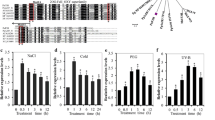
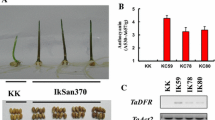
Abstract
Reaumuria trigyna, a Tamaricaceae archaic recretohalophyte, is an important feral forage plant in the desert steppe of northwestern China. We identified two significantly differentially expressed leucoanthocyanidin dioxygenase genes (RtLDOX/RtLDOX2) and investigated the function and characteristics of RtLDOX2. RtLDOX2 from R. trigyna was rapidly upregulated by salt, drought, and abscisic acid, consistent with the stress-related cis-regulatory elements in the promoter region. Recombinant RtLDOX2 converted dihydrokaempferol to kaempferol in vitro, and was thus interchangeable with flavonol synthase, a dioxygenase in the flavonoid pathway. Transgenic plants overexpressing RtLDOX2 accumulated more anthocyanin and flavonols under abiotic stresses, speculating that RtLDOX2 may act as a multifunctional dioxygenase in the synthesis of anthocyanins and flavonols. Overexpression of RtLDOX2 enhanced the primary root length, biomass accumulation, and chlorophyll content of salt-, drought-, and ultraviolet-B-stressed transgenic Arabidopsis. Antioxidant enzyme activity; proline content; and expression of antioxidant enzyme, proline biosynthesis, and ion-transporter genes were increased in transgenic plants. Therefore, RtLDOX2 confers tolerance to abiotic stress on transgenic Arabidopsis by promoting the accumulation of anthocyanins and flavonols. This in turn increases reactive oxygen species scavenging and activates other stress responses, such as osmotic adjustment and ion transport, and so improves tolerance to abiotic stresses.








Similar content being viewed by others
Abbreviations
- ORF:
-
Open reading frame
- RT-PCR:
-
Reverse transcription-polymerase chain reaction
- PA:
-
Proanthocyanidins
- SOD:
-
Superoxide dismutase
- POD:
-
Peroxidase
- CAT:
-
Catalase
- ROS:
-
Reactive oxygen species
- MDA:
-
Malondialdehyde
References
Abrahams S, Lee E, Walker AR, Tanner GJ, Larkin PJ, Ashton AR (2003) The Arabidopsis TDS4 gene encodes leucoanthocyanidin dioxygenase (LDOX) and is essential for proanthocyanidin synthesis and vacuole development. Plant J 35:624–636
Agati G, Azzarello E, Pollastri S, Tattini M (2012) Flavonoids as antioxidants in plants: location and functional significance. Plant Sci 196:67–76
Ahmed NU, Park JI, Jung HJ, Hur Y, Nou IS (2015) Anthocyanin biosynthesis for cold and freezing stress tolerance and desirable color in Brassica rapa. Funct Integr Genomics 15:383–394
Baba SA, Ashraf N (2019) Functional characterization of flavonoid 3′-hydroxylase, CsF3′ H, from Crocus sativus L.: Insights into substrate specificity and role in abiotic stress. Arch Biochem Biophys 667:70–78
Badawi GH, Kawano N, Yamauchi Y, Shimada E, Sasaki R, Kubo A, Tanaka K (2004) Overexpression of ascorbate peroxidase in tobacco chloroplasts enhances the tolerance to salt stress and water deficit. Physiol Plant 121:231–238
Baek YS, Song NY, Nam TG, Kim DO, Kang HC, Kwon OK, Baek NI (2015) Flavonoids from Fragaria ananassa calyx and their antioxidant capacities. J Korean Soc Appl Biol Chem 58:787–793
Benimhon Z, Judeinstein S, Trainin T, Harel-Beja R, Baraakov I, Borochoveori H, Holland D (2015) A “White” anthocyanin-less pomegranate (Punica granatum L.) caused by an insertion in the coding region of the Leucoanthocyanidin Dioxygenase (LDOX; ANS) gene. PLoS ONE 10:142777
Blumwald E (2000) Sodium transport and salt tolerance in plants. Curr Opin Cell Biol 12:431–434
Cao L, Xu X, Chen S, Ma H (2016) Cloning and expression analysis of Ficus carica anthocyanidin synthase 1 gene. Sci Hortic 211:369–375
Cui LG, Shan JX, Shi M, Gao JP, Lin HX (2014) ThemiR156-SPL9-DFRpathway coordinates the relationship between development and abiotic stress tolerance in plants. Plant J 80:1108–1117. https://doi.org/10.1111/tpj.12712
Dang ZH, Zheng LL, Wang J, Gao Z, Wu SB, Qi Z, Wang YC (2013) Transcriptomic profiling of the salt-stress response in the wild recretohalophyte Reaumuria trigyna. BMC Genomics 14:29
Eltayeb AE, Kawano N, Badawi GH, Kaminaka H, Sanekata T, Shibahara T, Inanaga S, Tanaka K (2007) Overexpression of monodehydroascorbate reductase in transgenic tobacco confers enhanced tolerance to ozone, salt and polyethylene glycol stresses. Planta 225:1255–1264
Falcone Ferreyra ML, Rius S, Emiliani J, Pourcel L, Feller A, Morohashi K, Grotewold E (2010) Cloning and characterization of a UV-B-inducible maize flavonol synthase. Plant J 62:77–91
Foyer CH, Noctor G (2005) Redox homeostasis and antioxidant signaling: a metabolic interface between stress perception and physiological responses. Plant Cell 17:1866–1875
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930
Gou JY, Felippes FF, Liu CJ, Weigel D, Wang JW (2011) Negative regulation of anthocyanin biosynthesis in Arabidopsis by a miR156-targeted SPL transcription factor. Plant Cell 23:1512–1522
Guo J, Han W, Wang M (2008) Ultraviolet and environmental stresses involved in the induction and regulation of anthocyanin biosynthesis: a review. Afr J Biotechnol 7:1–4
Halliwell B (2006) Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant Physiol 141:312–322
Han G, Wang Y, Fang S (2011) Climate change over the Qinghai-Tibet Plateau and its impacts on local agriculture and animal husbandry in the last 50 years. Resour Sci 33:1969–1975
Han XJ, Wu YF, Gao S, Yu HN, Xu RX, Lou HX, Cheng AX (2014) Functional characterization of a Plagiochasma appendiculatum flavone synthase I showing flavanone 2-hydroxylase activity. FEBS Lett 588:2307–2314
Harborne JB, Williams CA (2000) Advances in flavonoid research since 1992. Phytochemistry 55:481–504
Hazman M, Hause B, Eiche E, Nick P, Riemann M (2015) Increased tolerance to salt stress in OPDA-deficient rice ALLENE OXIDE CYCLASE mutants is linked to an increased ROS-scavenging activity. J Exp Bot 66:3339–3352
Heller W, Forkmann G, Britsch L, Grisebach H (1985) Enzymatic reduction of (+)-dihydroflavonols to flavan-3, 4-cis-diols with flower extracts from Matthiola incana and its role in anthocyanin biosynthesis. Planta 165:284–287
Hernández I, Chacón O, Rodriguez R, Portieles R, López Y, Pujol M, Borrás-Hidalgo O (2009) Black shank resistant tobacco by silencing of glutathione S-transferase. Biochem Biophys Res Commun 387:300–304
Jyothishwaran G, Kotresha D, Selvaraj T, Srideshikan SH, Rajvanshi PK, Jayabaskaran C (2007) A modified freeze–thaw method for efficient transformation of Agrobacterium tumefaciens. Curr Sci 93:770–772
Kim GT, Tsukaya H, Saito Y, Uchimiya H (1999) Changes in the shapes of leaves and flowers upon overexpression of the novel cytochromeP450 in Arabidopsis thaliana. Proc Natl Acad Sci USA 96:9433–9437
Kim YH, Kim CY, Song WK, Park DS, Kwon SY, Lee HS, Kwak S (2008) Overexpression of sweetpotato swpa4 peroxidase results in increased hydrogen peroxide production and enhances stress tolerance in tobacco. Planta 227:867–881
Kim J, Lee WJ, Vu TT, Jeong CY, Hong SW, Lee H (2017) High accumulation of anthocyanins via the ectopic expression of AtDFR confers significant salt stress tolerance in Brassica napus L. Plant Cell Rep 36:1215–1224
Koes R, Verweij W, Quattrocchio F (2005) Flavonoids: a colorful model for the regulation and evolution of biochemical pathways. Trends Plant Sci 10:236–242
Lee K, Jung YJ, Shin SY, Lee YH (2016) The natural flavone eupatorin induces cell cycle arrest at the G2/M phase and apoptosis in HeLa cells. Appl Biol Chem 59:193–199
Li N, Wang L, Zhang W, Takechi K, Takano H, Lin X (2014) Overexpression of UDP-glucose pyrophosphorylase from Larix gmelinii enhances vegetative growth in transgenic Arabidopsis thaliana. Plant Cell Rep 33:779–791
Li N, Wang X, Ma B, Du C, Zheng L, Wang Y (2017a) Expression of a Na+/H+ antiporter RtNHX1 from a recretohalophyte Reaumuria trigyna improved salt tolerance of transgenic Arabidopsis thaliana. J Plant Physiol 218:109–120
Li P, Li YJ, Zhang FJ, Zhang GZ, Jiang XY, Yu HM, Hou BK (2017b) The Arabidopsis UDP-glycosyltransferases UGT79B2 and UGT79B3, contribute to cold, salt and drought stress tolerance via modulating anthocyanin accumulation. Plant J 89:85–103
Li J, Zhao A, Yu M, Li Y, Liu X, Chen X (2018) Function analysis of anthocyanidin synthase from Morus alba L. by expression in bacteria and tobacco. Electr J Biotechnol 36:9–14
Li N, Du C, Ma B, Gao Z, Wu Z, Zheng L, Wang Y (2019) Functional analysis of ion transport properties and salt tolerance mechanisms of RtHKT1 from the Recretohalophyte Reaumuria trigyna. Plant Cell Physiol 60:85–106
Lillo C, Lea US, Ruoff P (2008) Nutrient depletion as a key factor for manipulating gene expression and product formation in different branches of the flavonoid pathway. Plant Cell Environ 31:587–601
Lin SQ, Zhou ZL, Zhang HL, Yin WQ (2015) Phenolic glycosides from the rhizomes of Cyperus rotundus and their antidepressant activity. J Korean Soc Appl Biol Chem 58:685–691
Liu Y, Shi Z, Maximova S, Payne MJ, Guiltinan MJ (2013) Proanthocyanidin synthesis in Theobroma cacao: genes encoding anthocyanidin synthase, anthocyanidin reductase, and leucoanthocyanidin reductase. BMC Plant Biol 13:202
Liu D, He S, Song X, Zhai H, Liu N, Zhang D, Liu Q (2015) IbSIMT1, a novel salt-induced methyltransferase gene from Ipomoea batatas, is involved in salt tolerance. Plant Cell Tissue Organ Cult 120:701–715
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods 25:402–408
Ma D, Sun D, Wang C, Li Y, Guo T (2014) Expression of flavonoid biosynthesis genes and accumulation of flavonoid in wheat leaves in response to drought stress. Plant Physiol Biochem 80:60–66
Mahajan M, Yadav SK (2014) Overexpression of a tea flavanone 3-hydroxylase gene confers tolerance to salt stress and Alternaria solani in transgenic tobacco. Plant Mol Biol 85:551–573
Martens S, Weisshaar B (2009) Metabolomic and genetic analyses of Favonol synthesis in Arabidopsis thaliana support the in vivo involvement of leucoanthocyanidin dioxygenase. Planta 229:427–445
Matsumura T, Tabayashi N, Kamagata Y, Souma C, Saruyama H (2002) Wheat catalase expressed in transgenic rice can improve tolerance against low temperature stress. Physiol Plant 116:317–327
Mittler R, Vanderauwera S, Gollery M, Van Breusegem F (2004) Reactive oxygen gene network ofplants. Trends Plant Sci 9:490–498
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annu Rev Plant Biol 59:651–681
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Plant Physiol 15:473–497
Nakabayashi R, Yonekura-Sakakibara K, Urano K, Suzuki M, Yamada Y, Nishizawa T (2014) Enhancement of oxidative and drought tolerance in arabidopsis by overaccumulation of antioxidant flavonoids. Plant J 77:367–379
Nakajima JI, Tanaka Y, Yamazaki M, Saito K (2001) Reaction mechanism from leucoanthocyanidin to anthocyanidin 3-glucoside, a key reaction for coloring in anthocyanin biosynthesis. J Biol Chem 276:25797–25803
Neugart S, Tobler M, Barnes PW (2019) Different irradiances of UV and PAR in the same ratios alter the flavonoid profiles of Arabidopsis thaliana wild-types and UV-signalling pathway mutants. Photochem Photobiol Sci 18:1685–1699. https://xs.scihub.ltd/https://doi.org/10.1039/C8PP00496J
Ning J, Li X, Hicks LM, Xiong L (2010) A Raf-like MAPKKK gene DSM1 mediates drought resistance through reactive oxygen species scavenging in rice. Plant Physiol 152:876–890
Pang Y, Peel GJ, Wright E, Wang Z, Dixon RA (2007) Early steps in proanthocyanidin biosynthesis in the model legume Medicago truncatula. Plant Physiol 145:601–615
Park S, Kim DH, Park BR, Lee JY, Lim SH (2019) Molecular and functional characterization of Oryza sativa flavonol synthase (OsFLS), a bifunctional dioxygenase. J Agric Food Chem 67:7399–7409. https://doi.org/10.1021/acs.jafc.9b02142
Qi X, Shuai Q, Chen H, Fan L, Zeng Q, He N (2014) Cloning and expression analyses of the anthocyanin biosynthetic genes in mulberry plants. Mol Genet Genomics 289:783–793
Reddy AM, Reddy VS, Scheffler BE, Wienand U, Reddy AR (2007) Novel transgenic rice overexpressing anthocyanidin synthase accumulates a mixture of flavonoids leading to an increased antioxidant potential. Metab Eng 9:95–111
Rice-Evans C, Miller N, Paganga G (1997) Antioxidant properties of phenolic compounds. Trends Plant Sci 2:152–159
Saito K, Kobayashi M, Gong Z, Tanaka Y, Yamazaki M (1999) Direct evidence for anthocyanidin synthase as a 2-oxoglutarate-dependent oxygenase: molecular cloning and functional expression of cDNA from a red forma of Perilla frutescens. Plant J 17:181–189
Selmar D, Kleinwächter M (2013) Stress enhances the synthesis of secondary plant products: the impact of stress-related over-reduction on the accumulation of natural products. Plant Cell Physiol 54:817–826
Shabala S, Mackay A (2011) Ion transport in halophytes. Adv Bot Res 57:151–199
Shi H, Liu G, Wei Y, Chan Z (2018) The zinc-finger transcription factor zat6 is essential for hydrogen peroxide induction of anthocyanin synthesis in Arabidopsis. Plant Mol Biol 97:1–12
Song X, Diao J, Ji J, Wang G, Guan C, Jin C, Wang Y (2016) Molecular cloning and identification of a flavanone 3-hydroxylase gene from Lycium chinense, and its overexpression enhances drought stress in tobacco. Plant Physiol Biochem 98:89–100
Tattini M, Loreto F, Fini A, Guidi L, Brunetti C, Velikova V, Ferrini F (2015) Isoprenoids and phenylpropanoids are part of the antioxidant defense orchestrated daily by drought-stressed P. latanus × acerifolia plants during Mediterranean summers. New Phytol 207:613–626
Toh HC, Wang SY, Chang ST, Chu FH (2013) Molecular cloning and characterization of flavonol synthase in Acacia confusa. Tree Genet Genomes 9:85–92
Turnbull JJ, Nakajima JI, Welford RW, Yamazaki M, Saito K, Schofield CJ (2004) Mechanistic studies on three 2-oxoglutarate-dependent oxygenases of flavonoid biosynthesis anthocyanidin synthase, flavonol synthase, and flavanone 3β-hydroxylase. J Biol Chem 279:1206–1216
Vu TT, Jeong CY, Nguyen HN, Lee D, Lee SA, Kim JH, Lee H (2015) Characterization of Brassica napus flavonol synthase involved in flavonol biosynthesis in Brassica napus L. J Agric Food Chem 63:7819–7829
Vu TNT, Le THT, Hoang PH, Sy DT, Vu TTT, Chu HM (2018) Overexpression of the Glycine max chalcone isomerase ( gmchi ) gene in transgenic talinum paniculatum plants. Turk J Bot 42:551–558
Winkel-Shirley B (2001) Flavonoid biosynthesis. A colorful model for genetics, biochemistry, cell biology, and biotechnology. Plant Physiol 126:485–493
Winkel-Shirley B (2002) Biosynthesis of flavonoids and effects of stress. Curr Opin Plant Biol 5:218–223
Xu Z, Mahmood K, Rothstein SJ (2017) ROS induces anthocyanin production via late biosynthetic genes and anthocyanin deficiency confers the hypersensitivity to ROS-generating stresses in Arabidopsis. Plant Cell Physiol 58:1364–1377
Xue Y, Wang YC (2008) Study of character of ions secretion from Reaumuria trigyna. J Desert Res 28:437–442
Yan M, Liu X, Guan C, Chen X, Liu Z (2011) Cloning and expression analysis of an anthocyanidin synthase gene homolog from Brassica juncea. Mol Breed 28:313–322
Yan M, Ding S, Liu L, Yin X, Shu J (2014) Cloning and expression analysis of an anthocyanidin synthase gene homologue from Brassica carinata. J Genet 93:513–516
Ye J, Xu F, Wang G, Chen Q, Tao T, Song Q (2017) Molecular cloning and characterization of an anthocyanidin synthase gene in Prunus persica (L.) Batsch. Notulae Botanicae Horti Agrobotanici Cluj-Napoca 45:28–35
You J, Zong W, Hu H, Li X, Xiao J, Xiong L (2014) A STRESS-RESPONSIVE NAC1-regulated protein phosphatase gene rice protein phosphatase18 modulates drought and oxidative stress tolerance through abscisic acid-independent reactive oxygen species scavenging in rice. Plant Physiol 166:2100–2114
Zhang J, Han ZY, Tian J, Zhang X, Song TT, Yao YC (2015) The expression level of anthocyanidin synthase determines the anthocyanin content of crabapple (Malus sp.) petals. Acta Physiol Plant 37:109
Zhang H, Du C, Wang Y, Wang J, Zheng L, Wang Y (2016) The Reaumuria trigyna leucoanthocyanidin dioxygenase (RtLDOX) gene complements anthocyanidin synthesis and increases the salt tolerance potential of a transgenic Arabidopsis ldox mutant. Plant Physiol Biochem 106:278–287
Zhishen J, Mengecheng T, Jianming W (1999) The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem 64:555–559
Zhou W, Huang C, Gong Y, Feng Q, Gao F (2010) Molecular cloning and expression analysis of an ANS gene encoding anthocyanidin synthase from purple-fleshed sweet potato [Ipomoea batatas (L.) Lam]. Plant Mol Biol Rep 28:112–121
Zhu JK (2016) Abiotic stress signaling and responses in plants. Cell 167:313–324
Acknowledgements
We would like to thank Professor Chaomei Ma at Inner Mongolia University, College of Life Sciences for kindly providing the Agilent 1290 Infinity UPLC system for determining recombinant protein activity in vitro.
Funding
This study was supported by the Science and Technology major project of Inner Mongolia Autonomous Region of China to State key laboratory of Reproductive Regulation and Breeding of Grassland Livestock (Grant number: zdzx2018065); the Advanced Talents Research Foundation of Inner Mongolia Agricultural University (Grant number: NDYB2018-11); National Natural Science Foundation of China (Grant numbers: 31760700 and 32060506); and the Natural Science Foundation of Inner Mongolia Autonomous Region (Grant number: 2019BS03035).
Author information
Authors and Affiliations
Contributions
YW, ZQ, LZ conceived the idea; NL, XW, BM performed the experiments; NL wrote the manuscript; ZW assisted in the recombinant protein in vitro activity assay; YW scrutinized the manuscript. All the authors approved the final version of the manuscript prior to submission.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, N., Wang, X., Ma, B. et al. A leucoanthocyanidin dioxygenase gene (RtLDOX2) from the feral forage plant Reaumuria trigyna promotes the accumulation of flavonoids and improves tolerance to abiotic stresses. J Plant Res 134, 1121–1138 (2021). https://doi.org/10.1007/s10265-021-01315-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10265-021-01315-2