Abstract
Somatic mutations in DNA methyltransferase 3A (DNMT3A) are among the most frequent alterations in clonal hematopoiesis (CH) and acute myeloid leukemia (AML), with a hotspot in exon 23 at arginine 882 (DNMT3AR882). Here, we demonstrate that DNMT3AR882H-dependent CH and AML cells are specifically susceptible to the hypomethylating agent azacytidine (AZA). Addition of AZA to chemotherapy prolonged AML survival solely in individuals with DNMT3AR882 mutations, suggesting its potential as a predictive marker for AZA response. AML and CH mouse models confirmed AZA susceptibility specifically in DNMT3AR882H-expressing cells. Hematopoietic stem cells (HSCs) and progenitor cells expressing DNMT3AR882H exhibited cell autonomous viral mimicry response as a result of focal DNA hypomethylation at retrotransposon sequences. Administration of AZA boosted hypomethylation of retrotransposons specifically in DNMT3AR882H-expressing cells and maintained elevated levels of canonical interferon-stimulated genes (ISGs), thus leading to suppressed protein translation and increased apoptosis.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
The following databases have been used in the study: LOLA (http://big.databio.org/regiondb/LOLACore_180412.tgz), MSigDB (http://www.gsea-msigdb.org/gsea/msigdb/collections.jsp) and HOMER (http://homer.ucsd.edu/homer/). RNA-seq and mouse WGBS data are stored at NCBI’s Gene Expression Omnibus (GEO) data repository with the accession code GSE146907. Human WGBS raw data have been deposited in the European Genome Archive (EGA; accession codes EGAD00001007504 (data set ID) and EGAS00001004825 (study ID)). Data cannot be made publicly available due to data protection regulations and will be shared upon reasonable request to the authors, with clearance by the ethics committee of University Heidelberg in charge of overseeing participant data-sharing requests
The human AML RNA-seq data were derived from the TCGA Research Network (http://cancergenome.nih.gov/). The compiled files consisting of normalized expression values from TCGA for selected DNMT3AWT and DNMT3AR882-mutant participant samples and the respective categorical class file (*.cls), which has been used for upload to GSEA, are available from the corresponding author upon request. Imaging source data are uploaded to figshare (microscopy data of Fig. 6b and Extended Data Fig. 7a (ref. 68); microscopy data of Extended Data Fig. 3b (ref. 69)). All other data are available from the corresponding author upon request. Source data are provided with this paper.
Code availability
All unpublished software packages and workflows used for the methylome analysis described in this paper are available on GitHub at https://github.com/stephenkraemer/ (bistro (for methylation calling), dss_workflow (for DMR calling), gtfanno (for gene annotation) and region_set_profiler (for enrichment analysis)) and https://github.com/DKFZ-ODCF/AlignmentAndQCWorkflows (bisulfite core workflow for WGBS alignment and methylation calling (methylctools was replaced by bistro)). The bistro code repository is currently private; access will be granted upon request. Jupyter notebooks with the code to reproduce the data analysis are available from the authors upon request.
References
Jaiswal, S. et al. Age-related clonal hematopoiesis associated with adverse outcomes. N. Engl. J. Med. 371, 2488–2498 (2014).
Genovese, G. et al. Clonal hematopoiesis and blood-cancer risk inferred from blood DNA sequence. N. Engl. J. Med. 371, 2477–2487 (2014).
McKerrell, T. et al. Leukemia-associated somatic mutations drive distinct patterns of age-related clonal hemopoiesis. Cell Rep. 10, 1239–1245 (2015).
Shlush, L. I. et al. Identification of pre-leukaemic haematopoietic stem cells in acute leukaemia. Nature 506, 328–333 (2014).
Ley, T. J. et al. DNMT3A mutations in acute myeloid leukemia. N. Engl. J. Med. 363, 2424–2433 (2010).
Dai, Y. J. et al. Conditional knockin of Dnmt3a R878H initiates acute myeloid leukemia with mTOR pathway involvement. Proc. Natl Acad. Sci. USA 114, 5237–5242 (2017).
Guryanova, O. A. et al. DNMT3A mutations promote anthracycline resistance in acute myeloid leukemia via impaired nucleosome remodeling. Nat. Med. 22, 1488–1495 (2016).
Loberg, M.A. et al. Sequentially inducible mouse models reveal that Npm1 mutation causes malignant transformation of Dnmt3a-mutant clonal hematopoiesis. Leukemia 33, 1635–1649 (2019).
Russler-Germain, D. A. et al. The R882H DNMT3A mutation associated with AML dominantly inhibits wild-type DNMT3A by blocking its ability to form active tetramers. Cancer Cell 25, 442–454 (2014).
Kim, S. J. et al. A DNMT3A mutation common in AML exhibits dominant-negative effects in murine ES cells. Blood 122, 4086–4089 (2013).
Ribeiro, A. F. et al. Mutant DNMT3A: a marker of poor prognosis in acute myeloid leukemia. Blood 119, 5824–5831 (2012).
Marcucci, G. et al. Age-related prognostic impact of different types of DNMT3A mutations in adults with primary cytogenetically normal acute myeloid leukemia. J. Clin. Oncol. 30, 742–750 (2012).
Fenaux, P. et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study. Lancet Oncol. 10, 223–232 (2009).
Fenaux, P. et al. Azacitidine prolongs overall survival compared with conventional care regimens in elderly patients with low bone marrow blast count acute myeloid leukemia. J. Clin. Oncol. 28, 562–569 (2010).
Dombret, H. et al. International phase 3 study of azacitidine vs conventional care regimens in older patients with newly diagnosed AML with >30% blasts. Blood 126, 291–299 (2015).
Christman, J. K. 5-Azacytidine and 5-aza-2′-deoxycytidine as inhibitors of DNA methylation: mechanistic studies and their implications for cancer therapy. Oncogene 21, 5483–5495 (2002).
Stresemann, C. & Lyko, F. Modes of action of the DNA methyltransferase inhibitors azacytidine and decitabine. Int. J. Cancer 123, 8–13 (2008).
Baylin, S. B. & Jones, P. A. A decade of exploring the cancer epigenome—biological and translational implications. Nat. Rev. Cancer 11, 726–734 (2011).
Tsai, H. C. et al. Transient low doses of DNA-demethylating agents exert durable antitumor effects on hematological and epithelial tumor cells. Cancer Cell 21, 430–446 (2012).
Treppendahl, M. B., Kristensen, L. S. & Gronbaek, K. Predicting response to epigenetic therapy. J. Clin. Invest. 124, 47–55 (2014).
Chiappinelli, K. B. et al. Inhibiting DNA methylation causes an interferon response in cancer via dsRNA including endogenous retroviruses. Cell 162, 974–986 (2015).
Roulois, D. et al. DNA-demethylating agents target colorectal cancer cells by inducing viral mimicry by endogenous transcripts. Cell 162, 961–973 (2015).
Mehdipour, P. et al. Epigenetic therapy induces transcription of inverted SINEs and ADAR1 dependency. Nature 588, 169–173 (2020).
Banerjee, S. et al. OAS-RNase L innate immune pathway mediates the cytotoxicity of a DNA-demethylating drug. Proc. Natl Acad. Sci. USA 116, 5071–5076 (2019).
Krug, U. et al. Feasibility of azacitidine added to standard chemotherapy in older patients with acute myeloid leukemia—a randomised SAL pilot study. PLoS ONE 7, e52695 (2012).
Muller-Tidow, C. et al. Azacitidine in combination with intensive induction chemotherapy in older patients with acute myeloid leukemia: The AML-AZA trial of the study alliance leukemia. Leukemia 30, 555–561 (2016).
Burnett, A.K. et al. The value of allogeneic bone marrow transplant in patients with acute myeloid leukaemia at differing risk of relapse: results of the UK MRC AML 10 trial. Br. J. Jaematol. 118, 385–400 (2002).
Lancet, J.E. et al. Phase 2 trial of CPX-351, a fixed 5:1 molar ratio of cytarabine/daunorubicin, vs cytarabine/daunorubicin in older adults with untreated AML. Blood 123, 3239–3246 (2014).
Löwenberg, B. et al. High-dose daunorubicin in older patients with acute myeloid leukemia. N. Engl. J. Med. 361, 1235–1248 (2009).
Bejar, R. et al. TET2 mutations predict response to hypomethylating agents in myelodysplastic syndrome patients. Blood 124, 2705–2712 (2014).
Kuhn, R., Schwenk, F., Aguet, M. & Rajewsky, K. Inducible gene targeting in mice. Science 269, 1427–1429 (1995).
Schulze, I. et al. Increased DNA methylation of Dnmt3b targets impairs leukemogenesis. Blood 127, 1575–1586 (2016).
Konopleva, M. et al. Mechanisms of apoptosis sensitivity and resistance to the BH3 mimetic ABT-737 in acute myeloid leukemia. Cancer Cell 10, 375–388 (2006).
Ohanian, M. et al. MYC protein expression is an important prognostic factor in acute myeloid leukemia. Leuk. Lymphoma 60, 37–48 (2019).
Meyer, S. E. et al. DNMT3A haploinsufficiency transforms FLT3ITD myeloproliferative disease into a rapid, spontaneous, and fully penetrant acute myeloid leukemia. Cancer Discov. 6, 501–515 (2016).
Thiede, C. et al. Analysis of FLT3-activating mutations in 979 patients with acute myelogenous leukemia: association with FAB subtypes and identification of subgroups with poor prognosis. Blood 99, 4326–4335 (2002).
Spencer, D. H. et al. CpG island hypermethylation mediated by DNMT3A is a consequence of AML progression. Cell 168, 801–816 (2017).
Tobiasson, M. et al. Comprehensive mapping of the effects of azacitidine on DNA methylation, repressive/permissive histone marks and gene expression in primary cells from patients with MDS and MDS-related disease. Oncotarget 8, 28812–28825 (2017).
Garcia-Manero, G. et al. Efficacy and safety of extended dosing schedules of CC-486 (oral azacitidine) in patients with lower-risk myelodysplastic syndromes. Leukemia 30, 889–896 (2016).
Stocking, C. & Kozak, C. A. Murine endogenous retroviruses. Cell. Mol. Life Sci. 65, 3383–3398 (2008).
Weber, F., Wagner, V., Rasmussen, S. B., Hartmann, R. & Paludan, S. R. Double-stranded RNA is produced by positive-strand RNA viruses and DNA viruses but not in detectable amounts by negative-strand RNA viruses. J. Virol. 80, 5059–5064 (2006).
Cancer Genome Atlas Research Network, et al. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N. Engl. J. Med. 368, 2059–2074 (2013).
Li, Y. et al. Ribonuclease L mediates the cell-lethal phenotype of double-stranded RNA editing enzyme ADAR1 deficiency in a human cell line. eLife 6, e25687 (2017).
Chitrakar, A. et al. Real-time 2-5A kinetics suggest that interferons β and λ evade global arrest of translation by RNase L. Proc. Natl Acad. Sci. USA 116, 2103–2111 (2019).
Liu, J., Xu, Y., Stoleru, D. & Salic, A. Imaging protein synthesis in cells and tissues with an alkyne analog of puromycin. Proc. Natl Acad. Sci. USA 109, 413–418 (2012).
Watson, C. J. et al. The evolutionary dynamics and fitness landscape of clonal hematopoiesis. Science 367, 1449–1454 (2020).
Metzeler, K. H. et al. DNMT3A mutations and response to the hypomethylating agent decitabine in acute myeloid leukemia. Leukemia 26, 1106–1107 (2012).
Falconi, G. et al. Somatic mutations as markers of outcome after azacitidine and allogeneic stem cell transplantation in higher-risk myelodysplastic syndromes. Leukemia 33, 785–790 (2019).
DiNardo, C. D. et al. Lack of association of IDH1, IDH2 and DNMT3A mutations with outcome in older patients with acute myeloid leukemia treated with hypomethylating agents. Leuk. Lymphoma 55, 1925–1929 (2014).
Wrangle, J. et al. Alterations of immune response of non-small cell lung cancer with azacytidine. Oncotarget 4, 2067–2079 (2013).
Juergens, R. A. et al. Combination epigenetic therapy has efficacy in patients with refractory advanced non-small cell lung cancer. Cancer Discov. 1, 598–607 (2011).
Rycaj, K. et al. Cytotoxicity of human endogenous retrovirus K-specific T cells toward autologous ovarian cancer cells. Clin. Cancer Res. 21, 471–483 (2015).
Johanning, G. L. et al. Expression of human endogenous retrovirus-K is strongly associated with the basal-like breast cancer phenotype. Sci. Rep. 7, 41960 (2017).
Krishnamurthy, J. et al. Genetic engineering of T cells to target HERV-K, an ancient retrovirus on melanoma. Clin. Cancer Res. 21, 3241–3251 (2015).
Espinet, E. et al. Aggressive PDACs show hypomethylation of repetitive elements and the execution of an intrinsic IFN program linked to a ductal cell-of-origin. Cancer Discov. https://doi.org/10.1158/2159-8290.CD-20-1202 (2020).
Libby, P. et al. Clonal hematopoiesis: crossroads of aging, cardiovascular disease, and cancer: JACC review topic of the week. J. Am. Coll. Cardiol. 74, 567–577 (2019).
Ross, R. Atherosclerosis–an inflammatory disease. N. Engl. J. Med. 340, 115–126 (1999).
Ridker, P. M. et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N. Engl. J. Med. 377, 1119–1131 (2017).
Tardif, J. C. et al. Efficacy and safety of low-dose colchicine after myocardial infarction. N. Engl. J. Med. 381, 2497–2505 (2019).
Nidorf, S. M. et al. Colchicine in patients with chronic coronary disease. N. Engl. J. Med. 383, 1838–1847 (2020).
Ventura, A. et al. Restoration of p53 function leads to tumour regression in vivo. Nature 445, 661–665 (2007).
Chung, Y.R., Kim, E. & Abdel-Wahab, O. Femoral bone marrow aspiration in live mice. J. Vis. Exp. 89, e51660 (2014).
Garg, S. et al. Hepatic leukemia factor is a novel leukemic stem cell regulator in DNMT3A, NPM1, and FLT3-ITD triple-mutated AML. Blood 134, 263–276 (2019).
Brambati, C. et al. Droplet digital polymerase chain reaction for DNMT3A and IDH1/2 mutations to improve early detection of acute myeloid leukemia relapse after allogeneic hematopoietic stem cell transplantation. Haematologica 101, e157–e161 (2016).
Scheller, M. et al. Cross talk between Wnt/β-catenin and Irf8 in leukemia progression and drug resistance. J. Exp. Med. 210, 2239–2256 (2013).
Li, H. et al. Efficient CRISPR–Cas9 mediated gene disruption in primary erythroid progenitor cells. Haematologica 101, e216–e219 (2016).
Scheller, M. et al. Nature Portfolio Protocol Exchange platform. https://doi.org/10.21203/rs.3.pex-1436/v1
Scheller, M. et al. Microscopy data of Fig. 6b and Extended Data Fig. 7a. Figshare. https://doi.org/10.6084/m9.figshare.13690093.v1 (2021).
Scheller, M. et al. Microscopy data of Extended Data Fig. 3b. Figshare. https://doi.org/10.6084/m9.figshare.13690258.v1 (2021).
Acknowledgements
We thank V. Eckstein for supporting the FACS sorting procedure, S. Garg for help in experiments with NSG mice and J. Kollan, K. Nerger, B. Besenbeck, M. Lotze and M. Horn for technical assistance. We thank S. Serba, K. Hillesheim and all animal keepers from the animal facility for supporting the mouse work. We gratefully acknowledge the data storage service SDS@hd supported by the Ministry of Science, Research and the Arts Baden-Württemberg (MWK) and the German Research Foundation (DFG) through grant INST 35/1314-1 FUG. Research reported in this publication was (in part) supported by funds from the German Research Foundation (DFG; MU1328/13-1, MU1328/15‐1 and MU1328/18‐1 to C.M.-T.; DFG Forschergruppe FOR2674 to M.D.M., D.B.L. and A.T.; and SFB873, Project B04 to A.T.), the German Cancer Aid (DKH; Projects 70112974 and 70113908 to C.M.-T. and Project 70112574 to D.B.L.), the Helmholtz Zukunftsthema ‘Aging and Metabolic Programming’ (AMPro) to M.D.M. and D.B.L., the German Jose-Carreras Leukemia Foundation (DJCLS; 22R/2017 to C.M.-T. and 04R/2017 to N.B.), the Innovative Medical Research of the University of Münster Medical School (IMF grant no. 121314 to N.B.), BMBF 031L0212A to C.M.-T., the RiskY-AML Joint Funding program of the German Cancer Consortium (DKTK) to C.M.-T. and A.T., and the Dietmar Hopp Foundation to A.T. We thank the High Throughput Sequencing Unit of the Genomics & Proteomics Core Facility, German Cancer Research Center (DKFZ), for providing excellent NGS services. We further thank the Omics IT and Data Management Core Facility (ODCF) for providing excellent data storage and computing infrastructure.
Author information
Authors and Affiliations
Contributions
M. Scheller, N.B. and C.M.-T. designed the DNMT3A KI mouse model. M. Scheller and J.-A.M. performed and analyzed all mouse experiments. L.H., A.K.L. and C. Pabst performed NSG experiments. M. Scheller performed cell culture experiments, western blotting, immunofluorescence staining, flow cytometry and cell sorting. M. Scheller and J.-A.M. performed RT–qPCR. M. Scheller, J.-A.M. and A.K.L. prepared samples for RNA-seq. A.K.L. performed fluorescence imaging and Rnasel knockdown. S.S. and D.B.L. established the 32D Dnmt3aR878H cell line and prepared RNA-seq and WGBS libraries. I.H. prepared samples for histological analysis. C.R., C.A., S.K., M. Schönung, S.S., D.B.L., M. Schlesner and J.Z. performed bioinformatic analysis of RNA-seq and WGBS data. C.T. performed diagnostic sequencing of primary AML samples. M.J., H.S., W.E.B., U.T., J.G., C.N., D.N. and C.M.-T. analyzed the clinical trial data. C. Plass, F.L., A.T., M.D.M., I.H. and F.L. contributed to study conception. M. Scheller and C.M.-T. designed the study. M. Scheller, D.B.L., S.G. and C.M.-T. analyzed the data and wrote the manuscript. All authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The original AML-AZA clinical trial was partially supported by Celgene and Amgen (principal investigator, C.M.T.). The Department of Medicine V is further supported by multiple biopharmaceutical companies for clinical trials and translational research projects. C.T. is co-owner and CEO of AgenDix GmbH, a company performing molecular diagnostics. C.T. has served as an adviser and provided educational support for Celgene, JAZZ, Novartis and Astellas. All other authors declare no competing interests.
Additional information
Peer review information Nature Cancer thanks the anonymous reviewers for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
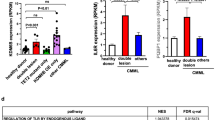
Extended Data Fig. 1 DNMT3A-R882 mutations predict response to AZA in human AML and clonal hematopoiesis.
a and b, Kaplan Meier plots depict all patients of the AML-AZA trial cohort for whom DNA was available to determine DNMT3A-mutation status and who received at least one day of chemotherapy within the trial (n = 166). Patients were not censored for allogeneic transplantation. Results are similar to the results for the entire patient cohort(n = 214) of the AML-AZA trial as described26. a, Event Free Survival (EFS) of all patients with AZA+ ‘7+3’ (n = 76 patients) versus ‘7+3’ (n = 90 patients). EFS times were 5.1 months for AZA+ ‘7+3’ compared to 5.8 months for the ‘7+3’ treatment group (P = 0.86). b, Overall Survival (OS) of all patients with AZA+ ‘7+3’ versus ‘7+3’. OS trended to be lower in patients receiving AZA+ ‘7+3’ (median OS=11.4 months) compared to 7+3 therapy only (median OS=21.4 months, P = 0.067). Survival probabilities for panels a and b were assessed using the Kaplan-Meier method and evaluated by log-rank Mantle- Cox test. c, Influence of DNMT3A-R882 mutation and AZA treatment on survival as calculated using univariate analysis and Cox proportional hazards regression model. The following parameters were included: treatment (AZA+ ‘7+3’ (n = 76) vs. ‘7+3’ (n = 90)) for all patients, DNMT3A-R882 status (yes (n = 34) vs. no (n = 132)) and DNMT3A-R882 mutation in AZA+ ‘7+3’ treatment (n = 13) vs. all others (n = 153). All patients from the AML-AZA trial with known DNMT3A mutation status and at least one day of received chemotherapy were included into these analyses. d, EFS of patients with DNMT3A-mutations outside of exon 23 treated with ‘7+3’ (n = 10) or AZA+ ‘7+3’ (n = 10) (P = 0.21). e, OS of patients with DNMT3A-mutations outside of exon 23 treated with ‘7+3’ (n = 10) or AZA+ ‘7+3’ (n = 10) (P = 0.36). Survival probabilities for panels d and e were assessed using the Kaplan-Meier method and then evaluated by log-rank Mantle- Cox test. A two-sided α of 0.05 was chosen for all comparisons using patient’s data. f, Variant allele frequency (VAF) of DNMT3A-R882H in bone marrow cells one, four and eight weeks after AZA or 0.9%NaCl control treatment as analyzed by Droplet Digital PCR (ddPCR). Data are presented as mean ± s.e.m. n.s. P = 0.3 for control mice (week 4 vs. week 10 post-transplantation; n = 11 mice), *P = 0.0193 for AZA-treated mice (week 7 vs. week 10 post-transplantation; n = 11 mice). Statistical significance was assessed using two-tailed Student´s unpaired t-test.
Extended Data Fig. 2 Characterization of the steady-state hematopoietic phenotype in DNMT3AR882H mice and effects of AZA treatment.
a, RNA-seq analyses of cDNA generated from peripheral blood/BM cells of +/m animals demonstrating the CGC to CAC mutation (the thymidine in the genome browser screenshot relates to the antisense sequence represented in the reads). b, Genotyping PCR showing DNMT3A wild-type mice (denoted as ‘+/+’), non-recombined floxed (fl) mice (functions as a null allele; denoted as ‘+/R882Hfl’) and Cre-mediated recombined mice with excised allele (denoted as ‘+/m’) as analyzed from peripheral blood and tail cut cells. c, Gating strategy applied for analyzing hematopoietic stem and progenitor cells from +/+ and +/m mice. d, Apoptotic cells in the peripheral blood of +/+ and +/m mice ten days after NaCl control- or AZA treatment (n = 5 mice per genotype and treatment). Percentages are shown for early apoptotic (AV+DAPI-) and late apoptotic/ necrotic (AV+DAPI+) cells. Data shown are the mean ± s.e.m. Myeloid cells: *P = 0.024 for +/+ AZA vs. +/m AZA. Statistical significance was assessed using a two-tailed Student´s unpaired t-tests. e, Number of donor-derived LMPPs in the bone marrow of recipients 32 weeks after treatment (n = 4 mice per group). Data are presented as mean ± s.e.m. +/+ NaCl vs. +/m NaCl: **P = 0.0044. Statistical significance was assessed using a two-tailed Student´s unpaired t-tests. All P-values not considered significant can be found in the Source Data.
Extended Data Fig. 3 Survival of leukemic mice after treatment.
a, Representative FACS profiles of leukemia in spleen from +/+:myc-bcl2 and +/m: myc-bcl2 mice. Data are representative for n = 9 mice. b, Hematoxylin and eosin (H&E)–stained spleen and liver from +/+, +/+: myc-bcl2 and +/m: myc-bcl2, +/+:FLT3-ITD and +/m:FLT3-ITD mice. Histologically, leukemic splenic tissue has a markedly distorted organization compared to tissue from healthy C57BL/6N mice (+/+), which’h has organized areas of white pulp (WP) and red pulp (RP). (b, top) staining of spleen (original magnification of 5x), and (b, bottom) staining of liver (original magnification of x10). n = 3 mice for each genotype. c, Representative FACS profiles of leukemia in peripheral blood of +/+:FLT3-ITD (n = 5) and +/m:FLT3-ITD (n = 11) mice.
Extended Data Fig. 4 TWGBS reveals preferential DNA hypomethylation of endogenous retroviral elements and interferon response genes in AZA treated DNMT3AR882H mutant mice.
a, Correlation analysis of DNA methylation levels in all 500 bp tiles using Pearson correlation comparing NaCl-treated +/+ with +/m LSK cells. PCC *100 = Pearson Correlation Coefficient multiplied by 100. b, Principal component analysis based on DNA methylation levels in genome-wide 500 bp tiling windows. red: +/+ NaCl control (n = 5 mice), blue: +/+ AZA (n = 5 mice), green: +/m NaCl control (n = 3 mice), purple: +/m AZA (n = 2 mice). c, Heatmap showing hierarchical clustering of mean beta values (i.e. % DNA methylation) for all 15,468 DMRs across all independent experiments (mice) analyzed. For mouse numbers per genotype and treatment see panel b. d and e, IGV browser tracks displaying DNA methylation data for all independent experiments analyzed by TWGBS in the present study. For mouse numbers per genotype and treatment see panel b. Each vertical line represents a CpG dinucleotide and the height of the line represents the DNA methylation in percent. Depicted is the (d) Ikzf1 gene locus which features 6 DMRs and the (e) Irf8 gene locus which features 8 DMRs (track ‘all_DMRs’). f-h, Enrichment analysis: depicted are –log10(qvalues)* sign(log_odds_ratio) from dark blue (depleted feature) to red (enriched feature) indicating the strength of enrichment of a given feature in a particular cluster as compared to all other clusters. (f) defined genomic regions, i.e. GENCODE transcription start sites (‘gencode_all_tss’), CpG islands (‘island’), CpG island shores (‘shore’), CpG island shelves (‘shelve’), intragenic regions (‘intragenic’), and intergenic regions (‘intergenic’), (g) hematopoietic enhancers as defined by published ChIP-seq data on primary murine hematopoietic cell types, and (h) transcription factor (TF) ChIP-seq peaks from hematopoietic cell types using the LOLA database (http://databio.org/regiondb).
Extended Data Fig. 5 DNA methylation data of individual DMRs at specific gene loci.
a-b, IGV browser tracks displaying DNA methylation data for all independent experiments (mice) analyzed by TWGBS in the present study. +/+ NaCl control (n = 5 mice), +/+ AZA (n = 5 mice), +/m NaCl control (n = 3 mice), +/m AZA (n = 2 mice). Each vertical line represents a CpG dinucleotide and the height of the line represents the DNA methylation in percent. Depicted are DMRs overlapping with LTR-ERV1 elements at the (a) Camk2b locus and at the (b) Cep85 locus.
Extended Data Fig. 6 DNA methylation changes upon AZA treatment in human AML patients.
a, WGBS was performed from human AML samples which either carried a DNMT3AR882H mutation (DNMT3A-R882H, n = 1) or which were wildtype for DNMT3A (WT, n = 2). For each patient a sample was available at diagnosis (Control) and at day 18 of treatment with AZA. Pairwise DMRs were called across genotypes and timepoints and categorized as having high or low levels of DNA methylation. DMRs from human AML samples were tested for enrichment of murine DMR clusters as described in Figure 5. Depicted are log10(qvalues)*sign(log_odds_ratio) from dark-blue (depleted features) to red (enriched features) indicating the strength of enrichment of a given murine DMR cluster as compared to all other clusters. Only DMR categories with significant enrichments are shown. b, DMRs from human AML samples were tested for enrichment of MSigDB hallmark gene sets. Depicted are –log10(q-values)*sign(log_odds_ratio) from dark-blue (depleted features) to red (enriched features) indicating the strength of enrichment of a given feature in a particular cluster as compared to all other clusters. Only DMR categories with significant enrichments are shown. c, ERV loci shown to be affected by AZA in human colorectal cancer cells6. IGV browser tracks display DNA methylation data for MER4A1 locus, MER50 locus, MER57 locus and MLT1C locus. Each vertical line represents a CpG dinucleotide and the height of the line represents the DNA methylation in percent.
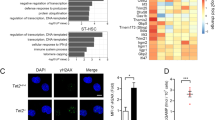
Extended Data Fig. 7 AZA induces dsRNA and ERV expression and reduces protein synthesis in DNMT3AR882H mutant cells.
a, Confocal immunofluorescence images of bone marrow cells from n = 1 mouse per genotype incubated with 2°AB (donkey anti mouse)-only as used for background normalization. Nuclei were counterstained with DAPI (blue). Scale bar 10 μm. b, Experimental scheme of tamoxifen treatment of primary mice.
Supplementary information
Supplementary Information
Study protocol of the AML-AZA clinical trial.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Fig. 6
Uncropped western blot source data.
Source Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Rights and permissions
About this article
Cite this article
Scheller, M., Ludwig, A.K., Göllner, S. et al. Hotspot DNMT3A mutations in clonal hematopoiesis and acute myeloid leukemia sensitize cells to azacytidine via viral mimicry response. Nat Cancer 2, 527–544 (2021). https://doi.org/10.1038/s43018-021-00213-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s43018-021-00213-9
This article is cited by
-
DNMT3A clonal hematopoiesis-driver mutations induce cardiac fibrosis by paracrine activation of fibroblasts
Nature Communications (2024)
-
Epigenetic regulation in hematopoiesis and its implications in the targeted therapy of hematologic malignancies
Signal Transduction and Targeted Therapy (2023)
-
Inflammatory signals from fatty bone marrow support DNMT3A driven clonal hematopoiesis
Nature Communications (2023)
-
Isoform-specific and ubiquitination dependent recruitment of Tet1 to replicating heterochromatin modulates methylcytosine oxidation
Nature Communications (2022)
-
The antileukemic activity of decitabine upon PML/RARA-negative AML blasts is supported by all-trans retinoic acid: in vitro and in vivo evidence for cooperation
Blood Cancer Journal (2022)