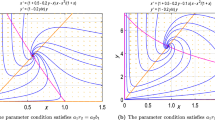
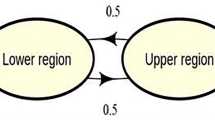
Abstract
Marine microbial primary production is influenced by the availability and uptake of essential nutrients, including iron. Although marine microbes have evolved mechanisms to scavenge sub-nanomolar concentrations of iron, recent observations suggest that viruses may co-opt these very same mechanisms to facilitate infection. The “Ferrojan Horse Hypothesis” proposes that viruses incorporate iron atoms into their tail fiber proteins to adsorb to target host receptors. Here, we propose an evolutionary game theoretic approach to consider the joint strategies of hosts and viruses in environments with limited nutrients (like iron). We analyze the bimatrix game and find that evolutionarily stable strategies depend on the stability and quality of nutrient conditions. For example, in highly stable iron conditions, virus pressure does not change host uptake strategies. However, when iron levels are dynamic, virus pressure can lead to fluctuations in the extent to which hosts invest in metabolic machinery that increases both iron uptake and susceptibility to viral infection. Altogether, this evolutionary game model provides further evidence that viral infection and nutrient dynamics jointly shape the fate of microbial populations.








Similar content being viewed by others
Data availability
All numerical integrations of ODEs were carried out via the fourth-order Runge–Kutta method as implemented by function ode45 in MATLAB v 2017a. Heteroclinic networks were visualized using R v 3.5.2’s [49] igraph v 1.2.2 [50] package. Scripts are available at https://doi.org/10.5281/zenodo.4750365
References
Field CB, Behrenfeld MJ, Randerson JT, Falkowski P (1998) Primary production of the biosphere: Integrating terrestrial and oceanic components. Science 281(5374):237–240
Morel FMM, Price NM (2003) The biogeochemical cycles of trace metals in the oceans. Science 300(5621):944–947
Saito MA, Bertrand EM, Dutkiewicz S, Bulygin VV, Moran DM, Monteiro FM, Follows MJ, Valois FW, Waterbury JB (2011) Iron conservation by reduction of metalloenzyme inventories in the marine diazotroph crocosphaera watsonii. Proc Natl Acad Sci 108(6):2184–2189
Boiteau RM, Mende DR, Hawco NJ, McIlvin MR, Fitzsimmons JN, Saito MA, Sedwick PN, DeLong EF, Repeta DJ (2016) Siderophore-based microbial adaptations to iron scarcity across the eastern pacific ocean. Proc Natl Acad Sci 113(50):14237–14242
Rue EL, Bruland KW (1995) Complexation of iron(iii) by natural organic ligands in the central north pacific as determined by a new competitive ligand equilibration/adsorptive cathodic stripping voltammetric method. Mar Chem 50(1):117–138.
Wu J, Boyle E, Sunda W, Wen L-S (2001) Soluble and colloidal iron in the oligotrophic north atlantic and north pacific. Science 293(5531):847–849
Hogle SL, Dupont CL, Hopkinson BM, King AL, Buck KN, Roe KL, Stuart RK, Allen AE, Mann EL, Johnson ZI, Barbeau KA (2018) Pervasive iron limitation at subsurface chlorophyll maxima of the california current. Proc Natl Acad Sci 115(52):13300–13305
Hutchins DA, Witter AE, Butler A, Luther III GW (1999) Competition among marine phytoplankton for different chelated iron species. Nature 400:858EP–
Pitchford JW, Brindley J (1999) Iron limitation, grazing pressure and oceanic high nutrient-low chlorophyll (HNLC) regions. J Plankton Res 21(3):525–547
Boyd PW (2002) Environmental factors controlling phytoplankton processes in the southern ocean1. J Phycol 38(5):844–861
Coale KH, Johnson KS, Fitzwater SE, Gordon RM, Tanner S, Chavez FP, Ferioli L, Sakamoto C, Rogers P, Millero F, Steinberg P, Nightingale P, Cooper D, Cochlan WP, Landry MR, Constantinou J, Rollwagen G, Trasvina A, Kudela R (1996) A massive phytoplankton bloom induced by an ecosystem-scale iron fertilization experiment in the equatorial pacific ocean. Nature 383(6600):495–501
Trick CG, Bill BD, Cochlan WP, Wells ML, Trainer VL, Pickell LD (2010) Iron enrichment stimulates toxic diatom production in high-nitrate, low-chlorophyll areas. Proc Natl Acad Sci 107(13):5887–5892
Maldonado MT, Boyd PW, LaRoche J, Strzepek R, Waite A, Bowie AR, Croot PL, Frew RD, Price NM (2001) Iron uptake and physiological response of phytoplankton during a mesoscale southern ocean iron enrichment. Limnol Oceanogr 46(7):1802–1808
Wells ML, Trick CG, Cochlan WP, Beall B (2009) Persistence of iron limitation in the western subarctic pacific SEEDS II mesoscale fertilization experiment. Deep-Sea Res II Top Stud Oceanogr 56(26):2810–2821
Cochlan WP (2001) The heterotrophic bacterial response during a mesoscale iron enrichment experiment (ironex ii) in the eastern equatorial pacific ocean. Limnol Oceanogr 46(2):428–435
Scott JE, Li K, Filkins LM, Zhu B, Kuchma SL, Schwartzman JD, OToole GA (2019) Pseudomonas aeruginosa can inhibit growth of streptococcal species via siderophore production. J Bacteriol
Saha M, Sarkar S, Sarkar B, Sharma BK, Bhattacharjee S, Tribedi P (2016) Microbial siderophores and their potential applications: a review. Environ Sci Pollut Res 23(5):3984–3999
Wilhelm SW, Trick CG (1994) Iron-limited growth of cyanobacteria: Multiple siderophore production is a common response. Limnol Oceanogr 39(8):1979–1984
Butler A, Theisen RM (2010) Iron(III)-siderophore coordination chemistry: Reactivity of marine siderophores. Coord Chem Rev 254(3–4):288–296
Hudson RJM, Morel FMM (1990) lron transport in marine phytoplankton: Kinetics of cellular and medium coordination reactions. Limnol Oceanogr 35(5):1002–1020
Niehus R, Picot A, Oliveira NM, Mitri S, Foster KR (2017) The evolution of siderophore production as a competitive trait. Evolution 71(6):1443–1455
Brown SP, Taddei F (2007) The durability of public goods changes the dynamics and nature of social dilemmas. PLoS One 2(7):1–7
Weitz JS, Eksin C, Paarporn K, Brown SP, Ratcliff WC (2016) An oscillating tragedy of the commons in replicator dynamics with game-environment feedback. Proc Natl Acad Sci
Bonnain C, Breitbart M, Buck KN (2016) The ferrojan horse hypothesis: Iron-virus interactions in the ocean. Front Mar Sci 3:82
Bertozzi Silva J, Storms Z, Sauvageau D (2016) Host receptors for bacteriophage adsorption. FEMS Microbiol Lett 363(4):01
Bartual SG, Otero JM, Garcia-Doval C, Llamas-Saiz AL, Kahn R, Fox GC, van Raaij MJ (2010) Structure of the bacteriophage t4 long tail fiber receptor-binding tip. Proc Natl Acad Sci 107(47):20287–20292
Caputi L, Carradec Q, Eveillard D, Kirilovsky A, Pelletier E, Pierella Karlusich JJ, Rocha Jimenez Vieira F, Villar E, Chaffron S, Malviya S, Scalco E, Acinas SG, Alberti A, Aury J-M, Benoiston A-S, Bertrand A, Biard T, Bittner L, Boccara M, Brum JR, Brunet C, Busseni G, Carratalá A, Claustre H, Coelho LP, Colin S, D’Aniello S, Da Silva C, Del Core M, Doré H, Gasparini S, Kokoszka F, Jamet J-L, Lejeusne C, Lepoivre C, Lescot M, Lima-Mendez G, Lombard F, Lukeš J, Maillet N, Madoui M-A, Martinez E, Mazzocchi MG, Néou MB, Paz-Yepes J, Poulain J, Ramondenc S, Romagnan J-B, Roux S, Salvagio Manta D, Sanges R, Speich S, Sprovieri M, Sunagawa S, Taillandier V, Tanaka A, Tirichine L, Trottier C, Uitz J, Veluchamy A, Veselá J, Vincent F, Yau S, Kandels-Lewis S, Searson S, Dimier C, Picheral M, Coordinators TO, Bork P, Boss E, de Vargas C, Follows MJ, Grimsley N, Guidi L, Hingamp P, Karsenti E, Sordino P, Stemmann L, Sullivan MB, Tagliabue A, Zingone A, Garczarek L, d’Ortenzio F, Testor P, Not F, d’Alcalà MR, Wincker P, Bowler C, Iudicone D (2019) Community-level responses to iron availability in open ocean plankton ecosystems. Glob Biogeochem Cycles 33(3):391–419
Fuhrman JA (1999) Marine viruses and their biogeochemical and ecological effects. Nature 399:541EP–
Thingstad TF (2000) Elements of a theory for the mechanisms controlling abundance, diversity, and biogeochemical role of lytic bacterial viruses in aquatic systems. Limnol Oceanogr 45(6):1320–1328
Menge DN, Weitz JS (2009) Dangerous nutrients: Evolution of phytoplankton resource uptake subject to virus attack. J Theor Biol 257(1):104–115
Lee W, van Baalen M, Jansen VA (2016) Siderophore production and the evolution of investment in a public good: An adaptive dynamics approach to kin selection. J Theor Biol 388:61–71
Hofbauer J (1996) Evolutionary dynamics for bimatrix games: A hamiltonian system? J Math Biol 34(5):675
Tilman AR, Plotkin J, Akcay E (2019) Evolutionary games with environmental feedbacks. bioRxiv
Rabsch W, Ma L, Wiley G, Najar FZ, Kaserer W, Schuerch DW, Klebba JE, Roe BA, Gomez JAL, Schallmey M, Newton SMC, Klebba PE (2007) Fepa- and tonb-dependent bacteriophage h8: Receptor binding and genomic sequence. J Bacteriol 189(15):5658–5674
Poorvin L, Rinta-Kanto JM, Hutchins DA, Wilhelm SW (2004) Viral release of iron and its bioavailability to marine plankton. Limnol Oceanogr 49(5):1734–1741
Poorvin L, Sander SG, Velasquez I, Ibisanmi E, LeCleir GR, Wilhelm SW (2011) A comparison of fe bioavailability and binding of a catecholate siderophore with virus-mediated lysates from the marine bacterium vibrio alginolyticus pwh3a. J Exp Mar Biol Ecol 399(1):43–47
Gong L, Gao J, Cao M (2018) Evolutionary Game Dynamics for Two Interacting Populations under Environmental Feedback. ArXiv e-prints
Krupa M (1997) Robust heteroclinic cycles. Journal of Nonlinear Science 7(2):129–176
Hofbauer J (1994) Heteroclinic cycles in ecological differential equations. Equadiff 8:105–116
Brannath W (1994) Heteroclinic networks on the tetrahedron. Nonlinearity 7(5):1367–1384
Lin Y-H, Weitz JS (2019) Spatial interactions and oscillatory tragedies of the commons. Phys Rev Lett 122:148102
Hilbe C, Šimsa Š, Chatterjee K, Nowak MA (2018) Evolution of cooperation in stochastic games. Nature 559(7713):246–249
Seymour J, Seuront L, Mitchell J (2007) Microscale gradients of planktonic microbial communities above the sediment surface in a mangrove estuary. Estuar Coast Shelf Sci 73(3):651–666
Satinsky BM, Crump BC, Smith CB, Sharma S, Zielinski BL, Doherty M, Meng J, Sun S, Medeiros PM, Paul JH, Coles VJ, Yager PL, Moran MA (2014) Microspatial gene expression patterns in the amazon river plume. Proc Natl Acad Sci 111(30):11085–11090
Seymour J, Seuront L, Doubell M, Waters R, Mitchell J (2006) Microscale patchiness of virioplankton. J Mar Biol Assoc U. K. 86(3):551–561
Palma M, Worgall S, Quadri LEN (2003) Transcriptome analysis of the pseudomonas aeruginosa response to iron. Arch Microbiol 180(5):374–379
Morrissey J, Bowler C (2012) Iron utilization in marine cyanobacteria and eukaryotic algae. Front Microbiol 3:43
Daughney CJ, Chtellier X, Chan A, Kenward P, Fortin D, Suttle CA, Fowle DA (2004) Adsorption and precipitation of iron from seawater on a marine bacteriophage (pwh3a-p1). Mar Chem 91(1):101–115
Core R (2018) Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria
Csardi G, Nepusz T (2006) The igraph software package for complex network research. InterJournal Complex Systems:1695
Acknowledgements
The authors would like to thank Stephen Abedon, David Talmy, Mya Breitbart, and Rachel Kuske for their helpful input on manuscript drafts. The authors also thank Stephen J Beckett for code review, and the Simons Collaboration on Ocean Processes and Ecology (SCOPE) community for their support.
Funding
This research was funded by the Simons Foundation (SCOPE Award ID 329018 and 721231).
Author information
Authors and Affiliations
Contributions
DM and JSW conceived the work, DM performed analysis, and DM and JSW wrote and edited the manuscript.
Corresponding authors
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Muratore, D., Weitz, J.S. Infect while the iron is scarce: nutrient-explicit phage-bacteria games. Theor Ecol 14, 467–487 (2021). https://doi.org/10.1007/s12080-021-00508-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12080-021-00508-8