, Cho SH, Kim EK (2016) Kojic acid peptide: a new compound with anti-tyrosinase potential. Ann Dermatol 28:555
Chiang HM, Chen HW, Huang YH, Chan SY, Chen CC, Wu WC, Wen KC (2012) Melanogenesis and natural hypopigmentation agents. Int J Med Biol Front 18:1e76
Lajis AFB, Ariff AB (2019) Discovery of new depigmenting compounds and their efficacy to treat hyperpigmentation: evidence from in vitro study. J Cosmetic Dermatol 18:703–727
Tanemura A, Yang L, Yang F, Nagata Y, Wataya-Kaneda M, Fukai K, Katayama I (2015) An immune pathological and ultrastructural skin analysis for rhododenol-induced leukoderma patients. J Dermatol Sci 77:185–188
Tian Y, Hoshino T, Chen CJ et al (2009) The evaluation of whitening efficacy of cosmetic products using a human skin pigmentation spot model. Skin Res Technol 15:218–223
Woo SH, Cho JS, Lee BS, Kim EK (2004) Decolorization of melanin by lignin peroxidase from Phanerochaete chrysosporium. Biotechnol Bioproc Eng 9:256
Shin SK, Hyeon JE, Joo YC, You SK, Han SO (2019) Effective melanin degradation by a synergistic laccase-peroxidase enzyme complex for skin whitening and other practical applications. Int J Biol Macromol 129:181–186
Eom MH, Kim YH (2014) Inactivating effect of phenolic unit structures on the biodegradation of lignin by lignin peroxidase from Phanerochaete chrysosporium. Enzyme Microb Technol 61:48–54
Passardi F, Theiler G, Zamocky M, Cosio C, Rouhier N, Teixera F, Dunand C (2007) PeroxiBase: the peroxidase database. Phytochemistry 68:1605–1611
Chung N, Aust SD (1995) Inactivation of lignin peroxidase by hydrogen peroxide during the oxidation of phenols. Arch Biochem Biophys 316:851–855
Mohorčič M, Friedrich J, Renimel I, André P, Mandin D, Chaumont JP (2007) Production of melanin bleaching enzyme of fungal origin and its application in cosmetics. Biotechnol Bioproc Eng 12:200–206
Khammuang S, Sarnthima R (2013) Decolorization of synthetic melanins by crude laccases of Lentinus polychrous Lév. Folia Microbiol 58:1–7
Sung HJ, Khan MF, Kim YH (2019) Recombinant lignin peroxidase-catalyzed decolorization of melanin using in-situ generated H2O2 for application in whitening cosmetics. Int J Biol Macromol 136:20–26
Rejinold NS, Shin JH, Seok HY, Kim YC (2016) Biomedical applications of microneedles in therapeutics: recent advancements and implications in drug delivery. Expert Opin Drug Deliv 13:109–131
Machado ECFA, Ambrosano L, Lage R, Abdalla BMZ, Costa A (2017) Nutraceuticals for healthy skin aging. Nutrition and functional foods for healthy aging. Academic Press, New York, pp 273–281
Muise A, Desmarais S (2010) Women’s perceptions and use of “anti-aging” products. Sex Roles 63:126–137
Ramos-e-Silva M, Celem LR, Ramos-e-Silva S, Fucci-da-Costa AP (2013) Anti-aging cosmetics: facts and controversies. Clin Dermatol 31:750–758
Karovičová J, Šimko P (2000) Determination of synthetic phenolic antioxidants in food by high-performance liquid chromatography. J Chromatogr A 882:271–281
Silva S, Ferreira M, Oliveira AS, Magalhaes C, Sousa ME, Pinto M, Almeida IF (2019) Evolution of the use of antioxidants in anti-ageing cosmetics. Int J Cosmet Sci 41:378–386
Ferreira-Dias S, Sandoval G, Plou F, Valero F (2013) The potential use of lipases in the production of fatty acid derivatives for the food and nutraceutical industries. Electron J Biotechnol 16:12–12
Ansorge-Schumacher MB, Thum O (2013) Immobilised lipases in the cosmetics industry. Chem Soc Rev 42:6475–6490
Montenegro L, Panico AM, Santagati LM, Siciliano EA, Intagliata S, Modica MN (2019) Solid lipid nanoparticles loading idebenone ester with pyroglutamic acid: in vitro antioxidant activity and in vivo topical efficacy. Nanomaterials 9:43
Ćorović M, Milivojević A, Simović M, Banjanac K, Pjanović R, Bezbradica D (2020) Enzymatically derived oil-based L-ascorbyl esters: synthesis, antioxidant properties and controlled release from cosmetic formulations. Sustain Chem Pharm 15:100231
Park SH, Kim HK (2020) Antibacterial activity of emulsions containing unsaturated fatty acid ergosterol esters synthesized by lipase-mediated transesterification. Enzyme Microb Techol 139:109581
Rmili F, Achouri N, Smichi N, Krayem N, Bayoudh A, Gargouri Y, Fendri A (2019) Purification and biochemical characterization of an organic solvent-tolerant and detergent-stable lipase from Staphylococcus capitis. Biotechnol Progress 35:e2833
Lima RN, dos Anjos CS, Orozco EV, Porto AL (2019) Versatility of Candida antarctica lipase in the amide bond formation applied in organic synthesis and biotechnological processes. Mol Catal 466:75–105
Mauricio T, Karmon Y, Khaiat A (2011) A randomized and placebo-controlled study to compare the skin-lightening efficacy and safety of lignin peroxidase cream vs. 2% hydroquinone cream. J Cosmet Dermatol 10:253–259
Duarte-Vázquez MA, Ortega-Tovar MA, García-Almendarez BE, Regalado C (2003) Removal of aqueous phenolic compounds from a model system by oxidative polymerization with turnip (Brassica napus L. var. purple top white globe) peroxidase. J Chem Technol Biotechnol 78:42–47
Cheng J, Yu SM, Zuo P (2006) Horseradish peroxidase immobilized on aluminum-pillared interlayered clay for the catalytic oxidation of phenolic wastewater. Water Res 40:283–290
Liu ZH, Kanjo Y, Mizutani S (2009) Removal mechanisms for endocrine disrupting compounds (EDCs) in wastewater treatment—physical means, biodegradation, and chemical advanced oxidation: a review. Sci Total Environ 407:731–748
Yu CP, Deeb RA, Chu KH (2013) Microbial degradation of steroidal estrogens. Chemosphere 91:1225–1235
Draelos ZD (2015) A split-face evaluation of a novel pigment-lightening agent compared with no treatment and hydroquinone. J Am Acad Dermatol 72:105–107
Ahuactzin-Pérez M, Tlecuitl-Beristain S, García-Dávila J, Santacruz-Juárez E, González-Pérez M, Gutiérrez-Ruíz MC, Sánchez C (2018) A novel biodegradation pathway of the endocrine-disruptor di (2-ethyl hexyl) phthalate by Pleurotus ostreatus based on quantum chemical investigation. Ecotoxicol Environ Safe 147:494–499
Zhong SM, Sun N, Liu HX, Niu YQ, Wu Y (2015) Reduction of facial pigmentation of melasma by topical lignin peroxidase: a novel fast-acting skin-lightening agent. Exp Ther Med 9:341–344
Acknowledgements
This work was supported by financial support from the Natural Science Foundation of Jiangsu Province (BK20170459), China, the National Natural Science Foundation of China (21808075). Consejo Nacional de Ciencia y Tecnología (CONACYT) is thankfully acknowledged for partially supporting this work under Sistema Nacional de Investigadores (SNI) program awarded to Hafiz M.N. Iqbal (CVU: 735340).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, L., Qamar, S.A., Bilal, M. et al. Broadening the Catalytic Role of Enzymes in Cosmeceutical Sector: A Robust Tool from White Biotechnology. Catal Lett 152, 707–719 (2022). https://doi.org/10.1007/s10562-021-03678-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-021-03678-6