Abstract
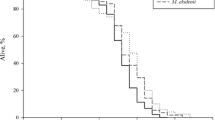
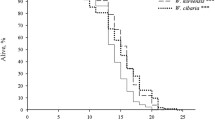
The aim of this study was to investigate the antioxidant activity of Weissella confusa CGMCC 19,308 and its influence on longevity and host defense against Salmonella Typhimurium of Caenorhabditis elegans. The CFCS (cell-free culture supernatant) of W. confusa CGMCC 19,308 possessed DPPH radicals, hydroxyl radicals, and superoxide anion scavenging activity. The lifespan of the C. elegans fed W. confusa CGMCC 19,308 was significantly (p < 0.001) longer than that of worms fed Escherichia coli OP50. Moreover, worms fed W. confusa CGMCC 19,308 were more resistant to oxidative stress induced by hydrogen peroxide and S. Typhimurium infection. RNA-seq analysis showed that the most significantly differentially expressed genes (DEGs) in C. elegans fed with W. confusa CGMCC 19,308 were mainly col genes (col-43, col-2, col-40, col-155, col-37), glutathione–S-transferase (GST)-related genes (gst-44, gst-9, gst-17, gst-18, gstk-2), cnc-9 (immune-related gene), and sod-5 (superoxide dismutase). These results indicated that cuticle collagen synthesis, immunity, and antioxidant defense (AOD) system of C. elegans were affected after being fed with W. confusa CGMCC 19,308 instead of E. coli OP50. Our study suggested W. confusa CGMCC 19,308 had the antioxidant activity and could prolong lifespan and enhance the host defense against S. Typhimurium of C. elegans. This study provided new evidences for the W. confusa CGMCC 19,308 as a potential probiotic candidate for anti-aging and anti-bacterial infection.




Similar content being viewed by others
Data Availability
The authors confirm that the data supporting the findings of this study are available within the article.
References
Joint FAO/WHO Working Group Report on Drafting Guidelines for the Evaluation of Probiotics in Food London, Ontario, Canada, April 30 and May 1, 2002. https://www.who.int/foodsafety/fs_management/en/probiotic_guidelines.pdf. Accessed on 1 May 2002.
Park KY, Jeong JK, Lee YE, DailyIII JW (2014) Health benefits of kimchi (Korean fermented vegetables) as a probiotic food. J Med Food 17:6–20. https://doi.org/10.1089/jmf.2013.3083
Nakagawa H, Shiozaki T, Kobatake E, Hosoya T, Moriya T, Sakai F, Taru H, Miyazaki T (2016) Effects and mechanisms of prolongevity induced by Lactobacillus gasseri SBT2055 in Caenorhabditis elegans. Aging Cell 15:227–236. https://doi.org/10.1111/acel.12431
Grompone G, Martorell P, Llopis S, Gonzalez N, Genoves S, Paula Mulet A, Fernandez-Calero T, Tiscornia I, Bollati-Fogolin M, Chambaud I, Foligne B, Montserrat A, Ramon D (2012) Anti-inflammatory Lactobacillus rhamnosus CNCM I-3690 strain protects against oxidative stress and increases lifespan in Caenorhabditis elegans. PLoS ONE 7:e52493. https://doi.org/10.1111/acel.12431
Bjorkroth KJ, Schillinger U, Geisen R, Weiss N, Hoste B, Holzapfel WH, Korkeala HJ, Vandamme P (2002) Taxonomic study of Weissella confusa and description of Weissella cibaria sp nov., detected in food and clinical samples. Int J Syst Evol Microbiol 52:141–148. https://doi.org/10.1099/00207713-52-1-141
Ikeda T, Yasui C, Hoshino K, Arikawa K, Nishikawa Y (2007) Influence of lactic acid bacteria on longevity of Caenorhabditis elegans and host defense against Salmonella entetica serovar Enteritidis. Appl Environ Microbiol 73:6404–6409. https://doi.org/10.1128/aem.00704-07
Collins MD, Samelis J, Metaxopoulos J, Wallbanks S (1993) Taxonomic studies on some leuconostoc-like organisms from fermented sausages: description of a new genus Weissella for the Leuconostoc paramesenteroides group of species. J Appl Bacteriol 75:595–603. https://doi.org/10.1111/j.1365-2672.1993.tb01600.x
Fairfax MR, Lephart PR, Salimnia H (2014) Weissella confusa: problems with identification of an opportunistic pathogen that has been found in fermented foods and proposed as a probiotic. Front Microbiol 5:254. https://doi.org/10.3389/fmicb.2014.00254
Quattrini M, Korcari D, Ricci G, Fortina MG (2020) A polyphasic approach to characterize Weissella cibaria and Weissella confusa strains. J Appl Microbiol 128:500–512. https://doi.org/10.1111/jam.14483
Benhouna IS, Heumann A, Rieu A, Guzzo J, Kihal M, Bettache G, Champion D, Coelho C, Weidmann S (2019) Exopolysaccharide produced by Weissella confusa: chemical characterisation, rheology and bioactivity. Int Dairy J 90:88–94. https://doi.org/10.1016/j.idairyj.2018.11.006
Rizzello CG, Coda R, Wang Y, Verni M, Kajala I, Katina K, Laitila A (2019) Characterization of indigenous Pediococcus pentosaceus, Leuconostoc kimchii, Weissella cibaria and Weissella confusa for faba bean bioprocessing. Int J Food Microbiol 302:24–34. https://doi.org/10.1016/j.ijfoodmicro.2018.08.014
Le B, Yang SH (2018) Isolation of Weissella strains as potent probiotics to improve antioxidant activity of salted squid by fermentation. J Appl Biol Chem 61:93–100. https://doi.org/10.3839/jabc.2018.014
Ahamefule CS, Qin Q, Odiba AS, Li S, Moneke AN, Ogbonna JC, Jin C, Wang B, Fang W (2020) Caenorhabditis elegans-based Aspergillus fumigatus infection model for evaluating pathogenicity and drug efficacy. Front Cell Infect Microbiol 10:320. https://doi.org/10.3389/fcimb.2020.00320
Hulme SE, Whitesides GM (2011) Chemistry and the worm: Caenorhabditis elegans as a platform for integrating chemical and biological research. Angew Chem Int Ed Engl 50:4774–4807. https://doi.org/10.1002/anie.201005461
Gruber J, Ng LF, Poovathingal SK, Halliwell B (2009) Deceptively simple but simply deceptive - Caenorhabditis elegans lifespan studies: considerations for aging and antioxidant effects. FEBS Lett 583:3377–3387. https://doi.org/10.1016/j.febslet.2009.09.051
Kurz CL, Tan MW (2004) Regulation of aging and innate immunity in C. elegans. Aging Cell 3:185–193. https://doi.org/10.1111/j.1474-9728.2004.00108.x
Wang W, Liu W, Chu W (2020) Isolation and preliminary screening of potentially probiotic Weissella confusa strains from healthy human feces by culturomics. Microb Pathog 147:104356. https://doi.org/10.1016/j.micpath.2020.104356
Brenner S (1974) The genetics of Caenorhabditis elegans. Genetics 77:71–94
Stiernagle T (2006) Maintenance of C. elegans. WormBook, ed. The C. elegans Research Community, WormBook, https://doi.org/10.1895/wormbook.1.101.1
Shi Y, Cui X, Gu S, Yan X, Li R, Xia S, Chen H, Ge J (2019) Antioxidative and probiotic activities of lactic acid bacteria isolated from traditional artisanal milk cheese from northeast China. Probiotics Antimicrob Proteins 11:1086–1099. https://doi.org/10.1007/s12602-018-9452-5
Yu HS, Jang HJ, Lee NK, Paik HD (2019) Evaluation of the probiotic characteristics and prophylactic potential of Weissella cibaria strains isolated from kimchi. LWT 112:108229. https://doi.org/10.1016/j.lwt.2019.05.127
Shivangi S, Devi PB, Ragul K, Shetty PH (2020) Probiotic potential of Bacillus strains isolated from an acidic fermented food Idli. Probiotics Antimicrob Proteins 12:1502–1513. https://doi.org/10.1007/s12602-020-09650-x
Azat R, Liu Y, Li W, Kayir A, Lin DB, Zhou WW, Zheng XD (2016) Probiotic properties of lactic acid bacteria isolated from traditionally fermented Xinjiang cheese. J Zhejiang Univ Sci B 17:597–609. https://doi.org/10.1631/jzus.B1500250
Martorell P, Forment JV, de Llanos R, Monton F, Llopis S, Gonzalez N, Genoves S, Cienfuegos E, Monzo H, Ramon D (2011) Use of Saccharomyces cerevisiae and Caenorhabditis elegans as model organisms to study the effect of cocoa polyphenols in the resistance to oxidative stress. J Agric Food Chem 59:2077–2085. https://doi.org/10.1021/jf104217g
Kato M, Hamazaki Y, Sun S, Nishikawa Y, Kage-Nakadai E (2018) Clostridium butyricum MIYAIRI 588 increases the lifespan and multiple-stress resistance of Caenorhabditis elegans. Nutrients 10:1921. https://doi.org/10.3390/nu10121921
Ling Y, Teng LL, Hua J, Li DS, Luo SH, Liu YC, Liu Y, Li SH (2019) Leucosceptroid B from glandular trichomes of Leucosceptrum canum reduces fat accumulation in Caenorhabditis elegans through suppressing unsaturated fatty acid biosynthesis. Chin J Nat Med 17:892–899. https://doi.org/10.1016/S1875-5364(19)30109-8
Dar MA, Ahmed R, Urwat U, Ahmad SM, Dar PA, Kushoo ZA, Dar TA, Mumtaz PT, Bhat SA, Amin U, Shabir N, Bhat HF, Shah RA, Ganai NA, Heidari M (2018) Expression kinetics of natural resistance associated macrophage protein (NRAMP) genes in Salmonella Typhimurium-infected chicken. BMC Vet Res 14:1746–6148. https://doi.org/10.1186/s12917-018-1510-4
Ankaiah D, Mitra S, Srivastava D, Madasamy S, Ayyanna R, Jha N, Arul V (2021) Probiotic characterization of bacterial strains from fermented South Indian tomato pickle and country chicken intestine having antioxidative and antiproliferative activities. J Appl Microbiol. https://doi.org/10.1111/jam.14991. Online ahead of print.
Xiong L, Ni X, Niu L, Zhou Y, Wang Q, Khalique A, Liu Q, Zeng Y, Shu G, Pan K, Jing B, Zeng D (2019) Isolation and preliminary screening of a Weissella confusa strain from giant panda (Ailuropoda melanoleuca). Probiotics Antimicrob Proteins 11:535–544. https://doi.org/10.1007/s12602-018-9402-2
Lakra AK, Domdi L, Hanjon G, Tilwani YM, Arul V (2020) Some probiotic potential of Weissella confusa MD1 and Weissella cibaria MD2 isolated from fermented batter. LWT 125:109261. https://doi.org/10.1016/j.lwt.2020.109261
Lakra AK, Ramatchandirane M, Kumar S, Suchiang K, Arul V (2021) Physico-chemical characterization and aging effects of fructan exopolysaccharide produced by Weissella cibaria MD2 on Caenorhabditis elegans. LWT 143:111100. https://doi.org/10.1016/j.lwt.2021.111100
Martorell P, Llopis S, González N, Chenoll E, López-Carreras N, Aleixandre A, Chen Y, Karoly ED, Ramon D, Genovés S (2016) Probiotic strain Bifidobacterium animalis subsp. lactis CECT 8145 reduces fat content and modulates lipid metabolism and antioxidant response in Caenorhabditis elegans. J Agric Food Chem 4:3462–3472. https://doi.org/10.1021/acs.jafc.5b05934
Tsubone TM, Martins WK, Franco MSF, Silva MN, Itri R, Baptista MS (2021) Cellular compartments challenged by membrane photo-oxidation. Arch Biochem Biophys 697:108665. https://doi.org/10.1016/j.abb.2020.108665
Jin X, He Y, Liu Z, Zhou Y, Chen W (2020) Lactic acid bacteria exhibit similar antioxidant capacities in: Caenorhabditis elegans - and Campylobacter jejuni -infected mice. RSC Adv 10: 3329–3342. https://doi.org/10.1039/C9RA06105C
Schifano E, Zinno P, Guantario B, Roselli M, Marcoccia S, Devirgiliis C, Uccelletti D (2019) The foodborne strain Lactobacillus fermentum MBC2 triggers pept-1-dependent pro-longevity effects in Caenorhabditis elegans. Microorganisms 7:45. https://doi.org/10.3390/microorganisms7020045
Sim I, Park KT, Kwon G, Koh JH, Lim YH (2018) Probiotic potential of Enterococcus faecium isolated from chicken cecum with immunomodulating activity and promoting longevity in Caenorhabditis elegans. J Microbiol Biotechnol 28:883–892. https://doi.org/10.4014/jmb.1802.02019
Kwon G, Lee J, Lim YH (2016) Dairy propionibacterium extends the mean lifespan of Caenorhabditis elegans via activation of the innate immune system. Sci Rep 6:31713. https://doi.org/10.1038/srep31713
Zhou M, Liu X, Yu H, Yin X, Nie SP, Xie MY, Chen W, Gong J (2018) Cell signaling of Caenorhabditis elegans in response to enterotoxigenic Escherichia coli infection and Lactobacillus zeae protection. Front Immunol 9:1745. https://doi.org/10.3389/fimmu.2018.01745
Komura T, Ikeda T, Yasui C, Saeki S, Nishikawa Y (2013) Mechanism underlying prolongevity induced by bifidobacteria in Caenorhabditis elegans. Biogerontology 14:73–87. https://doi.org/10.1007/s10522-012-9411-6
Noureen S, Riaz A, Arshad M, Arshad N (2018) In vitro selection and in vivo confirmation of the antioxidant ability of Lactobacillus brevis MG000874. J Appl Microbiol 126:1221–1232. https://doi.org/10.1111/jam.14189
Kamaladevi A, Ganguli A, Kumar M, Balamurugan K (2013) Lactobacillus casei protects malathion induced oxidative stress and macromolecular changes in Caenorhabditis elegans. Pestic Biochem Physiol 105:213–223. https://doi.org/10.1016/j.pestbp.2013.02.005
Engelmann I, Griffon A, Tichit L, Montañana-Sanchis F, Wang J, Reinke V, Waterston RH, Hillier LW, Ewbank JJ (2011) A comprehensive analysis of gene expression changes provoked by bacterial and fungal infection in C. elegans. PLoS One 6: e19055. https://doi.org/10.1371/journal.pone.0019055
Zugasti O, Ewbank JJ (2009) Neuroimmune regulation of antimicrobial peptide expression by a noncanonical TGF-beta signaling pathway in Caenorhabditis elegans epidermis. Nat Immunol 10:249–256. https://doi.org/10.1038/ni.1700
Remondi SAC, Burgwyn FB, Souza AVD, Elamparithi J, Lopes CA, Eleftherios MJG (2018) Pathogenesis of the Candida parapsilosis complex in the model host Caenorhabditis elegans. Genes 9:401. https://doi.org/10.3390/genes9080401
Mesbahi H, Pho KB, Tench AJ, Guerrero VLL, Macneil LT (2020) Cuticle collagen expression is regulated in response to environmental stimuli by the GATA transcription factor ELT-3 in Caenorhabditis elegans. Genetics 215:483–495. https://doi.org/10.1534/genetics.120.303125
Ewald CY, Landis JN, Abate JP, Murphy CT, Blackwell TK (2014) Dauer-independent insulin/IGF-1-signalling implicates collagen remodelling in longevity. Nature 519:97–101. https://doi.org/10.1038/nature14021
Teuscher AC, Statzer C, Pantasis S, Bordoli MR, Ewald CY (2019) Assessing collagen deposition during aging in mammalian tissue and in Caenorhabditis elegans. In: Sagi I, Afratis N (ed) Collagen. Methods in Molecular Biology. Humana Press, New York, pp 169–188. https://doi.org/10.1007/978-1-4939-9095-5_13
Brunquell J, Morris S, Lu Y, Cheng F, Westerheide SD (2016) The genome-wide role of HSF-1 in the regulation of gene expression in Caenorhabditis elegans. BMC Genom 17:559. https://doi.org/10.1186/s12864-016-2837-5
Funding
The study presented in the manuscript was funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Author information
Authors and Affiliations
Contributions
W. C. conceived and designed the study. W. W., S. L., and X. H. performed the experiments. W. W. and W. C. analyzed the data and wrote the manuscript with input from all authors.
Corresponding author
Ethics declarations
Competing Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, W., Li, S., Heng, X. et al. Weissella confusa CGMCC 19,308 Strain Protects Against Oxidative Stress, Increases Lifespan, and Bacterial Disease Resistance in Caenorhabditis elegans. Probiotics & Antimicro. Prot. 14, 121–129 (2022). https://doi.org/10.1007/s12602-021-09799-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-021-09799-z