Abstract
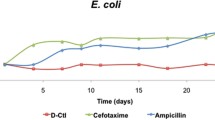
Antimicrobial peptides (AMPs), which hold tremendous promise in overcoming the emergence of drug resistance, are limited in wide clinical applications due to their instability, especially against trypsin. Herein, we designed six peptide mutants based on the cathelicidin CATHPb2, followed by screening. Pb2-1, which showed the best activity against drug-resistant bacteria among these mutants, was selected to be combined with the trypsin inhibitory loop ORB-C to obtain two hybrid peptides: PCL-1 and Pb2-1TI. Notably, both of the hybrid peptides exhibited a remarkable enhancement in trypsin resistance compared with Pb2-1. The tests showed that PCL-1 displayed broad-spectrum antimicrobial activity that was superior to that of Pb2-1TI. In addition, PCL-1 had relatively lower cytotoxicity than Pb2-1TI towards the L02 and HaCaT cell lines and negligible hemolysis, as well as tolerance to high concentrations of salt, extreme pH, and temperature variations. In vivo, PCL-1 effectively improved the survival rate of mice that were systemically infected with drug-resistant Escherichia coli through efficient bacterial clearance from the blood and organs. With regard to mode of action, PCL-1 damaged the integrity of the bacterial cell membrane and attached to the membrane surface while bound to bacterial genomic DNA to eventually kill the bacteria. Altogether, the trypsin-resistant peptide PCL-1 is expected to be a candidate for the clinical treatment of bacterial infections.






Similar content being viewed by others
Data Availability
All data generated or analyzed during this study are included in this published article.
References
Reddy KV, Yedery RD, Aranha C (2004) Antimicrobial peptides: premises and promises. Int J Antimicrob Agents 24(6):536–547. https://doi.org/10.1016/j.ijantimicag.2004.09.005
Yeaman MR, Yount NY (2003) Mechanisms of antimicrobial peptide action and resistance. Pharmacol Rev 55(1):27–55. https://doi.org/10.1124/pr.55.1.2
Fox JL (2013) Antimicrobial peptides stage a comeback. Nat Biotechnol 31(5):379–382. https://doi.org/10.1038/nbt.2572
Zaiou M (2007) Multifunctional antimicrobial peptides: therapeutic targets in several human diseases. J Mol Med (Berl) 85(4):317–329. https://doi.org/10.1007/s00109-006-0143-4
Baba MS, Zin NM, Hassan ZA, Latip J, Pethick F, Hunter IS, Edrada-Ebel R, Herron PR (2015) In vivo antimalarial activity of the endophytic actinobacteria, Streptomyces SUK 10. J Microbiol 53(12):847–855. https://doi.org/10.1007/s12275-015-5076-6
Kim H, Kim HR, Kim NR, Jeong BJ, Lee JS, Jang S, Chung DK (2015) Oral administration of Lactobacillus plantarum lysates attenuates the development of atopic dermatitis lesions in mouse models. J Microbiol 53(1):47–52. https://doi.org/10.1007/s12275-015-4483-z
Gennaro R, Zanetti M (2000) Structural features and biological activities of the cathelicidin-derived antimicrobial peptides. Biopolymers 55(1):31–49. https://doi.org/10.1002/1097-0282(2000)55:1%3c31::AID-BIP40%3e3.0.CO;2-9
Lehrer RI, Ganz T (2002) Cathelicidins: a family of endogenous antimicrobial peptides. Curr Opin Hematol 9(1):18–22. https://doi.org/10.1097/00062752-200201000-00004
Wang J, Chou S, Xu L, Zhu X, Dong N, Shan A, Chen Z (2015) High specific selectivity and membrane-active mechanism of the synthetic centrosymmetric α-helical peptides with Gly-Gly pairs. Sci Rep 5:15963. https://doi.org/10.1038/srep15963
Moncla BJ, Pryke K, Rohan LC, Graebing PW (2011) Degradation of naturally occurring and engineered antimicrobial peptides by proteases. Adv Biosci Biotechnol 2(6):404–408. https://doi.org/10.4236/abb.2011.26059
Rawlings ND, Barrett AJ (1994) Families of serine peptidases. Methods Enzymol 244:19–61. https://doi.org/10.1016/0076-6879(94)44004-2
Wang J, Song J, Yang Z, He S, Yang Y, Feng X, Dou X, Shan A (2019) Antimicrobial peptides with high proteolytic resistance for combating Gram-negative bacteria. J Med Chem 62(5):2286–2304. https://doi.org/10.1021/acs.jmedchem.8b01348
Bode W, Huber R (1992) Natural protein proteinase inhibitors and their interaction with proteinases. Eur J Biochem 204(2):433–451. https://doi.org/10.1111/j.1432-1033.1992.tb16654.x
Hernandez JF, Gagnon J, Chiche L, Nguyen TM, Andrieu JP, Heitz A, Trinh Hong T, Pham TT, Le Nguyen D (2000) Squash trypsin inhibitors from Momordica cochinchinensis exhibit an atypical macrocyclic structure. Biochemistry 39(19):5722–5730. https://doi.org/10.1021/bi9929756
Debowski D, Lukajtis R, Legowska A, Karna N, Pikula M, Wysocka M, Maliszewska I, Sienczyk M, Lesner A, Rolka K (2012) Inhibitory and antimicrobial activities of OGTI and HV-BBI peptides, fragments and analogs derived from amphibian skin. Peptides 35(2):276–284. https://doi.org/10.1016/j.peptides.2012.04.001
Li J, Zhang C, Xu X, Wang J, Yu H, Lai R, Gong W (2007) Trypsin inhibitory loop is an excellent lead structure to design serine protease inhibitors and antimicrobial peptides. FASEB J 21(10):2466–2473. https://doi.org/10.1096/fj.06-7966com
Cai S, Qiao X, Feng L, Shi N, Wang H, Yang H, Guo Z, Wang M, Chen Y, Wang Y, Yu H (2018) Python cathelicidin CATHPb1 protects against multidrug-resistant staphylococcal infections by antimicrobial-immunomodulatory duality. J Med Chem 61(5):2075–2086. https://doi.org/10.1021/acs.jmedchem.8b00036
Wiegand I, Hilpert K, Hancock RE (2008) Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat Protoc 3(2):163–175. https://doi.org/10.1038/nprot.2007.521
Zgoda JR, Porter JR (2001) A convenient microdilution method for screening natural products against bacteria and fungi. Pharm Biol 39(3):221–225. https://doi.org/10.1076/phbi.39.3.221.5934
Amit C, Muralikumar S, Janaki S, Lakshmipathy M, Therese KL, Umashankar V, Padmanabhan P, Narayanan J (2019) Designing and enhancing the antifungal activity of corneal specific cell penetrating peptide using gelatin hydrogel delivery system. Int J Nanomedicine 14:605–622. https://doi.org/10.2147/IJN.S184911
Pinto SN, Dias SA, Cruz AF, Mil-Homens D, Fernandes F, Valle J, Andreu D, Prieto M, Castanho M, Coutinho A, Veiga AS (2019) The mechanism of action of pepR, a viral-derived peptide, against Staphylococcus aureus biofilms. J Antimicrob Chemother 74(9):2617–2625. https://doi.org/10.1093/jac/dkz223
Khara JS, Lim FK, Wang Y, Ke XY, Voo ZX, Yang YY, Lakshminarayanan R, Ee PLR (2015) Designing alpha-helical peptides with enhanced synergism and selectivity against Mycobacterium smegmatis: discerning the role of hydrophobicity and helicity. Acta Biomater 28:99–108. https://doi.org/10.1016/j.actbio.2015.09.015
Ma L, Xie X, Liu H, Huang Y, Wu H, Jiang M, Xu P, Ye X, Zhou C (2020) Potent antibacterial activity of MSI-1 derived from the magainin 2 peptide against drug-resistant bacteria. Theranostics 10(3):1373–1390. https://doi.org/10.7150/thno.39157
Kim H, Jang JH, Kim SC, Cho JH (2014) De novo generation of short antimicrobial peptides with enhanced stability and cell specificity. J Antimicrob Chemother 69(1):121–132. https://doi.org/10.1093/jac/dkt322
Ji S, Li W, Zhang L, Zhang Y, Cao B (2014) Cecropin A-melittin mutant with improved proteolytic stability and enhanced antimicrobial activity against bacteria and fungi associated with gastroenteritis in vitro. Biochem Biophys Res Commun 451(4):650–655. https://doi.org/10.1016/j.bbrc.2014.08.044
Chen HL, Su PY, Chang YS, Wu SY, Liao YD, Yu HM, Lauderdale TL, Chang K, Shih C (2013) Identification of a novel antimicrobial peptide from human hepatitis B virus core protein arginine-rich domain (ARD). PLoS Pathog 9(6):e1003425. https://doi.org/10.1371/journal.ppat.1003425
Zhang Q, Xu Y, Wang Q, Hang B, Sun Y, Wei X, Hu J (2015) Potential of novel antimicrobial peptide P3 from bovine erythrocytes and its analogs to disrupt bacterial membranes in vitro and display activity against drug-resistant bacteria in a mouse model. Antimicrob Agents Chemother 59(5):2835–2841. https://doi.org/10.1128/AAC.04932-14
Han HM, Gopal R, Park Y (2016) Design and membrane-disruption mechanism of charge-enriched AMPs exhibiting cell selectivity, high-salt resistance, and anti-biofilm properties. Amino Acids 48(2):505–522. https://doi.org/10.1007/s00726-015-2104-0
Takahashi D, Shukla SK, Prakash O, Zhang G (2010) Structural determinants of host defense peptides for antimicrobial activity and target cell selectivity. Biochimie 92(9):1236–1241. https://doi.org/10.1016/j.biochi.2010.02.023
Travis SM, Anderson NN, Forsyth WR, Espiritu C, Conway BD, Greenberg EP, McCray PB Jr, Lehrer RI, Welsh MJ, Tack BF (2000) Bactericidal activity of mammalian cathelicidin-derived peptides. Infect Immun 68(5):2748–2755. https://doi.org/10.1128/iai.68.5.2748-2755.2000
Chou S, Shao C, Wang J, Shan A, Xu L, Dong N, Li Z (2016) Short, multiple-stranded beta-hairpin peptides have antimicrobial potency with high selectivity and salt resistance. Acta Biomater 30:78–93. https://doi.org/10.1016/j.actbio.2015.11.002
Clifton LA, Skoda MW, Le Brun AP, Ciesielski F, Kuzmenko I, Holt SA, Lakey JH (2015) Effect of divalent cation removal on the structure of gram-negative bacterial outer membrane models. Langmuir 31(1):404–412. https://doi.org/10.1021/la504407v
Travkova OG, Moehwald H, Brezesinski G (2017) The interaction of antimicrobial peptides with membranes. Adv Colloid Interface Sci 247:521–532. https://doi.org/10.1016/j.cis.2017.06.001
Singh S, Nimmagadda A, Su M, Wang M, Teng P, Cai J (2018) Lipidated alpha/alpha-AA heterogeneous peptides as antimicrobial agents. Eur J Med Chem 155:398–405. https://doi.org/10.1016/j.ejmech.2018.06.006
Zhang Z, Cherryholmes G, Chang F, Rose DM, Schraufstatter I, Shively JE (2009) Evidence that cathelicidin peptide LL-37 may act as a functional ligand for CXCR2 on human neutrophils. Eur J Immunol 39(11):3181–3194. https://doi.org/10.1002/eji.200939496
Funding
This work was funded by the National Key Research and Development Program of China (2018YFA0902000), the National Natural Science Foundation of China (No. 81803591), the National Science and Technology Major Project Foundation of China (2019ZX09721001-004–005), the fellowship of China Postdoctoral Science Foundation (2020T130723), the Natural Science Foundation of Jiangsu Province of China (No. BK20180563; No. BK20201327), and the Basic Scientific Research Business Expense Project of China Pharmaceutical University (No. 2632021ZD07).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethical Approval
All experimental procedures were approved by the animal care and management committee of China Pharmaceutical University (2020–03-001).
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wu, H., Xu, P., Huang, Y. et al. PCL-1, a Trypsin-Resistant Peptide, Exerts Potent Activity Against Drug-Resistant Bacteria. Probiotics & Antimicro. Prot. 13, 1467–1480 (2021). https://doi.org/10.1007/s12602-021-09801-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-021-09801-8