Abstract
AMP-activated protein kinase (AMPK) is a critical sensor of energy status that coordinates cell growth with energy balance. In non-small cell lung cancer (NSCLC) the role of AMPKα is controversial and its contribution to lung carcinogenesis is not well-defined. Furthermore, it remains largely unknown whether long non-coding RNAs (lncRNAs) are involved in the regulation of AMPK-mediated pathways. Here, we found that loss of AMPKα in combination with activation of mutant KRASG12D increased lung tumour burden and reduced survival in KrasLSLG12D/+/AMPKαfl/fl mice. In agreement, functional in vitro studies revealed that AMPKα silencing increased growth and migration of NSCLC cells. In addition, we identified an AMPKα-modulated lncRNA, KIMAT1 (ENSG00000228709), which in turn regulates AMPKα activation by stabilizing the lactate dehydrogenase B (LDHB). Collectively, our study indicates that AMPKα loss promotes KRAS-mediated lung tumorigenesis and proposes a novel KRAS/KIMAT1/LDHB/AMPKα axis that could be exploited for therapeutic purposes.
Similar content being viewed by others
Introduction
Despite recent significant advances in the management of lung cancer, NSCLC still has a poor five-year survival rate of approximately 15%, primarily attributable to late diagnosis, chemoresistance and resistance to targeted therapies [1].
The Kirsten rat sarcoma viral oncogene homologue (KRAS) is one of the main oncogenic drivers in NSCLC, but effective targeted therapies for patients with mutant KRAS remain limited [2]. Recently, novel studies have highlighted the importance of metabolic alterations in KRAS mutant cancers, which have high energetic needs and require biosynthetic precursors to support cell growth [3]. Specifically, KRAS has been shown to directly control the rewiring of tumour metabolism and promote aerobic glycolysis [4]. However, whether specific metabolic pathways are necessary for KRAS-mediated tumour maintenance has not been fully elucidated.
A central metabolic regulator of energy balance is the AMP-activated protein kinase (AMPK), whose role as tumour suppressor or promoter is still controversial [5]. AMPK functions as a cellular energy sensor that detects decreases in the ATP level and acts to maintain energy homeostasis when the concentration of ADP and AMP increases in the cell [6]. Therefore, AMPK up-regulates catabolic pathways that generate ATP such as glucose uptake, autophagy and fatty acid oxidation, while suppressing anabolic pathways that consume ATP such as fatty acid synthesis, cell growth and protein synthesis [7]. AMPK is characterised by a catalytic alpha subunit containing the threonine site of phosphorylation (Thr 172) essential for activation of the kinase. In mammals there are two isoforms of the alpha subunit, AMPKα1 and AMPKα2, respectively, encoded by two different genes, PRKAA1 and PRKAA2, with potential distinct regulatory properties [8]. By acting as a metabolic checkpoint that coordinates cell growth with energy status, AMPK has been considered to mediate the tumour suppressor activity of the liver kinase LKB1 [9,10,11] that is inactivated in 20% of lung adenocarcinoma, often in concurrence with KRAS mutations [12]. However, evidence reported that the role of AMPK in cancer can be LKB1-independent [13, 14] and other members of the AMPK family might be mediators of the LKB1 function [15]. Furthermore, recent studies highlighted a context-specific tumour promoter role in the tumour microenvironment [16, 17], leaving numerous questions on the AMPK behaviour in tumorigenesis unsolved.
LncRNAs are non-protein-coding RNA molecules longer than 200 nucleotides that bind to DNA, RNA and proteins to control gene regulation at every level [18]. Because of their potential to modulate multiple genes in cis or in trans, they are now receiving great attention as attractive targets in cancer treatment. LncRNAs not only contribute to proliferation, viability and motility of tumour cells [19] but are also associated with metabolic reprogramming in cancer [20]. Nevertheless, only a few functional lncRNAs have been characterized to date and their involvement in the regulation of kinases signalling remains largely unknown.
In this study, we show that AMPKα knockout (KO) promotes KRAS-induced tumorigenesis in vivo, therefore supporting a tumour suppressor role for AMPKα in non-small cell lung cancer. Moreover, our study reveals a new regulatory mechanism of AMPKα activation mediated by lncRNAs. We identified the lncRNA KIMAT1 (ENSG00000228709) as an lncRNA modulated by AMPKα that in turn regulates AMPKα phosphorylation and activity. Our work sheds light on the role of AMPKα in cancer, and on a new level of regulation of AMPKα activation controlled by lncRNAs that can potentially be exploited for therapeutic intervention.
Results
Inactivation of AMPKα promotes lung cancer progression in Kras LSLG12D/+ /AMPKα fl/fl mice
To study the role of AMPKα in vivo we have employed genetically engineered mouse models with conditional KO of both AMPK alpha subunits, alone or in combination. As homozygous deletion of AMPKα subunits is embryonic lethal, we used conditional KO mouse models with homozygous deletions of the single AMPKα1 (AMPKα1fl/fl, Prkaa1 gene) and AMPKα2 (AMPKα2fl/fl, Prkaa2 gene) subunits specifically deleted in the lungs upon intranasal administration of the AdenoCre recombinase (Ad-Cre). To achieve the complete loss of AMPKα, we crossed the two strains to produce a conditional KO mouse model with deletion of both alpha subunits (AMPKα1fl/fl /AMPKα2fl/fl). AMPKα+/+ (WT), AMPKα1fl/fl (α1KO), AMPKα2fl/fl (α2KO), and AMPKα1fl/fl/AMPKα2fl/fl (α1/α2KO) mice were dosed with Ad-Cre and monitored for lung cancer development up to 24 months (Fig. 1a). To verify the efficient deletion of AMPK alpha, immunohistochemistry analysis (IHC) of phospho-Acetyl-CoA carboxylase (p-ACCSer79), which is a direct substrate and a well-established indicator of the AMPK activity [21], was used as a readout of AMPKα activity and deletion. p-ACCSer79 expression was lower in α1KO, α2KO and α1/α2 KO mice compared to WT mice indicating a decrease of AMPKα activity (Fig. 1b and Supplementary Fig. S1a). Histological analysis performed on haematoxylin & eosin (H&E) stained lung sections showed an increase in the mucosa-associated lymphoid tissue in the KO mice, although a similar phenotype was observed in the wild-type AMPKα+/+ mice (Fig. 1c) suggesting a possible side effect of the Ad-Cre [22]. However, 3.6% of AMPKα1fl/fl mice, 1.8% of AMPKα2fl/fl mice and 1.8% of AMPKα1fl/fl/AMPKα2fl/fl mice developed early adenomas between 10 and 18 months, and 1.8% of AMPKα1fl/fl/AMPKα2fl/fl mice developed lung adenocarcinoma at 21 months after treatment (Fig. 1c, d), denoting a possible low tumour incidence caused by loss of AMPKα.
a Schematic representation of the experimental design for AMPKα+/+ (WT), AMPKα1fl/fl (α1KO), AMPKα2fl/fl (α2KO), and AMPKα1fl/fl/AMPKα2fl/fl (α1/α2KO) conditional KO mouse models utilised in the study. Homozygous deletion of AMPKα1 and AMPKα2 subunits was specifically induced in the lungs by intranasal inhalation of Ad-Cre recombinase. b Quantification of IHC staining for p-ACCSer79 in WT (n = 7), α1KO (n = 7), α2KO (n = 21) and α1/α2KO (n = 15) mice. Error bars show SD. *P < 0.05, **P < 0.01 by a 2-tailed Student’s t-test (Fig. S1). c Representative H&E stained lung sections of AMPKα conditional KO mice sacrificed between 10 and 21 months. The WT#1, #2, #3, α1KO#1, α2KO#1, #2 and α1/α2KO#1 mice presented mucosa-associated lymphoid tissue. The α1KO#2, #3, α2KO#3 and α1/α2KO#2 mice developed adenoma, while the α1/α2KO#3 mouse developed a well-differentiated adenocarcinoma (scale bar 400 μm). d Table reporting the percentage of lung adenoma and adenocarcinoma developed in AMPKα KO mice upon treatment with Ad-Cre.
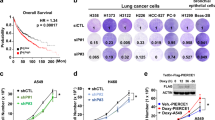
Next, we investigated whether the absence of AMPKα could accelerate KRAS-induced lung tumourigenesis. AMPKα1, AMPKα2 and AMPKα1/α2 KO mice were crossed with a conditional Lox-Stop-Lox KRASG12D mouse model. Ad-Cre intranasal instillation resulted in concomitant activation of KrasG12D, and simultaneous deletion of Prkaa1 and Prkaa2 in the lung epithelium. Mice wild-type for AMPKα have been indicated as “K”, mice with deletion of AMPKα1 or AMPKα2 alone as “Kα1” or “Kα2”, respectively, and mice with loss of both subunits as “Kα2”. To validate the model and assess the efficiency of AMPKα deletion, we used a combination of laser capture micro-dissection, western blot analysis and immunohistochemistry (Fig. 2a). We isolated DNA and RNA from tumours using laser capture micro-dissection and verified recombination at the DNA level, and expression of AMPKα1 and AMPKα2 at the RNA level, confirming deletion of both AMPKα subunits (Supplementary Fig. S2a–c). Western blot analysis was performed on tumour lysates for p-AMPKαThr172 and p-ACCSer79, whose expressions were lower in Kα tumours than in K tumours (Supplementary Fig. S2d). Immunohistochemistry for p-ACCSer79 expression was performed on Ad-Cre lung sections as a readout of AMPKα activity and deletion. p-ACCSer79 expression was lower in Kα1, Kα2 and Kα mice compared to K mice, proving efficient deletion of AMPKα (Fig. 2b and Supplementary Fig. S2e). To evaluate the effect of AMPKα loss on lung cancer development, H&E staining was performed on lung sections following Ad-Cre inhalation. Histological analysis showed higher tumour burden in Kα1, Kα2 and Kα mice than in KRASG12D mice alone (K), suggesting that loss of AMPKα promotes tumour development (Fig. 2c, d). Intriguingly, deletion of the single AMPKα1 or AMPKα2 subunit or both resulted in significantly reduced survival compared to KRASG12D mice (Fig. 2e), with a median survival of 12.5 weeks for Kα1 mice, 15 weeks for Kα2 mice and 13.5 weeks for the double KO mice Kα, compared to 17.5 weeks of K mice, indicating a possible important effect for AMPKα in KRAS-mediated lung tumourigenesis. Altogether, these results demonstrate that AMPKα has a tumour suppressor role in NSCLC and its loss promotes KRAS-mediated lung tumorigenesis.
a Schematic representation of the experimental design for KrasLSLG12D/+/AMPKα+/+ (K), KrasLSLG12D/+/AMPKα1fl/fl (Kα1), KrasLSLG12D/+/AMPKα2fl/fl (Kα2) and KrasLSLG12D/+/AMPKα1fl/fl/AMPKα2fl/fl (Kα) mice administered with Ad-Cre virus. b Quantification of IHC staining for p-ACCSer79 in K (n = 7), Kα1 (n = 26), Kα2 (n = 13) and Kα (n = 8) mice. Error bars show SD. **P < 0.01, ***P < 0.001 by a 2-tailed Student’s t-test. c Representative H&E stained lung sections of two K mice and two Kα mice sacrificed at 19 weeks after treatment (scale bar 500 μm for whole lung section and 100 μm for insert). d Quantification of the tumour/lung ratio of H&E lung sections for K, Kα1, Kα2 and Kα mice. *P < 0.05, ***P < 0.001 by a 2-tailed Student’s t-test. e Overall survival of KrasLSLG12D/+/AMPKαfl/fl mice following treatment with Ad-Cre. Kaplan–Meier survival curve for AMPKα wild-type (K mice n = 10, median survival 17.5 weeks), AMPKα1 KO (Kα1 mice n = 28, median survival 12.5 weeks), AMPKα2 KO (Kα2 mice n = 19, median survival 15 weeks) and AMPKα KO (Kα mice n = 17, median survival 13.5 weeks) mice treated with Ad-Cre at 6 weeks and sacrificed when showing signs of distress such as heavy breathing, hunched posture, and lethargic appearance. **P < 0.01, ***P < 0.001 by log-rank test (Fig. S2).
AMPKα silencing promotes cell growth and cell migration in vitro
To further investigate the role of AMPKα in NSCLC tumorigenesis, the effect of AMPKα loss on cell growth and migration was evaluated by loss-of-function studies in vitro. First, AMPKα expression was analysed in a panel of NSCLC cell lines including normal lung, primary and metastatic cell lines (Supplementary Table S1). In general, all the cell lines expressed AMPKα with the AMPKα1 subunit more abundant than the AMPKα2 subunit (Supplementary Fig. S3a). For the purpose of this study, lung adenocarcinoma KRAS mutant (Calu1 and A549) and KRAS wild-type (H1299) cell lines were chosen to further investigate the role of AMPKα. Cells were stably transduced with AMPKα1 and/or AMPKα2 shRNA lentiviruses. We generated stable AMPKα knockdown (KD) cell lines for the single AMPKα1 (shRNAα1) and AMPKα2 (shRNAα2) subunits and for total AMPKα (shRNAα1/α2). Cells were kept in selection with puromycin to isolate positive clones. Western blot and qRT-PCR analysis confirmed down-regulation of AMPKα in shAMPKα1 and shAMPKα2 cells compared to control cells (Supplementary Fig. S3b, c). Similarly, decreased expression of total AMPKα is evident in AMPKα1/α2 KD compared to control cells in all the cell lines analysed (Supplementary Fig. S3b, c).
Next, we tested whether down-regulation of AMPKα would affect the growth of NSCLC cell lines using colony formation assay. AMPKα silencing increased colony formation in A549 and H1299 compared to control cells (Fig. 3a, b). An Oris migration assay was performed to assess cell migration. Down-regulation of AMPKα1, AMPKα2 and total AMPKα caused a significant increase in cell migration in A549, H1299 and Calu1 cells (Fig. 3c, d and Supplementary Fig. S4a). To rule out the possibility that cell migration was affected by changes in cell proliferation, we monitored cell confluence upon silencing of AMPKα, using an IncuCyte Zoom live-cell imaging systems, and did not observe significant changes between shAMPKα KD cells and control cells up to 64 h (Fig. S4b). Furthermore, overexpression of AMPKα1 and AMPKα2 subunits (AMPKα OE) rescued the increase in cell growth and cell migration observed after AMPKα silencing, confirming a tumour suppressor role (Fig. S5a–c). Although we observed similar effects in both KRAS mutant and KRAS wild-type cells, we should note that H1299 cells harbour wild-type KRAS copy number gain which has been shown to be another means of KRAS activation [23]. Altogether, these results suggest that AMPKα plays a tumour suppressor role in NSCLC cells and both AMPKα1 and AMPKα2 subunits work similarly.
a, b Representative pictures of colony formation assay of AMPKα1, AMPKα2 and total AMPKα1/α2 A549 (a) and H1299 (b) KD cells eight days after cell seeding (top) and quantification (bottom) represented as average number of colonies of three biological replicates ± SD. c Representative fluorescence images of Oris migration assay for H1299 cells stably transduced with shRNA lentiviruses for AMPKα1, AMPKα2 and total AMPKα1/α2. Photos were taken at the beginning (time 0) and at the end of the experiments (24 h). Scale bar 500 μm. d Quantification of cell migration described in c. Data show mean ± SD of three biological replicates. *P < 0.05, **P < 0.01, ***P < 0.001 by a 2-tailed Student’s t-test (Figs. S3–S5).
KRAS/KIMAT1/AMPKα axis
To understand the correlation between KRAS and AMPKα observed in vivo, we asked whether KRAS could affect AMPKα activation in vitro. KRAS mutant NSCLC cell lines were treated with trametinib, an inhibitor of the mitogen-activated protein kinase enzymes (MEK1 and/or MEK2) [24], and AMPKα phosphorylation was assessed by western blot analysis. MEK inhibition, evaluated through phosphorylation of its downstream target ERK, caused an increase in the phosphorylation levels of AMPKα in all the cell lines analysed (Fig. 4a). In accordance with this finding, Calu1 cells transfected with a plasmid expressing the mutant KRASG12D had decreased AMPKα phosphorylation (Fig. 4b), confirming that KRAS affects AMPKα activation.
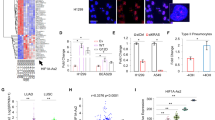
a Immunoblot of AMPKα upon inhibition of KRAS with trametinib (20 nM) for 24 h in A549, Calu1, CORL23 and Calu6 cells. The numbers below the p-AMPKαThr172 lane represent protein quantification relative to total AMPKα and GAPDH, which was determined from the band intensity using Image J software and normalised to the DMSO samples. b Immunoblot of AMPKα in Calu1 cells transfected with empty vector (EV) or plasmid overexpressing mutant KRAS(G12D). c Over-representation analysis (ORA) for RNA-Seq of AMPKα1/α2 KD H1299 cells compared to control cells. d Heatmap showing lncRNAs differentially expressed in H1299 AMPKα1/α2 KD cells relative to control cells. KIMAT1 (AP001065.15) is indicated by a red star. e Schematic representation of the KIMAT1 location on Chr.21. f qRT-PCR analysis of KIMAT1 expression in TMAs containing lung adenocarcinoma samples with mutant KRASG12D (n = 8, P = patient) and matched normal lungs (n = 8). KIMAT1 expression analysed by qRT-PCR after AMPKα1/α2 down-regulation (g) and AMPKα1/α2 overexpression (AMPKα OE) (h) in NSCLC cells. Error bars show SD of three and two biological replicates, respectively. LncRNA expression analysed by qRT-PCR (i) and AMPKα status checked by western blot (j) after inhibition of total AMPKα in H1299 and Calu1 cells treated with dorsomorphin (10 μM) for 24 h, and after activation with A769662 (100 nM) for six hours. Data show mean ± SD of three independent experiments. *P < 0.05, **P < 0.01 by a 2-tailed Student’s t-test. All blots are representative of at least three biological replicates. β-actin and GAPDH were used as loading control.
To shed light on the molecular mechanisms through which AMPKα could be involved in lung tumorigenesis, we analysed by RNA-sequencing the whole transcriptome upon AMPKα1, AMPKα2 or AMPKα1/α2 KD in H1299 cells. Interestingly, over-representation analysis (ORA) showed enrichment for the ERK pathway (Fig. 4c), further supporting a potential KRAS/AMPKα axis. Given the pleiotropic function of lncRNAs, we next searched for lncRNAs differentially expressed in the same conditions (AMPKα1, AMPKα2 or AMPKα1/α2 KD versus control) to possibly establish a link between AMPKα and the KRAS signalling (Fig. 4d). Nine lncRNAs were up-regulated and nine were down-regulated in shAMPKα1 cells compared to control cells, while seven lncRNAs were up-regulated and eight were down-regulated in shAMPKα2 cells compared to control cells (Supplementary Table S2). In the double AMPKα1/α2 KD cells, eight lncRNAs were up-regulated and 12 lncRNAs down-regulated (Supplementary Table S2). The majority of lncRNAs were commonly regulated by the two subunits, further confirming that they have similar roles and modulate the same pathways.
AP001065.15 (ENSG00000228709) was identified as the top up-regulated lncRNA upon depletion of AMPKα1 and AMPKα2 subunits alone or in combination (Fig. 4d and Supplementary Table S2). AP001065.15 is a 912 nt long intergenic non-coding RNA on chromosome 21 (Fig. 4e) that we previously identified and characterized as a KRAS-responsive lncRNA and named KIMAT1 (KRAS-Induced Metastasis-Associated Transcript 1) [23]. Notably, KIMAT1 was up-regulated in a majority of KRASG12D mutant lung adenocarcinomas compared to matched normal lung (Fig. 4f). AMPKα depletion induced up-regulation of KIMAT1 in three different cell lines (Fig. 4g), while AMPKα overexpression decreased KIMAT1 expression level in H1299 and Calu1 cells as assessed by qRT-PCR (Fig. 4h). Furthermore, KIMAT1 was significantly up-regulated in cells treated with the AMPKα inhibitor dorsomorphin [25] compared to untreated cells, while activation of AMPKα through the A769662 compound [26] caused a decrease in the level of KIMAT1 (Fig. 4i, j), confirming that AMPKα regulates KIMAT1 expression.
To elucidate the mechanism through which AMPK could modulate KIMAT1, we looked at mTOR, a critical effector of AMPK-mediated cell growth and proliferation [27]. As previously reported, AMPK activation halts the mTOR pathway by directly phosphorylating its regulatory subunit Raptor [28] and the upstream regulator TSC2 [29] (Fig. 5a). Interestingly, NSCLC cells treated with the mTOR inhibitor rapamycin showed a reduction in the level of KIMAT1 (Fig. 5b). Moreover, rapamycin prevented the increase of KIMAT1 induced by AMPKα KD (Fig. 5c) suggesting that AMPK might regulate KIMAT1 through the mTOR signalling.
a Immunoblot of mTOR upon activation of AMPK in H1299 cells treated with A769662 (100 nM) for six hours. b qRT-PCR analysis of KIMAT1 expression following inhibition of mTOR with rapamycin (200 nM) for 24 h in NSCLC cells. Error bars show SD of three independent experiments. c qRT-PCR for KIMAT1 (top) and immunoblot of AMPKα and mTOR (bottom) upon inhibition of mTOR pathway with rapamycin in AMPKα KD Calu1 cells. Error bars show SD of three independent experiments. d Immunoblot of AMPKα (left) and qRT-PCR for KIMAT1 (right) upon silencing of KIMAT1 through LNATM-GapmeR (GpR-KIMAT1) in H1299 and Calu1 cells, represented as average of three biological replicates ± SD. e Immunoblot of AMPKα (left) and qRT-PCR for KIMAT1 (right) upon overexpression of KIMAT1 (pCDH-KIMAT1) in Calu1 cells. Error bars show SD of two independent experiments. f Immunoblot of AMPKα and ACC (left) and qRT-PCR for KIMAT1 (right) upon treatment with trametinib (20 nM) for 24 h and overexpression of KIMAT1 in Calu1 cells. Error bars show SD of two independent experiments. n.s. = not significant, *P < 0.05, **P < 0.01, ***P < 0.001 by a 2-tailed Student’s t-test. All blots are representative of at least three biological replicates. β-actin and GAPDH were used as loading control.
We also investigated whether silencing of KIMAT1 through LNATM-GapmeRs, antisense oligonucleotides that trigger RNase-H-mediated degradation [30], affected AMPKα status. Results revealed an increase in the phosphorylation status of AMPKα upon KIMAT1 depletion (GpR-KIMAT1) (Fig. 5d) and a decrease of AMPKα phosphorylation upon KIMAT1 overexpression (pCDH-KIMAT1) (Fig. 5e). In a rescue experiment, KIMAT1 overexpression and simultaneous inhibition of KRAS with trametinib reduced both AMPKα phosphorylation level and phosphorylation of ACC, which reports on activity downstream of AMPKα (Fig. 5f). Together these findings indicate that KIMAT1 can rescue the phenotype caused by KRAS and is responsible for AMPKα activation. In summary, AMPKα KD induces KIMAT1, which in turn represses AMPKα activation, establishing a negative feedback loop.
KIMAT1 directly interacts with LDHB
To understand how KIMAT1 could regulate AMPKα activation, we looked at KIMAT1 interacting proteins. Through RNA antisense purification (RAP) coupled with quantitative mass spectrometry (RAP-MS) using biotinylated KIMAT1 antisense probes, we have previously identified 45 KIMAT1 interacting proteins [23], including the lactate dehydrogenase B (LDHB), a glycolytic enzyme involved in glucose metabolism, whose expression correlates with both KRAS genomic copy number gain and KRAS mutation [31]. To confirm the association between KIMAT1 and LDHB we performed cross-linked RNA Immunoprecipitation (CLIP) using a specific antibody against LDHB and a non-specific antibody (immunoglobulin G, IgG) as a negative control, followed by qRT-PCR for KIMAT1. We observed a significant enrichment of KIMAT1 in the LDHB antibody-bound complex compared to the IgG-bound complex, proving a direct interaction between KIMAT1 and LDHB (Fig. 6a). Importantly, KIMAT1 overexpression (pCDH-KIMAT1) in H460 cells, with low expression of endogenous KIMAT1, increased LDHB protein level compared to control cells (pCDH-CTL) (Fig. 6b). Conversely, KIMAT1 KD (GpR-KIMAT1) reduced LDHB expression (Fig. 6c). These results prove that KIMAT1 is important for LDHB protein stability. The overexpression of AMPKα decreased LDHB expression (Fig. 6d). However, silencing of AMPKα in combination with KIMAT1 down-regulation was insufficient to induce a significant increase in LDHB expression as the absence of KIMAT1 destabilises LDHB (Fig. 6e).
a Cross-linked RNA Immunoprecipitation (CLIP) performed in H1299 cells with an antibody against LDHB (top) followed by qRT-PCR for KIMAT1 (bottom) showed as mean ± SD of three biological replicates. Ubiquitin C (UBC) was used as a negative control. H-chain=Heavy chain. b Immunoblot of LDHB (top) and qRT-PCR for KIMAT1 (bottom) in H460 cells stably transduced with control (pCDH-CTL) or KIMAT1 (pCDH-KIMAT1) lentiviral vector to overexpress the lncRNA. Error bars show SD of three independent experiments c Immunoblot of LDHB upon transfection with control (GpR-CTL) or anti-KIMAT1 (GpR-KIMAT1) LNATM-GapmeRs in H1299 cells. b, c The numbers below the LDHB lane represent the protein levels, relative to their β-actin, which were determined from the band intensity using Image J software, and normalised to controls. d Immunoblot of LDHB upon overexpression of AMPKα in H1299 cells. The lower band corresponds to the AMPKα1 subunit, while the upper band corresponds to the GFP-tagged AMPKα2 subunit. e Immunoblot of LDHB upon silencing of KIMAT1 and AMPKα in H1299 cells. f Schematic representation of the oxidation of lactate into pyruvate and concomitant conversion of NAD+ into NADH catalysed by LDHB. LDHA preferentially catalyses the opposite reduction of pyruvate into lactate. g Lactate concentration measured by lactate assay upon silencing of KIMAT1 in Calu1 cells, showed as mean ± SD of three biological replicates. h Immunoblot of AMPKα upon silencing of LDHB in Calu1 cells. i Immunoblot of LDHB upon inhibition of mTOR with rapamycin (200 nM) for 24 h in H1299 and CORL23 cells. *P < 0.05, **P < 0.01 by a 2-tailed Student’s t-test. All blots are representative of at least three biological replicates. β-actin was used as loading control.
LDHB, together with LDHA, catalyses the interconversion of pyruvate into lactate and concomitant interconversion of NADH into NAD+, with a preference for the lactate oxidation reaction (Fig. 6f) [32]. To assess whether silencing of KIMAT1 affected the activity of LDHB other than its expression, we analysed lactate intracellular level upon KIMAT1 KD and found an increase in lactate concentration (Fig. 6g). This indicates a reduction in pyruvate conversion that supplies energy to the cells, thus reducing ATP levels [33]. In agreement, LDHB silencing increased the phosphorylation level of AMPKα, suggesting that KIMAT1 influences AMPKα phosphorylation via LDHB (Fig. 6h). Moreover, the expression of LDHB decreased upon rapamycin treatment supporting the involvement of the mTOR pathway in this mechanism (Fig. 6i).
In vitro depletion of LDHB through shRNA lentiviruses caused a decrease in cell proliferation and colony formation, indicating that LDHB acts as an oncogene in NSCLC cells (Fig. 7a–c). Notably, LDHB was up-regulated in 540 lung adenocarcinoma samples (LuAd) compared to normal lung samples (n = 59) from the TCGA dataset, supporting a tumour promoter role (Fig. 7d). In addition, Kaplan–Meier survival analysis revealed that patients with high LDHB expression had worse overall survival compared to patients with low LDHB expression (Fig. 7e). Finally, IHC analysis showed that LDHB expression was up-regulated in Kα mice compared to K mice alone denoting that LDHB could be a mediator of the AMPKα signalling in vivo (Fig. 7f).
a Immunoblot of LDHB silencing in A549 and H1299 cells stably transduced with two different shRNAs. b, c Representative pictures of colony formation assay (b) and representative curves of proliferation assay measured by IncuCyte analysis (c) of A549 and H1299 cells stably transduced with LDHB shRNAs. Error bars show SD for technical replicates. *P < 0.05 by a 2-tailed Student’s t-test. Data are representative of at least two independent experiments. d Analysis of LDHB expression in 59 normal and 540 tumour specimens of lung adenocarcinoma (LuAd) from the TCGA. e Univariate Kaplan–Meier survival curve for lung adenocarcinoma patients with low and high expression of LDHB from the LuAd TCGA dataset. f Representative IHC staining for LDHB (left) in two K and two Kα mice and quantification (right) for K (n = 7) and Kα (n = 11) mice. Error bars show SD. Scale bar 100 μm. *P < 0.05 by a 2-tailed Student’s t-test.
In summary, we have identified a novel KRAS/KIMAT1/LDHB/AMPKα axis (Fig. 8) where KRAS activation and/or mutation activate KIMAT1. KIMAT1 binds and stabilizes LDHB, which, by promoting the interconversion of lactate into pyruvate, affects the level of ATP and consequently reduces AMPKα activation. AMPKα depletion, in turn, induces KIMAT1 through the mTOR pathway establishing a negative feedback loop.
a Schematic model of the human KRAS/KIMAT1/LDHB/AMPKα axis. KRAS activation and/or mutation activate KIMAT1. KIMAT1 binds and stabilizes LDHB, which by regulating lactate oxidation affects the ATP level and reduces AMPKα activation. AMPKα depletion, in turn, induces KIMAT1 through the mTOR pathway, establishing a negative feedback loop.
Discussion
AMPK is a key sensor of cellular energy and a metabolic checkpoint for cancer cell growth. However, its role in cancer progression is controversial because AMPK can act as a tumour suppressor or tumour promoter depending on the specific context [16, 34]. Furthermore, the two AMPKα subunits may exhibit independent oncogenic or tumour suppressive functions [8]. Our in vitro and in vivo studies support a common role for AMPKα1 and AMPKα2 as a tumour suppressor in NSCLC. Although loss of AMPKα alone induced lung cancer development with low incidence, simultaneous deletion of AMPKα and KRAS activation promoted tumour progression in a genetically engineered mouse model of lung cancer. In the KRASG12D background, the single subunit knockout mice Kα1 and Kα2 had a similar phenotype to Kα mice lacking both subunits, showing in each case increased tumour burden and reduced overall survival compared to mice carrying the KRASG12D mutation alone. In contrast with our findings, Eichner LJ and colleagues published that AMPKα loss decreased tumour growth in a mouse model of lung adenocarcinoma driven by KRASG12D, although the difference was not statistically significant compared to KRASG12D tumours alone [35]. Despite the fact that the KRASG12D mouse model is similar to the one used in our study, the different FVB strain they employed and the diverse genetic background could be responsible for the different phenotype observed. It is worth mentioning that AMPK pro-tumorigenic effect was only noted in low glucose conditions [35]. Therefore, while in tumours with energy deprivation AMPK may be fundamental for tumour growth and its inhibition could be therapeutically beneficial, in tumours with high energy and metabolism, which do not depend on AMPK, its activation would be a good strategy to halt tumour growth [35]. Accordingly, the role of AMPK in cancer is context dependent and may rely on the temporal energetic demands of the tumour. This could explain why AMPKα loss halted tumour growth in KrasLSLG12D/+/p53−/− (KP) tumours, which have a higher proliferation rate compared to KRASG12D alone tumours [36, 37] and thus likely to encounter more metabolic stress. In agreement with our findings, a recent work by Murray CW and colleagues reported tumour growth following AMPKα depletion in KRASG12D-driven lung tumours [15]. Of note, the KrasG12D mouse strain is the same we used in our study. However, while the authors focused on a mouse model harbouring the simultaneous deletion of both AMPKα1 and AMPKα2, we dissected the functional role of the single AMPKα subunits alone or in combination with KRASG12D activation and proved that they both act as tumour suppressor genes. In accordance with our in vivo results, in vitro data also showed increased cell growth and migration upon silencing of the single AMPKα1 and AMPKα2 subunits and total AMPKα in NSCLC cell lines. These effects were rescued upon AMPKα enforced expression.
LncRNAs are important players in the cancer landscape and regulate numerous pathways, including multiple metabolic processes [20]. To get insights into the mechanism behind the contribution of AMPKα to KRAS-mediated tumorigenesis, we searched for AMPKα-modulated lncRNAs. We focused on the top induced lncRNA upon AMPKα KD, KIMAT1, a KRAS-responsive lncRNA recently characterised by our group and essential for cancer cell survival [23]. We demonstrated that AMPKα silencing or inhibition increased the levels of KIMAT1, while AMPKα overexpression or activation reduced the levels of the lncRNA. A reduction in KIMAT1 expression was observed upon rapamycin treatment confirming the involvement of the mTOR pathway in KIMAT1 regulation. We have shown that KIMAT1 is transcriptionally regulated by MYC [23] and MYC has been proved as a downstream effector of the mTOR pathway [38]. Therefore, we speculate that AMPK could modulate KIMAT1 through the recruitment of transcription factors such as MYC that bind to KIMAT1 and are activated by mTOR.
KIMAT1 is not only repressed by AMPKα but it also modulates AMPKα phosphorylation by regulating LDHB, a glycolytic enzyme that converts lactate into pyruvate. LDHB is overexpressed or amplified in human lung adenocarcinoma and positively correlates with both KRAS genomic copy number gain and KRAS mutation, representing a potential therapeutic target for KRAS-driven lung cancer [31]. Importantly, LDHB was required for the growth of KRAS mutant lung tumours in vivo [31]. We demonstrated that LDHB promoted cancer cell proliferation and correlated with poor patient survival. KIMAT1 by binding and stabilizing LDHB promotes the conversion of lactate into pyruvate that fuels energy to the cells through the Krebs cycle or aerobic glycolysis [39], thus inducing an increase in ATP level and consequently decreasing AMPKα phosphorylation status.
As many other lncRNAs, KIMAT1 is not conserved in mouse. Therefore, although AMPKα KO has the same pro-tumorigenic effect in mouse and human, this effect is not directly mediated by KIMAT1 in mouse. Nevertheless, LDHB expression increased in KrasLSLG12D/+/AMPKαfl/fl mice, suggesting that a similar mechanism involving AMPKα and LDHB might regulate KRAS-driven lung tumorigenesis in mouse. In conclusion, our work shed light on the function of the AMPKα subunits in NSCLC and on a novel level of regulation of the AMPK signalling controlled by lncRNAs and identifies a KRAS/KIMAT1/LDHB/AMPKα axis that could be exploited therapeutically in patients with KRAS-driven cancers.
Materials and methods
Mouse studies
All procedures involving animals were approved by the CRUK Manchester Institute’s Animal Welfare and Ethical Review Body in accordance with the Animals Scientific Procedures Act 1986, the ARRIVE guidelines and the Committee of the National Cancer Research Institute guidelines, and conducted under project license number P72E31537 (M.G.).
The AMPKα1fl/fl (STOCK Prkaa1tm1.1Sjm/J; loxP sites flanking exon 3 of the Prkaa1 gene, encoding the AMPKα1 catalytic subunit) and AMPKα2fl/fl (STOCK Prkaa2tm1.1Sjm/J; loxP sites flanking exon 2 of the Prkaa2 gene, encoding the AMPKα2 catalytic subunit) mouse models were purchased from the Jackson Laboratories (Bar Harbour, ME), and housed according to institutional guidelines. Lox-Stop-Lox KRASG12D mouse model has been described previously [40].
All experiments utilised a mixture of female and male mice. Six weeks old littermates were instilled intranasally with Ad5-CMV-Cre recombinase (University of Iowa) at a viral titre of 1 ×107 PFU (in 50 μl of PBS) according to the protocol by DuPage et al. [41]. Body weight was measured once a week and mice were observed for signs of illness or distress as rapid weight loss (>20%) and laboured respiration. At the designated time-points, or when showing signs of breathing abnormalities, mice were euthanized by cervical dislocation, lungs excised, weighed, photographed and bisected. Part of the lungs was fixed in formalin and subjected to H&E staining for further analysis. The remaining part was snap-frozen in liquid nitrogen and stored at −80 oC. Groups allocation and monitoring were performed by colleagues of the CRUK Biological Resources Unit who were blind about treatments. Mice groups were unknown to the investigators performing histological analysis. No randomisation was used and no animals were excluded from the analysis.
Histology and immunohistochemistry
Mice lungs were fixed in 4% formalin, transferred into 70% EtOH after 24 h and embedded in paraffin blocks (FFPE). Sections were prepared and stained with haematoxylin and eosin using standard methods. Quantification was performed using Definiens Developer XD 2.0.4 software (Definiens AG, Munich, Germany) and the total burden area was calculated with the following formula: tumour area / total area × 100%. Immunohistochemistry was performed with the Refine Kit and run on the Leica bond Rx. The phospho-Acetyl-CoA Carboxylase (Ser79) antibody (#3661, CST) and the LDHB antibody (#14824-1-AP, Proteintech) were used at a dilution of 1/1000. Slides were scanned on the Leica SCN400 (Leica Microsystems, Milton Keynes, United Kingdom,) using a 20X objective lens and analysed with the Definiens software for quantification. Briefly, for each slide the tissue was segmented, the cells were segmented and then the staining intensity was scored. Haematoxylin-stained nuclei were identified by the software to generate a total cell count per tumour. A ratio between the number of stained-positive cells and total cell number was used to calculate the percentage of cells staining positive. The analysis was performed on individual slides and all lung images were analysed by the same algorithm simultaneously.
Laser Capture Micro-dissection (LCM)
Tumour areas were identified using haematoxylin-eosin staining and were micro-dissected with the Leica LMD6000 system according to the standard procedure. Briefly, the lung tissue was cut using an ultraviolet laser and specific tumour areas were collected into LCM cap. The dissected material was deparaffinised and digested with proteinase K (P8107, NEB). Genomic DNA and total RNA were extracted with the Norgen kit (#54300) according to the manufacturer’s protocol, including on-column DNase treatment. To validate AMPK recombination, 50 ng of DNA was used for PCR amplification and PCR products were analysed on a 1% agarose gel. Primers sequences (5′-3′) used for PCR are as follow: LCM_Prkaa1 Fwd: CCCACCATCACTCCATCTCT, LCM_Prkaa1 Rev: CCCACATAGGAAAGCGTGTT, LCM_Rec_Prkaa1 Rev: AGCCTGCTTGGCACACTTAT. LCM_Prkaa2 Fwd: CAATCCAGTGAGGTGAGCAT, LCM_Prkaa2 Rev: GACTAGCAAATGCTTGGGAG, LCM_Rec_Prkaa2 Rev: TCCCCTTGAACAAGCATACC.
For RNA expression, 15 ng of RNA was reverse transcribed and amplified with the iTaqTM Universal SYBR Green One-Step kit (#172-5151, Bio-Rad) according to the manufacturer’s protocol using the following primers (5′-3′): Mouse Prkaa1 Fwd: AAGCTGTTCAGGCACCCTCACA, Mouse Prkaa1 Rev: CGGCTTTCCTTTTCGTCCAACTAGG, Mouse Prkaa2 Fwd: CGCCACTCTGCTGATGCA, Mouse Prkaa2 Rev: AAATAGGTAGCTGGGCAAATCCT. Mouse Actb Fwd: TTCACCACCACAGCTGAGAG, Mouse Actb Rev: ATAGTGATGACCTGGCCGTC.
Cell lines
Lung adenocarcinoma cell line H1299, A549, H460, H292, Calu1 and Calu6, lung fibroblasts HEL299, normal human bronchial epithelium BEAS2B and HEK-293T cells were purchased from American Type Culture Collection (ATCC). Lung large cell carcinoma CORL23 cells were purchased from Sigma-Aldrich. Cell lines were cultured in RPMI-1640 (Gibco) or Dulbecco’s modified Eagles medium DMEM (Calu1, Calu6 and HEK-293T) supplemented with 10% Foetal Bovine Serum (FBS) and 100 U/mL penicillin/streptomycin (Sigma, St. Louis, MO). Cell cultures were maintained at 37 °C in a humidified incubator at 5% CO2. All cell lines were validated by STR profiling using PowerPlex 21 (Promega) and were periodically checked for Mycoplasma through in-house testing at the CRUK MI Molecular Biology Core Facility. Cell lines were carried for no more than 15 cell passages.
Stable shRNA-mediated AMPKα knockdown
Lentiviral particles with short hairpin RNA (shRNA) targeting human PRKAA1 (V3SVHS00_5032150), PRKAA2 (V3SVHS00_7245856) and a negative non-targeting control (RH54348) were purchased from Dharmacon (Lafeyette, CO, USA). Cells were seeded in 96-well plates at a density of 3.5×103 cells/well and transduced with purified lentiviral shRNA particles in 50 μl of Transduced Media containing 5 μg/mL polybrene (SC134220, Santa-Cruz Biotechnology). After a 16 h transduction, 100 μl of RPMI Growth Medium was added directly to each well without removing medium, and cells incubated for 24 h. To select for NSCLC cells with stably integrated shRNA, cells were kept in puromycin (1 μg/mL for A549 and Calu1, 1.5 μg/mL for H1299) for a week and then harvested for immunoblot analysis.
Stable shRNA-mediated LDHB knockdown
Lentiviral particles with short hairpin RNA (shRNA) targeting human LDHB and a negative non-targeting control were self-produced using the lentiviral vectors pLKO.1 (#8453, Addgene) [42]. HEK-293T cells were co-transfected with 3 μg of lentiviral vectors and the lentiviral packaging plasmids psPAX2 (#12260, Addgene) and pMD2.G (#12259, Addgene) in antibiotic-free medium using Lipofectamine 3000 (L3000-008, Thermo Fisher Scientific). Lentiviral medium was collected after 48 h of transfection, passed through a 0.45 μm filter and added to cells. Cells were incubated with the virus-containing medium for eight h and then the medium was replaced with puromycin for selection of positively transduced cells.
LNATM-GapmeRs, plasmid transfection and other reagents
LNATM-GapmeRs were designed and synthesized from Qiagen (GpR-KIMAT1: AACGAGTGCAAAGTGT, GpR-CTL: AACACGTCTATACGC). 50 nM of LNATM-GapmeRs were transfected using Lipofectamine RNAiMax (#13778-075, Thermo Fisher Scientific) according to the manufacturer’s instructions for 48 h. KIMAT1 full-length was cloned into a pCDH-GFP plasmid (CD511B-1, System Biosciences). KRAS(G12D) plasmid has been previously described [43]. AMPKα was overexpressed using the AMPKα1 (#27632, Addgene) [44] and AMPKα2 (RG210226, Origene) plasmids. The AMPKα2 plasmid expresses the complete ORF with a GFP expression tag encoding a protein with a predicted molecular weight around 80 KDa. Plasmids were transiently transfected into the cells using Lipofectamine 3000 reagent for 48 h. Commercially available ON-TARGET plus smart pool siRNA for LDHB (L-009779-00-0005) and non-targeting control (D-001810-10-05) were purchased from Dharmacon (Lafayette, CO, USA) and transfected using Lipofectamine RNAiMax according to the manufacturer’s instructions for 72 h. Dhorsomorphin (S7306), A769662 (S2697), Trametinib (S2673), Puromycin (S7417) and Rapamycin (S1039) were purchased from Selleckchem.
Colony formation, cell confluence and migration assays
For the colony formation assay, stably transduced cells were seeded in a six-well plate at a density of 800 cells/well and maintained in RPMI-1640 containing 10% FBS for eight days. Colonies were fixed with ice-cold methanol and stained with 0.05% Crystal violet (#32909, Sigma-Aldrich). Colonies were counted and quantified using ImageJ software. Cell confluence was analysed using the IncuCyte Zoom live-cell imaging systems from Essen Bioscience. Phase-contrast images of cells seeded into a 96-well plate were taken every two hours and the percentage of confluence was calculated with the IncuCyte Zoom software.
Cell migration was evaluated using an Oris 96-well cell migration assay kit (PROCMA5, Platypus Technologies, Madison, WI), according to the manufacturer’s instructions. 5 × 104 cells were seeded into a 96-well plate to attach in a monolayer around a non-toxic biocompatible gel that dissolves to reveal a cell-free detection zone. Migration in the detection zone was analysed in real-time on the PerkinElmer Opera Phenix (PerkinElmer Waltham, MA) high content imaging system. Images were acquired one hour after cell-seeding (time zero) and after 24 h using the Zeiss EC Plan Neofluar 20X air objective NA 0.4 WD 8.28 mm, and the 488 nm 50 mW laser. Multiple image tiles were stitched together using the Harmony software (PerkinElmer). Images were then analysed using ImageJ software. Quantification was performed by calculating the percentage of gap closure with the following equation: (Migration area) - (Pre-Migration area)/(Migration area) x 100.
Protein extraction and Immunoblot
Cells were washed with cold phosphate-buffered saline and lysed in 1X RIPA buffer (R0278, Sigma-Aldrich) supplemented with Complete Protease Inhibitor (#4693116001, Roche) and anti-phosphatase PhosSTOP Cocktail Tablets (#4906845001, Roche). Protein concentration was determined using the Pierce BCA Protein Assay kit (#23225, Thermo Fisher Scientific) according to the manufacturer’s instructions. Equal amounts of protein lysates and molecular weight marker (#161-0374, BioRad) were loaded on 4-12% SDS–polyacrylamide gels and transferred to PVDF membranes. The membranes were blocked in 5% non-fat dry milk in Tris-buffered saline with 0.1% Tween-20 for one h and incubated overnight with antibodies at 4 °C. After a second wash, membranes were incubated with peroxidase-conjugated secondary antibodies (GE Healthcare, Amersham, Pittsburg, PA, USA) and developed with an enhanced chemiluminescence (ECL) detection reagent (K-12049-D50, Advansta). The chemiluminescence was captured with a Chemidoc Touch imager (#733BR2370, Bio-Rad). β-actin (sc-376421, Santa Cruz Biotechnology, Santa Cruz, CA, USA) and GAPDH (14C10) (#2118, Cell Signalling Technologies) were used as loading control. 5′AMP-activated protein kinase AMPKα1 (#2795), AMPKα2 (#2757), AMPKα (#2532), Phospho-AMPKα (Thr172) (40H9) (#2535), Acetyl-CoA Carboxylase (#3662), Phospho-Acetyl-CoA Carboxylase (Ser79) (#3661), p44/42 MAPK (Erk1/2) (#9102), Phospho-p44/42 MAPK (Erk1/2) (Thr202/Tyr204) (#9101), mTOR (#2972), Phospho-mTOR (#5536) and RAS (#8955) antibodies were purchased from Cell Signalling Technologies. Human Lactate Dehydrogenase B/LDHB Antibody (MAB9205) was purchased from R&D System. To assess proteins with similar molecular weights and the phosphorylation/total levels, the membranes were stripped and/or re-probed.
Cross-linked RNA Immunoprecipitation (CLIP)
Cells were UV irradiated in ice-cold PBS at 0.8 J/cm2 using a UVP Crosslinker (#849-3010-2, Analytik Jena), and lysed in RIPA buffer with 1X protease inhibitor cocktail and RNase inhibitor (M0314, NEB) for 10 minutes on ice. Lysates were then precleared with Protein G beads (#100AD, Thermo Fisher Scientific) for one h at 4 °C, and incubated with IgG (#2729, Cell Signalling Technologies) or the indicated antibodies for three h at 4 °C. Immuno-complexes were precipitated with Protein G beads and washed six times with washing buffer (50 mM Tris-HCl pH 7.5, 150 mM NaCl, 1 mM MgCl2, and 0.05% IGEPAL CA-630). 10% of beads were boiled with 1X Laemli buffer (#1610747, Bio-Rad) at 95 °C for five minutes and loaded on a polyacrylammide gel to verify immunoprecipitation efficiency. The remaining beads were treated with TurboDNase (AM2238, Thermo Fisher Scientific) and Proteinase K and RNA was isolated using the TRIzol reagent. Ubiquitin (UBC) was used as a negative control.
Lactate assay
Intracellular level of lactate was measured with a lactate assay kit (MAK064, Sigma-Aldrich) where the lactate concentration is determined by an enzymatic assay that results in a colorimetric product proportional to the lactate present. Absorbance was read at 570 nm using a Spectramax spectrophotometer microplate reader (Molecular Devices) and lactate concentration was normalised to protein concentration using the Pierce BCA Protein Assay kit according to the manufacturer’s instructions.
Expression of LDHB in TCGA
Analysis of The Cancer Genome Atlas (TCGA) dataset of Lung Adenocarcinoma (LuAd) was performed on data acquired from the Cancer Genomics Cloud. The raw read counts were first converted to RPKM values and then transformed to log2 scale. A total of 59 normal and 540 tumour specimens of lung adenocarcinoma were analysed.
Overall survival analysis
Overall survival analysis was performed on LuAd dataset. Kaplan–Meier curve was generated at 20 years follow-up period. BAM files for the dataset were downloaded from GDC (https://portal.gdc.cancer.gov/) and counts were extracted from the BAM files using feature Counts.
RNA extraction and quantitative real-time PCR
Cells and tissues were lysed using TRIzol reagent (#15596026, Thermo Fisher Scientific) and total RNA was isolated according to manufacturer’s instructions. For genes and lncRNA detection, 500 ng of total RNA was reverse-transcribed using the Verso cDNA synthesis kit (AB1453A, Thermo Fisher Scientific) according to the manufacturer’s protocol. Then, 1 μl cDNA from each reaction was amplified with the FS Universal SYBR Green Master Rox mix (#4913850001, Roche). The reaction was run on a Lighcycler 96 instrument (Roche) and normalised for relative expression to ACTB (β-actin). The fold change was calculated using the comparative ΔCt method. All the primers used were custom-designed and sequences (5′-3′) are listed below. Human PRKAA1 Fwd: AGGAGAGCTATTTGATTATATCTGTAAGAATG, Human PRKAA1 Rev: ACACCAGAAAGGATCTGTTGGAA, Human PRKAA2 Fwd: CGGCTCTTTCAGCAGATTCTGT, Human PRKAA2 Rev: ATCGGCTATCTTGGCATTCATG. KIMAT1 Fwd: AAAACAACGCCCCCTGTC, KIMAT1 Rev: TCCCCTCTCTGAAAACGTACA, Human ACTB Fwd: TGACATTAAGGAGAAGCTGTGCTAC, Human ACTB Rev: GAGTTGAAGGTAGTTTCGTGGATG, LDHB Fwd: GATGGATTTTGGGGGAACAT, LDHB Rev: AACACCTGCCACATTCACAC, UBC Fwd: TAAGGAAGGCATCCCTCCTGAC, UBC Rev: TTCACGAAGATTTGCATCCCAC.
RNA-sequencing
Poly-A libraries were prepared with a Sureselect PolyA kit (G9691-90010, Agilent) from 1 μg of total RNA. Samples were sequenced using a Nextseq500 instrument (Illumina) for 75 bp. Sequencing was between 20 and 30 million reads per sample. RNA-Seq reads were quality checked and aligned to the human genome assembly (GRCh37) using the RSubread package aligner [45] with the default settings. Differential expression (DE) was evaluated using the DESeq2 Bioconductor package [46] by comparing the gene level integer read count for the knockdown and control samples.
Statistical analysis
Statistics and graphs were generated using Prism 7 (GraphPad). Graphs show mean ± SD from three biological replicates for all experiments unless indicated otherwise in the figure legend. Statistical significance was assessed by a paired, 2-tailed Student’s t-test. Log-rank test was used for the survival curve (Kaplan–Meier) analysis. n.s. = not significant, *P < 0.05, **P < 0.01, ***P < 0.001.
For RNA-Seq analysis, the resulting P values were adjusted using the Benjamini and Hochberg approach for controlling the false discovery rate. Genes with an adjusted P value determined to be < 0.05 (FDR < 0.05) by DESeq2 between two groups were considered to be differentially expressed.
Data availability
RNA-Seq data generated in this study have been deposited in the NCBI Gene Expression Omnibus database under accession code: GSE165843.
References
Moran C. Importance of molecular features of non-small cell lung cancer for choice of treatment. Am J Pathol. 2011;178:1940–8.
Yang H, Liang SQ, Schmid RA, Peng RW. New horizons in KRAS-mutant lung cancer: dawn after darkness. Front Oncol. 2019;9:1–13.
Pupo E, Avanzato D, Middonti E, Bussolino F, Lanzetti L. KRAS-driven metabolic rewiring reveals novel actionable targets in cancer. Front Oncol. 2019;9:1–9.
Yun J, Rago C, Cheong I, Pagliarini R, Angenendt P, Rajagopalan H, et al. Glucose deprivation contributes to the development of KRAS pathway mutations in tumor cells. Science. 2009;325:1555–9.
Faubert B, Vincent EE, Poffenberger MC, Jones RG. The AMP-activated protein kinase (AMPK) and cancer: Many faces of a metabolic regulator. Cancer Lett. 2015;356:165–70.
Carling D, Thornton C, Woods A, Sanders MJ. AMP-activated protein kinase: new regulation, new roles? Biochem J. 2012;445:11–27.
Hardie DG. AMP-activated protein kinase: an energy sensor that regulates all aspects of cell function. Genes Dev. 2011;25:1895–908.
Ross FA, MacKintosh C, Hardie DG. AMP-activated protein kinase: a cellular energy sensor that comes in 12 flavours. FEBS J. 2016;283:2987–3001.
Hawley SA, Boudeau J, Reid JL, Mustard KJ, Udd L, Makela TP, et al. Complexes between the LKB1 tumor suppressor, STRAD alpha/beta and MO25 alpha/beta are upstream kinases in the AMP-activated protein kinase cascade. J Biol. 2003;2:28.
Woods A, Johnstone SR, Dickerson K, Leiper FC, Fryer LGD, Neumann D, et al. LKB1 Is the Upstream Kinase in the AMP-Activated Protein Kinase Cascade. Curr Biol. 2003;13:2004–8.
Shaw RJ, Kosmatka M, Bardeesy N, Hurley RL, Witters LA, DePinho RA, et al. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc Natl Acad Sci USA. 2004;101:3329–35.
Skoulidis F, Byers LA, Diao L, Papadimitrakopoulou VA, Tong P, Izzo J, et al. Co-occurring genomic alterations define major subsets of KRAS-mutant lung adenocarcinoma with distinct biology, immune profiles, and therapeutic vulnerabilities. Cancer Discov. 2015;5:861–78.
Hawley SA, Pan DA, Mustard KJ, Ross L, Bain J, Edelman AM, et al. Calmodulin-dependent protein kinase kinase-beta is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab. 2005;2:9–19.
Woods A, Dickerson K, Heath R, Hong S-P, Momcilovic M, Johnstone SR, et al. Ca2+/calmodulin-dependent protein kinase kinase-beta acts upstream of AMP-activated protein kinase in mammalian cells. Cell Metab. 2005;2:21–33.
Murray CW, Brady JJ, Tsai MK, Li C, Winters IP, Tang R, et al. An LKB1–SIK axis suppresses lung tumor growth and controls differentiation. Cancer Disco. 2019;9:1590–605.
Jeon S-M, Chandel NS, Hay N. AMPK regulates NADPH homeostasis to promote tumour cell survival during energy stress. Nature. 2012;485:661–5.
Saha M, Kumar S, Bukhari S, Balaji SA, Kumar P, Hindupur SK, et al. AMPK-AKT double negative feedback loop in breast cancer cells regulates their adaptation to matrix deprivation. Cancer Res. 2018;78:1497–510.
Fang Y, Fullwood MJ. Roles, functions, and mechanisms of long non-coding RNAs in cancer. Genomics Proteom Bioinforma. 2016;14:42–54.
Schmitt AM, Chang HY. Long noncoding RNAs in cancer pathways. Cancer Cell. 2016;29:452–63.
Sun H, Huang Z, Sheng W, Xu MD. Emerging roles of long non-coding RNAs in tumor metabolism. J Hematol Oncol. 2018;11:1–16.
Howell JJ, Hellberg K, Turner M, Talbott G, Kolar MJ, Ross DS, et al. Metformin Inhibits Hepatic mTORC1 Signaling via Dose-Dependent Mechanisms Involving AMPK and the TSC Complex. Cell Metab. 2017;25:463–71.
Pfeifer A, Brandon EP, Kootstra N, Gage FH, Verma IM. Delivery of the Cre recombinase by a self-deleting lentiviral vector: Efficient gene targeting in vivo. Proc Natl Acad Sci USA. 2001;98:11450–5.
Shi L, Magee P, Fassan M, Sahoo S, Leong HS, Lee D, et al. A KRAS-responsive long non-coding RNA controls microRNA processing. Nat Commun. 2021;12:1–19. https://doi.org/10.1038/s41467-021-22337-3.
Yamaguchi T, Kakefuda R, Tajima N, Sowa Y, Sakai T. Antitumor activities of JTP-74057 (GSK1120212), a novel MEK1/2 inhibitor, on colorectal cancer cell lines in vitro and in vivo. Int J Oncol. 2011;39:23–31.
Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108(Oct):1167–74.
Cool B, Zinker B, Chiou W, Kifle L, Cao N, Perham M, et al. Identification and characterization of a small molecule AMPK activator that treats key components of type 2 diabetes and the metabolic syndrome. Cell Metab. 2006;3(Jun):403–16.
Shaw RJ. LKB1 and AMP-activated protein kinase control of mTOR signalling and growth. Acta Physiol (Oxf). 2009;196(May):65–80.
Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, et al. AMPK phosphorylation of Raptor mediates a metabolic checkpoint. Mol Cell. 2008;30:214–26.
Inoki K, Zhu T, Guan K-L. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–90.
Kurreck J, Wyszko E, Gillen C, Erdmann VA. Design of antisense oligonucleotides stabilized by locked nucleic acids. Nucleic Acids Res. 2002;30:1911–8.
McCleland ML, Adler AS, Deming L, Cosino E, Lee L, Blackwood EM, et al. Lactate dehydrogenase B is required for the growth of KRAS-dependent lung adenocarcinomas. Clin Cancer Res. 2013;19:773–84.
Markert CL, Shaklee JB, Whitt GS. Evolution of a gene. Multiple genes for LDH isozymes provide a model of the evolution of gene structure, function, and regulation. Science. 1975;189:102–14.
Porporato PE, Dhup S, Dadhich RK, Copetti T, Sonveaux P. Anticancer targets in the glycolytic metabolism of tumors: A comprehensive review. Front Pharm. 2011;2:1–18.
Jeon S-M, Hay N. The double-edged sword of AMPK signaling in cancer and its therapeutic implications. Arch Pharm Res. 2015;38:346–57.
Eichner LJ, Brun SN, Herzig S, Young NP, Curtis SD, Shackelford DB, et al. Genetic analysis reveals AMPK is required to support tumor growth in murine Kras-dependent lung cancer models. Cell Metab. 2019;29:285–302.
Jackson EL, Olive KP, Tuveson DA, Bronson R, Crowley D, Brown M, et al. The differential effects of mutant p53 alleles on advanced murine lung cancer. Cancer Res. 2005;65:10280–8.
Sutherland KD, Song J-Y, Kwon MC, Proost N, Zevenhoven J, Berns A. Multiple cells-of-origin of mutant K-Ras–induced mouse lung adenocarcinoma. Proc Natl Acad Sci. 2014;111:4952–7.
Gera JF, Mellinghoff IK, Shi Y, Rettig MB, Tran C, Hsu JH, et al. AKT activity determines sensitivity to mammalian target of rapamycin (mTOR) inhibitors by regulating cyclin D1 and c-myc expression. J Biol Chem. 2004;279:2737–46.
Kroemer G, Pouyssegur J. Tumor cell metabolism: cancer’s Achilles’ Heel. Cancer Cell. 2008;13:472–82.
Jackson EL, Willis N, Mercer K, Bronson RT, Crowley D, Montoya R, et al. Analysis of lung tumor initiation and progression using conditional expression of oncogenic K-ras. Genes Dev. 2001;15:3243–8.
DuPage M, Dooley AL, Jacks T. Conditional mouse lung cancer models using adenoviral or lentiviral delivery of Cre recombinase. Nat Protoc. 2009;4:1064–72.
Stewart SA, Dykxhoorn DM, Palliser D, Mizuno H, Yu EY, An DS, et al. Lentivirus-delivered stable gene silencing by RNAi in primary cells. Rna. 2003;9:493–501.
Shi L, Middleton J, Jeon YJ, Magee P, Veneziano D, Laganà A, et al. KRAS induces lung tumorigenesis through microRNAs modulation. Cell Death Dis. 2018;9:1–15.
Egan DF, Shackelford DB, Mihaylova MM, Gelino SR, Rebecca A, Mair W, et al. Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science. 2011;331:456–61.
Liao Y, Smyth GK, Shi W. The subread aligner: fast, accurate and scalable read mapping by seed-and-vote. Nucleic Acids Res. 2013;41:e108.
Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550.
Acknowledgements
We are grateful to the CRUK Biological Resources Unit for the help with the in vivo studies. We also thank the Imaging & Cytometry, Histology, Computational Biology, and Molecular Biology Core Facilities in the CRUK Manchester Institute for their help.
Funding
Work in the MG lab is funded by a CRUK MI core grant (C5759/A20971) and the Lung Cancer Centre (C5759/A20465).
Author information
Authors and Affiliations
Contributions
MLM and MG designed the project. MLM performed in vitro and in vivo experiments. LS and PM helped with in vitro experiments. SS was responsible for the bioinformatics analysis. MLM and MF performed histopathological analysis. MLM and MG wrote the manuscript. MG conceived the study and supervised experiments.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interest.
Ethics
TMAs containing lung adenocarcinoma samples with mutant KRASG12D (n = 8) and matched normal lungs (n = 8) were obtained from the Manchester Cancer Research Centre (MCRC) Biobank under the Christie Hospital Human Tissue License no. 18/NW/0092 and written informed consent was obtained from all patients.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Edited by S. Fulda
Rights and permissions
About this article
Cite this article
La Montagna, M., Shi, L., Magee, P. et al. AMPKα loss promotes KRAS-mediated lung tumorigenesis. Cell Death Differ 28, 2673–2689 (2021). https://doi.org/10.1038/s41418-021-00777-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41418-021-00777-0
This article is cited by
-
New insights into activation and function of the AMPK
Nature Reviews Molecular Cell Biology (2023)
-
Total neoadjuvant therapy for the treatment of locally advanced rectal cancer: a systematic minireview
Biology Direct (2022)