Abstract
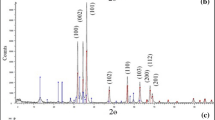
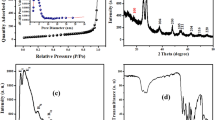
PVA-co-AAm/TiO2/SiO2 nanocomposites adsorbents synthesized by γ-irradiation copolymerization of polyvinyl alcohol (PVA) and acrylamide (AAm) incorporated TiO2/SiO2 nanopowders, aiming to enhance the removal of basic blue 3 dye (BB3) and Cu (II) ions from aqueous solutions. Properties of nanocomposites were analyzed by different techniques. FTIR results showed successful incorporation of nanoparticles and copolymerization of PVA and AAM. SEM/EDS confirm the peaks belonging to C, O, Si, and Ti. TEM investigation illustrated that TiO2/SiO2 nanoparticles were well dispersion, uniform, and homogeneous shape of particle size of 60–70 nm. XRD data specified characteristic diffraction peaks of TiO2 and the calculated crystalline size was 43 nm. Adsorption results confirm that PVA-co-AAm/TiO2/SiO2 nanocomposites provide better adsorption capacities of both BB3 and Cu (II) was three-folds rather than PVA-co-AAm-30. The nanocomposite prepared at 30 kGy (PVA-co-AAm/TiO2/SiO2-30) showed high swelling, gelation, and highest adsorption capacity and it was selected as the best adsorbent for batch experiments. The optimum adsorption was achieved using 0.4 g PVA-co-AAm/TiO2/SiO2-30 adsorbent dosage. The adsorption capacity of BB3 and Cu (II) was 140.9 and 190.3 mg/g with a removal efficiency of 93.5 and 95.2% after 7 h and 6 h, pH 11 and pH 6, and initial concentration of 150 and 200 mg/L, respectively. The adsorption of BB3 or Cu (II) was endothermic and spontaneous, well described by the pseudo-second-order adsorption kinetic and fit Langmuir isotherm. The results revealed that the as-synthesized PVA-co-AAm/TiO2/SiO2 nanocomposites could be employed as effective adsorbents for the adsorption of BB3 and Cu (II) ions from wastewater with high adsorption capacity and recovery.











Similar content being viewed by others
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
J.P. Vareda, A.J.M. Valente, L. Durães, Assessment of heavy metal pollution from anthropogenic activities and remediation strategies: A review. J. Environ. Manage. 246, 101–118 (2019)
S.T. Akar, T. Akar, Z. Kaynak, B. Anilan, A. Cabuk, Ö. Tabak, T.A. Demir, T. Gedikbey, Removal of copper (II) ions from synthetic solution and real wastewater by the combined action of dried Trametes versicolor cells and montmorillonite. Hydrometallurgy 97(1–2), 98–104 (2009)
M.K. Uddin, A review on the adsorption of heavy metals by clay minerals, with special focus on the past decade. Chem. Eng. J. 308, 438–462 (2017)
M.N. Khalaf, Green Polymers and Environmental Pollution Control (CRC Press, Boca Raton, 2016).
W. Tanan, S. Saengsuwan, A one-pot microwave-assisted synthesis of IPN hydrogels based on HEMA/AM/PVA blend for enhancing Cu (II) and Pb (II) ions removal. J. Environ. Chem. Eng. 8(2), 103469 (2020)
S. Zinatloo-Ajabshir, S.A. Heidari-Asil, M. Salavati-Niasari, Recyclable magnetic ZnCo2O4-based ceramic nanostructure materials fabricated by simple sonochemical route for effective sunlight-driven photocatalytic degradation of organic pollution. Ceram. Int. 47(7), 8959–8972 (2021)
V. Dulman, S.-M. Cucu-Man, I. Bunia, M. Dumitras, Batch and fixed bed column studies on removal of Orange G acid dye by a weak base functionalized polymer. Desalin. Water Treat. 57(31), 14708–14727 (2016)
M. Mousavi-Kamazani, S. Zinatloo-Ajabshir, M. Ghodrati, One-step sonochemical synthesis of Zn (OH)2/ZnV3O8 nanostructures as a potent material in electrochemical hydrogen storage. J. Mater. Sci.: Mater. Electron. 31(20), 17332–17338 (2020)
S. Zinatloo-Ajabshir, M. Mousavi-Kamazani, Effect of copper on improving the electrochemical storage of hydrogen in CeO2 nanostructure fabricated by a simple and surfactant-free sonochemical pathway. Ceram. Int. 46(17), 26548–26556 (2020)
S. Zinatloo-Ajabshir, M.S. Morassaei, O. Amiri, M. Salavati-Niasari, Green synthesis of dysprosium stannate nanoparticles using Ficus carica extract as photocatalyst for the degradation of organic pollutants under visible irradiation. Ceram. Int. 46(5), 6095–6107 (2020)
S. Zinatloo-Ajabshir, M. Baladi, M. Salavati-Niasari, Enhanced visible-light-driven photocatalytic performance for degradation of organic contaminants using PbWO4 nanostructure fabricated by a new, simple and green sonochemical approach. Ultrason. Sonochem. 72, 105420 (2021)
P. Maijan, P. Amornpitoksuk, S. Chantarak, Synthesis and characterization of poly (vinyl alcohol-g-acrylamide)/SiO2@ ZnO photocatalytic hydrogel composite for removal and degradation of methylene blue. Polymer 203, 122771 (2020)
T.M. Morsi, A.M. Elbarbary, M.M. Ghobashy, S.H. Othman, Surface decontamination in fuel manufacture plants by chelating solution of nanoparticles. Radiochim. Acta 106(5), 383–392 (2018)
S.H. Othman, A.M. Elbarbary, G. Rashad, T.W. Fasih, Radio-iodide uptake by modified poly (glycidyl methacrylate) as anion exchange resin. Radiochim. Acta 105(1), 75–84 (2017)
H.-P. Feng, L. Tang, G.-M. Zeng, Y. Zhou, Y.-C. Deng, X. Ren, B. Song, C. Liang, M.-Y. Wei, J.-F. Yu, Core-shell nanomaterials: Applications in energy storage and conversion. Adv. Coll. Interface. Sci. 267, 26–46 (2019)
Y. Zhang, M. Wu, M. Wu, J. Zhu, X. Zhang, Multifunctional carbon-based nanomaterials: applications in biomolecular imaging and therapy. ACS Omega 3(8), 9126–9145 (2018)
A.M. Elbarbary, I.A. Ibrahim, H.M. Shafik, S.H. Othman, Magnetic 99mTc-core-shell of polyethylene glycol/polyhydroxyethyl methacrylate based on Fe3O4 nanoparticles: Radiation synthesis, characterization and biodistribution study in tumor bearing mice. Adv. Powder Technol. 28(8), 1898–1910 (2017)
S.H. Othman, I.A. Ibrahim, M.H. Hatab, A.M. Elbarbary, Preparation, characterization and biodistribution in quails of 99mTc-folic acid/chitosan nanostructure. Int. J. Biol. Macromol. 92, 550–560 (2016)
W. Park, H. Shin, B. Choi, W.-K. Rhim, K. Na, D.K. Han, Advanced hybrid nanomaterials for biomedical applications. Prog. Mater. Sci. 114, 100686 (2020)
G. Lofrano, M. Carotenuto, G. Libralato, R.F. Domingos, A. Markus, L. Dini, R.K. Gautam, D. Baldantoni, M. Rossi, S.K. Sharma, Polymer functionalized nanocomposites for metals removal from water and wastewater: an overview. Water Res. 92, 22–37 (2016)
M. Ghorbani, H. Eisazadeh, Removal of COD, color, anions and heavy metals from cotton textile wastewater by using polyaniline and polypyrrole nanocomposites coated on rice husk ash. Compos. B Eng. 45(1), 1–7 (2013)
S. Akbarnejad, A.A. Amooey, S. Ghasemi, High effective adsorption of acid fuchsin dye using magnetic biodegradable polymer-based nanocomposite from aqueous solutions. Microchem. J. 149, 103966 (2019)
A.K. Sarkar, A. Saha, A.B. Panda, S. Pal, pH Triggered superior selective adsorption and separation of both cationic and anionic dyes and photocatalytic activity on a fully exfoliated titanate layer–natural polymer based nanocomposite. Chem. Commun. 51(89), 16057–16060 (2015)
N. Gong, Y. Liu, R. Huang, Simultaneous adsorption of Cu2+ and Acid fuchsin (AF) from aqueous solutions by CMC/bentonite composite. Int. J. Biol. Macromol. 115, 580–589 (2018)
M. Tanzifi, M.T. Yaraki, M. Karami, S. Karimi, A.D. Kiadehi, K. Karimipour, S. Wang, Modelling of dye adsorption from aqueous solution on polyaniline/carboxymethyl cellulose/TiO2 nanocomposites. J. Colloid Interface Sci. 519, 154–173 (2018)
E. Binaeian, S.B. Zadvarzi, D. Yuan, Anionic dye uptake via composite using chitosan-polyacrylamide hydrogel as matrix containing TiO2 nanoparticles; comprehensive adsorption studies. Int. J. Biol. Macromol. 162, 150–162 (2020)
A. Awwad, M. Amer, M. Al-aqarbeh, TiO2-kaolinite nanocomposite prepared from the Jordanian Kaolin clay: Adsorption and thermodynamics of Pb (II) and Cd (II) ions in aqueous solution, Chem. Int. (2020).
M. Rezakazemi, M. Sadrzadeh, T. Mohammadi, T. Matsuura, Methods for the Preparation of Organic–Inorganic Nanocomposite Polymer Electrolyte Membranes for Fuel Cells (Springer, Organic-Inorganic Composite Polymer Electrolyte Membranes, 2017), pp. 311–325
G.S. El-Sayyad, M. Abd Elkodous, A.M. El-Khawaga, M.A. Elsayed, A.I. El-Batal, M. Gobara, Merits of photocatalytic and antimicrobial applications of gamma-irradiated CoxNi1− x Fe2O4/SiO2/TiO2 x= 0.9 nanocomposite for pyridine removal and pathogenic bacteria/fungi disinfection implication for wastewater treatment, RSC Adv. 10(9) 5241-5259 (2020)
C.H. Zhu, Z.B. Hai, C.H. Cui, H.H. Li, J.F. Chen, S.H. Yu, In situ controlled synthesis of thermosensitive poly (N-isopropylacrylamide)/Au nanocomposite hydrogels by gamma radiation for catalytic application. Small 8(6), 930–936 (2012)
A.M. Elbarbary, N.M. El-Sawy, Radiation synthesis and characterization of polyvinyl alcohol/chitosan/silver nanocomposite membranes: antimicrobial and blood compatibility studies. Polym. Bull. 74(1), 195–212 (2017)
M. Micutz, R.M. Lungu, V. Circu, M. Ilis, T. Staicu, Hydrogels obtained via γ-irradiation based on poly (acrylic acid) and its copolymers with 2-hydroxyethyl methacrylate. Appl. Sci. 10(14), 4960 (2020)
A.G. Chmielewski, M. Haji-Saeid, S. Ahmed, Progress in radiation processing of polymers. Nucl. Instrum. Methods Phys. Res. Sect. B 236(1–4), 44–54 (2005)
E. Marin, J. Rojas, Y. Ciro, A review of polyvinyl alcohol derivatives: promising materials for pharmaceutical and biomedical applications. Afr. J. Pharm. Pharmacol 8(24), 674–684 (2014)
A. Rabiee, Acrylamide-based anionic polyelectrolytes and their applications: A survey. J. Vinyl Add. Tech. 16(2), 111–119 (2010)
X. Chen, A. Selloni, Introduction: titanium dioxide (TiO2) nanomaterials. Chem. Rev. 114(19), 9281–9282 (2014)
M. Hdidar, S. Chouikhi, A. Fattoum, M. Arous, A. Kallel, Influence of TiO2 rutile doping on the thermal and dielectric properties of nanocomposite films based PVA. J. Alloy. Compd. 750, 375–383 (2018)
C. Anderson, A.J. Bard, An improved photocatalyst of TiO2/SiO2 prepared by a sol-gel synthesis. J. Phys. Chem. 99(24), 9882–9885 (1995)
Y.H. Gad, A.M. Elbarbary, Radiation synthesis of Fe3O4/SiO2/glycidyl methacrylate/acrylonitrile nanocomposite for adsorption of basic violet 7 dye: kinetic, isotherm, and thermodynamic study. Appl. Organomet. Chem. (2021). https://doi.org/10.1002/aoc.6258
N. Mahmud, A. Benamor, M.S. Nasser, M.M. Ba-Abbad, M.H. El-Naas, A.W. Mohammad, Effective heterogeneous fenton-Like degradation of malachite green dye using the core-shell Fe3O4@ SiO2 nano-catalyst. ChemistrySelect 6(4), 865–875 (2021)
W. Zhang, Q. Deng, Q. He, J. Song, S. Zhang, H. Wang, J. Zhou, H. Zhang, A facile synthesis of core-shell/bead-like poly (vinyl alcohol)/alginate@ PAM with good adsorption capacity, high adaptability and stability towards Cu (II)removal. Chem. Eng. J. 351, 462–472 (2018)
E. Sonker, R. Tiwari, P. Adhikary, K. Kumar, S. Krishnamoorthi, Preparation of ultra-high-molecular-weight polyacrylamide by vertical solution polymerization technique. Polym. Eng. Sci. 59(6), 1175–1181 (2019)
E.S. Agorku, H. Mittal, B.B. Mamba, A.C. Pandey, A.K. Mishra, Fabrication of photocatalyst based on Eu3+-doped ZnS–SiO2 and sodium alginate core shell nanocomposite. Int. J. Biol. Macromol. 70, 143–149 (2014)
S. Pourjafar, A. Rahimpour, M. Jahanshahi, Synthesis and characterization of PVA/PES thin film composite nanofiltration membrane modified with TiO2 nanoparticles for better performance and surface properties. J. Ind. Eng. Chem. 18(4), 1398–1405 (2012)
M.M. Ba-Abbad, A.A.H. Kadhum, A.B. Mohamad, M.S. Takriff, K. Sopian, Synthesis and catalytic activity of TiO2 nanoparticles for photochemical oxidation of concentrated chlorophenols under direct solar radiation. Int. J. Electrochem. Sci 7(6), 4871–4888 (2012)
K. Thamaphat, P. Limsuwan, B. Ngotawornchai, Phase characterization of TiO2 powder by XRD and TEM. Agric. Nat. Resour. 42(5), 357–361 (2008)
X. Cao, J. Ma, X. Shi, Z. Ren, Effect of TiO2 nanoparticle size on the performance of PVDF membrane. Appl. Surf. Sci. 253(4), 2003–2010 (2006)
S. Zinatloo-Ajabshir, M. Salavati-Niasari, Preparation and characterization of nanocrystalline praseodymium oxide via a simple precipitation approach. J. Mater. Sci.: Mater. Electron. 26(8), 5812–5821 (2015)
S. Zinatloo-Ajabshir, M. Salavati-Niasari, Synthesis of pure nanocrystalline ZrO2 via a simple sonochemical-assisted route. J. Ind. Eng. Chem. 20(5), 3313–3319 (2014)
F. Chen, J. Zhao, H. Hidaka, Highly selective deethylation of rhodamine B: adsorption and photooxidation pathways of the dye on the TiO2/SiO2 composite photocatalyst. Int. J. Photoenergy 5(4), 209–217 (2003)
L. Zou, Y. Luo, M. Hooper, E. Hu, Removal of VOCs by photocatalysis process using adsorption enhanced TiO2–SiO2 catalyst. Chem. Eng. Process. 45(11), 959–964 (2006)
K.A. Gebru, C. Das, Removal of Pb (II) and Cu (II) ions from wastewater using composite electrospun cellulose acetate/titanium oxide (TiO2) adsorbent. J. Water Process Eng. 16, 1–13 (2017)
S. Sakarkar, S. Muthukumaran, V. Jegatheesan, Evaluation of polyvinyl alcohol (PVA) loading in the PVA/titanium dioxide (TiO2) thin film coating on polyvinylidene fluoride (PVDF) membrane for the removal of textile dyes. Chemosphere 257, 127144 (2020)
P.M. Pakdel, S.J. Peighambardoust, Review on recent progress in chitosan-based hydrogels for wastewater treatment application. Carbohyd. Polym. 201, 264–279 (2018)
A.M. Elbarbary, M.M. Ghobashy, Phosphorylation of chitosan/HEMA interpenetrating polymer network prepared by γ-radiation for metal ions removal from aqueous solutions. Carbohyd. Polym. 162, 16–27 (2017)
K.-W. Jung, S.Y. Lee, J.-W. Choi, Y.J. Lee, A facile one-pot hydrothermal synthesis of hydroxyapatite/biochar nanocomposites: adsorption behavior and mechanisms for the removal of copper (II) from aqueous media. Chem. Eng. J. 369, 529–541 (2019)
A. Masoumi, K. Hemmati, M. Ghaemy, Structural modification of acrylonitrile–butadiene–styrene waste as an efficient nanoadsorbent for removal of metal ions from water: isotherm, kinetic and thermodynamic study. RSC Adv. 5(3), 1735–1744 (2015)
Acknowledgements
Authors would to thank National Center for Radiation Research and Technology, Egyptian Atomic Energy Authority for facilitating experiments of preparation, irradiation and apparatus used for characterization.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
AME was responsible for the conception and design, testing, data acquisition, data interpretation, writing—review, and editing. YHG was responsible for analysis and data interpretation, writing—review, and editing manuscript. All authors read, revise, and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests and non-financial competing interests.
Consent to publish
All authors approved for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Elbarbary, A.M., Gad, Y.H. Radiation Synthesis and Characterization of Poly (vinyl alcohol)/acrylamide/TiO2/SiO2 Nanocomposite for Removal of Metal Ion and Dye from Wastewater. J Inorg Organomet Polym 31, 4103–4125 (2021). https://doi.org/10.1007/s10904-021-02029-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-021-02029-7