Abstract
Urea, as a significant molecule in biology, chemistry and agriculture, is extensively present both in industrial production and daily life. However, its excessively releasing into water and soil could bring about a serious threat to the environment and ecology due to the potential eutrophication. Considering the potential hydrogen content in urea, urea-rich wastewater is also regarded as a strategic energy storage resource. Among all technologies, the electrochemical treatment of urea wastewater behaves superior advantages both on environment protection and energy recovery, causing tremendous attention in recent years. Herein, this review summarized electrochemical methods for urea conversions for pollutant control and energy harvesting. As the kernel role in the electrochemical systems, the latest development of advanced electrodes is presented with the basic design principles described. The relationships between the electrocatalysts and their urea oxidation performance have been discussed thoroughly. Additionally, recent advances about novel applications for energy production and resource recovery are also displayed. Finally, the prospects and challenges are still to be addressed, orienting a clear direction for the electrochemical hydrogen harvesting from urea-containing wastewater in the future.

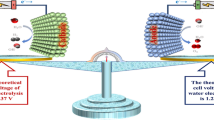
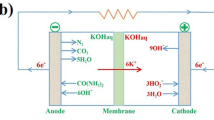
Reproduced with permission from Ref. [26]. b Comparison of urea electrolysis and water splitting from electrochemical reaction and standard potentials required

Reproduced with permission from Ref. [34]. c Optimized structure for bridge-coordinated urea on nickel oxyhydroxide. (Reproduced with permission from Ref. [35]). d LSV of NiClO-D and NiOH-D catalysts on GCEs in 1 m KOH aqueous electrolyte with 0.33 m urea, and illustration of lattice-oxygen involved mechanism and calculated Gibbs free energy profiles. (Reproduced with permission from Ref. [36])

Reproduced with permission from Ref. [47]) c Schematic illustration of the metallic sulfur incorporation Ni(OH)2 nanosheets. d Urea oxidation LSV plots of M-Ni(OH)2 electrode (the inset shows the onset potential) and e comparison between M-Ni(OH)2 electrode and the P-Ni(OH)2 electrode. (Reproduced with permission from Ref. [48]). f Open-ended Ni(OH)2 nanotubes grown on 3D nickel foam for enhanced urea electrocatalytic oxidation. (Reproduced with permission from Ref. [49]). g The formation energy calculated from Ni(OH)2 and NiClOH to NiOO models (Reproduced with permission from Ref. [36]). h Schematic illustration of deep reconstruction of Ni-based electrodes by a lithiation-induced strategy. (Reproduced with permission from Ref. [54])

Reproduced with permission from Ref. [58. c LSV curves of several electrodes in a two-electrode systems in 1 M KOH with and without 0.33 M urea. (Reproduced with permission from Ref. [68]). d The synthesis process for Ni-MOF nanosheets, and e their corresponding SEM (i) and TEM (ii) images. f LSV curves of Ni-MOF, Ni(OH)2 and 20% Pt/C in 1 M KOH electrolyte with and without 0.33 M urea, and g the corresponding Nyquist plots of Ni-MOF and Ni(OH)2. (Reproduced with permission from Ref. [70])

Reproduced with permission from Ref. [84]) c Illustration of an oxygen vacancy in NiMoO4 structure. d CV curves comparison of several electrodes in 1 M KOH and 0.5 M urea electrolyte and long-time operation of r-NiMoO4/NF. (Reproduced with permission from Ref. [85]) e Chronoamperometry experiments of LaNiO3 and NiO performed at 0.45, 0.50, and 0.58 V in 5 M KOH and 0.33 M urea solution. (Reproduced with permission from Ref. [93]) f Cycling stability tests for La0.5Sr1.5NiO4+δ performed in Ar-saturated 1 M KOH and 1 M urea solution at a scan rate of 10 mV s−1 over a potential window of 0.41 to 0.7 V. (Reproduced with permission from Ref. [94])

Reproduced with permission from Ref. [118]. b Images of Ni–Fe2O3/rGO/PVA aerogel without (i) and with (ii) a load of 100 g, and the CV plots of NiO/rGO/PVA and c NiO–Fe2O3/rGO/PVA electrodes in absence and presence of 0.33 M urea in 1.0 M KOH electrolyte. (Reproduced with permission from Ref. [121]) d Schematic illustration of the fabrication processof the Ni-WC/C catalyst, and e their corresponding CV in the electrolyte of 1 M KOH and 0.33 M urea. (Reproduced with permission from Ref. [122])

Reproduced with permission from Ref. [137]) b TEM images of small and large size MnO2 nanolayers with the size distribution histograms inset. The LSV plots of S-MnO2-G-NF in 1 M KOH electrolyte in the absence and presence of 0.5 M urea, and LSV comparison with other electrodes in 1 M KOH with 0.5 M urea. (Reproduced with permission from Ref. [145]) c SEM image (i), cross image (ii) and (iii) EDS mapping of the fabricated Zn0.08Co0.92P/TM electrode. Polarization curves (iv) for Zn0.08Co0.92P/TM||Zn0.08Co0.92P/TM as bifunctional catalysts in overall electrolysis in the presence and absence of 0.5 m urea in 1.0 m KOH. Photograph of overall urea electrolysis driven by a 1.0 V DC power supply (V). (Reproduced with permission from Ref. [147])
Similar content being viewed by others
References
Lubitz W, Tumas W (2007) Hydrogen: an overview. Chem Rev 107(10):3900–3903. https://doi.org/10.1021/cr050200z
Seh ZW, Kibsgaard J, Dickens CF, Chorkendorff IB, Norskov JK, Jaramillo TF (2017) Combining theory and experiment in electrocatalysis: insights into materials design. Science. https://doi.org/10.1126/science.aad4998
Winsche W, Hoffman KC, Salzano F (1973) Hydrogen: its future role in the nation’s energy economy. Science 180(4093):1325–1332. https://doi.org/10.1126/science.180.4093.1325
Jain IP (2009) Hydrogen the fuel for 21st century. Int J Hydrogen Energ 34(17):7368–7378. https://doi.org/10.1016/j.ijhydene.2009.05.093
Sartbaeva A, Kuznetsov VL, Wells SA, Edwards PP (2008) Hydrogen nexus in a sustainable energy future. Energ Environ Sci 1(1):79–85. https://doi.org/10.1039/b810104n
Wentorf R, Hanneman R (1974) Thermochemical hydrogen generation. Science 185(4148):311–319. https://doi.org/10.1126/science.185.4148.311
Parlett CMA, Durndell LJ, Isaacs MA, Liu XT, Wu CF (2020) Ethanol steam reforming for hydrogen production over hierarchical macroporous mesoporous SBA-15 supported nickel nanoparticles. Top Catal 63(3–4):403–412. https://doi.org/10.1007/s11244-020-01265-4
Johnson TC, Morris DJ, Wills M (2010) Hydrogen generation from formic acid and alcohols using homogeneous catalysts. Chem Soc Rev 39(1):81–88. https://doi.org/10.1039/b904495g
Avci AK, Onsan ZI, Trimm DL (2003) On-board hydrogen generation for fuel cell-powered vehicles: the use of methanol and propane. Top Catal 22(3–4):359–367. https://doi.org/10.1023/A:1023504826480
Zahmakiran M, Ozkar S (2013) Transition metal nanoparticles in catalysis for the hydrogen generation from the hydrolysis of ammonia-borane. Top Catal 56(13–14):1171–1183. https://doi.org/10.1007/s11244-013-0083-5
Zhang LN, Lang ZL, Wang YH, Tan HQ, Zang HY, Kang ZH, Li YG (2019) Cable-like Ru/WNO@C nanowires for simultaneous high-efficiency hydrogen evolution and low-energy consumption chlor-alkali electrolysis. Energ Environ Sci 12(8):2569–2580. https://doi.org/10.1039/c9ee01647c
Karunadasa HI, Montalvo E, Sun YJ, Majda M, Long JR, Chang CJ (2012) A molecular MoS2 edge site mimic for catalytic hydrogen generation. Science 335(6069):698–702. https://doi.org/10.1126/science.1215868
Kuehnel MF, Reisner E (2018) Solar hydrogen generation from lignocellulose. Angew Chem Int Ed 57(13):3290–3296. https://doi.org/10.1002/anie.201710133
Morales-Perez AA, Garcia-Perez R, Tabla-Vazquez CG, Ramirez-Zamora RM (2021) Simultaneous hydrogen production and acetic acid degradation by heterogeneous photocatalysis using a metallurgical waste as catalyst. Top Catal 64(1–2):17–25. https://doi.org/10.1007/s11244-020-01346-4
Logan BE (2004) Extracting hydrogen electricity from renewable resources. Environ Sci Technol 38(9):160a–167a. https://doi.org/10.1021/es040468s
Lu L, Williams NB, Turner JA, Maness PC, Gu J, Ren ZJ (2017) Microbial photoelectrosynthesis for self-sustaining hydrogen generation. Environ Sci Technol 51(22):13494–13501. https://doi.org/10.1021/acs.est.7b03644
Dotan H, Landman A, Sheehan SW, Malviya KD, Shter GE, Gravel DA, Arzi Z, Yehudai N, Halabi M, Gal N, Hadari N, Cohen C, Rothschild A, Grader GS (2019) Decoupled hydrogen and oxygen evolution by a two-step electrochemical-chemical cycle for efficient overall water splitting. Nat Energy 4(9):786–795. https://doi.org/10.1038/s41560-019-0462-7
Gong M, Li YG, Wang HL, Liang YY, Wu JZ, Zhou JG, Wang J, Regier T, Wei F, Dai HJ (2013) An advanced Ni–Fe layered double hydroxide electrocatalyst for water oxidation. J Am Chem Soc 135(23):8452–8455. https://doi.org/10.1021/ja4027715
Mitra D, Narayanan SR (2018) A stable and electrocatalytic iron electrode for oxygen evolution in alkaline water electrolysis. Top Catal 61(7–8):591–600. https://doi.org/10.1007/s11244-018-0971-9
Randall DG, Naidoo V (2018) Urine: the liquid gold of wastewater. J Environ Chem Eng 6(2):2627–2635. https://doi.org/10.1016/j.jece.2018.04.012
Rollinson AN, Jones J, Dupont V, Twigg MV (2011) Urea as a hydrogen carrier: a perspective on its potential for safe, sustainable and long-term energy supply. Energ Environ Sci 4(4):1216. https://doi.org/10.1039/c0ee00705f
Kim J, Choi WJK, Choi J, Hoffmann MR, Park H (2013) Electrolysis of urea and urine for solar hydrogen. Catal Today 199:2–7. https://doi.org/10.1016/j.cattod.2012.02.009
Botte GG (2015) Electrolytic cells and methods for the production of ammonia and hydrogen. Google Patents,
Botte GG (2012) Electrolytic cells and methods for the production of ammonia and hydrogen. Google Patents,
Boggs BK, King RL, Botte GG (2009) Urea electrolysis: direct hydrogen production from urine. Chem Commun 32:4859–4861. https://doi.org/10.1039/B905974A
Miller HA, Lavacchi A, Vizza F (2020) Storage of renewable energy in fuels and chemicals through electrochemical reforming of bioalcohols. Curr Opin Electrochem 21:140–145. https://doi.org/10.1016/j.coelec.2020.02.001
Bezerra ÂC, de Sá EL, Nart FC (1997) In situ vibrational study of the initial steps during urea electrochemical oxidation. J Phys Chem B 101(33):6443–6449. https://doi.org/10.1021/jp9700793
Rubel M, Rhee C, Wieckowski A, Rikvold P (1991) Cyclic voltammetry of platinum single crystal electrodes in solutions containing urea. J Electroanal Chem Interf Electrochem 315(1–2):301–306. https://doi.org/10.1016/0022-0728(91)80078-5
Cho K, Hoffmann MR (2014) Urea degradation by electrochemically generated reactive chlorine species: products and reaction pathways. Environ Sci Technol 48(19):11504–11511. https://doi.org/10.1021/es5025405
Zöllig H, Remmele A, Fritzsche C, Morgenroth E, Udert KM (2015) Formation of chlorination byproducts and their emission pathways in chlorine mediated electro-oxidation of urine on active and nonactive type anodes. Environ Sci Technol 49(18):11062–11069. https://doi.org/10.1021/acs.est.5b01675
Song HJ, Yoon H, Ju B, Lee D-Y, Kim D-W (2019) Electrocatalytic selective oxygen evolution of carbon-coated Na2Co1-xFexP2O7 nanoparticles for alkaline seawater electrolysis. ACS Catal 10(1):702–709. https://doi.org/10.1021/acscatal.9b04231
Hernlem BJ (2005) Electrolytic destruction of urea in dilute chloride solution using DSA electrodes in a recycled batch cell. Water Res 39(11):2245–2252. https://doi.org/10.1021/acscatal.9b04231
Jin H, Wang X, Tang C, Vasileff A, Li L, Slattery A, Qiao SZ (2021) Stable and highly efficient hydrogen evolution from seawater enabled by an unsaturated nickel surface nitride. Mater Adv. https://doi.org/10.1002/adma.202007508
Barrios AM, Lippard SJ (2000) Interaction of urea with a hydroxide-bridged dinuclear nickel center: an alternative model for the mechanism of urease. J Am Chem Soc 122(38):9172–9177. https://doi.org/10.1021/ja000202v
Daramola DA, Singh D, Botte GG (2010) Dissociation rates of urea in the presence of NiOOH catalyst: a DFT analysis. J Phys Chem A 114(43):11513–11521. https://doi.org/10.1021/jp105159t
Zhang L, Wang L, Lin H, Liu Y, Ye J, Wen Y, Chen A, Wang L, Ni F, Zhou Z (2019) A lattice-oxygen-involved reaction pathway to boost urea oxidation. Angew Chem Int Ed 58(47):16820–16825. https://doi.org/10.1002/anie.201909832
Chen W, Xu L, Zhu X, Huang YC, Zhou W, Wang D, Zhou Y, Du S, Li Q, Xie C (2021) Unveiling the electrooxidation of urea: intramolecular coupling of the N-N Bond. Angew Chem Int Ed 133(13):7373–7383. https://doi.org/10.1002/ange.202015773
Chen W, Xie C, Wang Y, Zou Y, Dong C-L, Huang Y-C, Xiao Z, Wei Z, Du S, Chen C (2020) Activity origins and design principles of nickel-based catalysts for nucleophile electrooxidation. Chem 6(11):2974–2993. https://doi.org/10.1016/j.chempr.2020.07.022
Suárez D, Díaz N, Merz KM (2003) Ureases: quantum chemical calculations on cluster models. J Am Chem Soc 125(50):15324–15337. https://doi.org/10.1021/ja030145g
Guo F, Ye K, Du M, Huang X, Cheng K, Wang G, Cao D (2016) Electrochemical impedance analysis of urea electro-oxidation mechanism on nickel catalyst in alkaline medium. Electrochim Acta 210:474–482. https://doi.org/10.1016/j.electacta.2016.05.149
Vedharathinam V, Botte GG (2014) Experimental investigation of potential oscillations during the electrocatalytic oxidation of urea on Ni catalyst in alkaline medium. J Phys Chem C 118(38):21806–21812. https://doi.org/10.1021/jp5052529
Vedharathinam V, Botte GG (2013) Direct evidence of the mechanism for the electro-oxidation of urea on Ni(OH)2 catalyst in alkaline medium. Electrochim Acta 108:660–665. https://doi.org/10.1016/j.electacta.2013.06.137
Zhang LS, Wang LP, Lin HP, Liu YX, Ye JY, Wen YZ, Chen A, Wang L, Ni FL, Zhou ZY, Sun SG, Li YY, Zhang B, Peng HS (2019) A Lattice-oxygen-involved reaction pathway to boost urea oxidation. Angew Chem Int Ed 58(47):16820–16825. https://doi.org/10.1002/anie.201909832
Kim J, Kwon D, Kim K, Hoffmann MR (2014) Electrochemical production of hydrogen coupled with the oxidation of arsenite. Environ Sci Technol 48(3):2059–2066. https://doi.org/10.1021/es4046814
Ding Y, Li Y, Xue YY, Miao BQ, Li SN, Jiang YC, Liu X, Chen Y (2019) Atomically thick Ni(OH)2 nanomeshes for urea electrooxidation. Nanoscale 11(3):1058–1064. https://doi.org/10.1039/c8nr08104b
Yang WL, Yang XP, Hou CM, Li BJ, Gao HT, Lin JH, Luo XL (2019) Rapid room-temperature fabrication of ultrathin Ni(OH)2 nanoflakes with abundant edge sites for efficient urea oxidation. Appl Catal B-Environ 259:118020. https://doi.org/10.1016/j.apcatb.2019.118020
Wang D, Yan W, Botte GG (2011) Exfoliated nickel hydroxide nanosheets for urea electrolysis. Electrochem Commun 13(10):1135–1138. https://doi.org/10.1016/j.elecom.2011.07.016
Zhu X, Dou X, Dai J, An X, Guo Y, Zhang L, Tao S, Zhao J, Chu W, Zeng XC (2016) Metallic nickel hydroxide nanosheets give superior electrocatalytic oxidation of urea for fuel cells. Angew Chem Int Ed 55(40):12465–12469. https://doi.org/10.1002/anie.201606313
Ji R-Y, Chan D-S, Jow J-J, Wu M-S (2013) Formation of open-ended nickel hydroxide nanotubes on three-dimensional nickel framework for enhanced urea electrolysis. Electrochem Commun 29:21–24. https://doi.org/10.1016/j.elecom.2013.01.006
Zhan S, Zhou Z, Liu M, Jiao Y, Wang H (2019) 3D NiO nanowalls grown on Ni foam for highly efficient electro-oxidation of urea. Catal Today 327:398–404. https://doi.org/10.1016/j.cattod.2018.02.049
Wang D, Yan W, Vijapur SH, Botte GG (2012) Enhanced electrocatalytic oxidation of urea based on nickel hydroxide nanoribbons. J Power Sources 217:498–502. https://doi.org/10.1016/j.jpowsour.2012.06.029
Wu M-S, Ji R-Y, Zheng Y-R (2014) Nickel hydroxide electrode with a monolayer of nanocup arrays as an effective electrocatalyst for enhanced electrolysis of urea. Electrochim Acta 144:194–199. https://doi.org/10.1016/j.electacta.2014.08.098
Wu M-S, Sie Y-J, Yang S-B (2019) Hollow mesoporous nickel dendrites grown on porous nickel foam for electrochemical oxidation of urea. Electrochim Acta 304:131–137. https://doi.org/10.1016/j.electacta.2019.02.100
Liu X, Ni K, Wen B, Guo RT, Niu CJ, Meng JS, Li Q, Wu PJ, Zhu YW, Wu XJ, Mai LQ (2019) Deep reconstruction of nickel-based precatalysts for water oxidation catalysis. ACS Energy Lett 4(11):2585–2592. https://doi.org/10.1021/acsenergylett.9b01922
He Q, Wan Y, Jiang H, Pan Z, Wu C, Wang M, Wu X, Ye B, Ajayan PM, Song L (2018) Nickel vacancies boost reconstruction in nickel hydroxide electrocatalyst. ACS Energy Lett 3(6):1373–1380. https://doi.org/10.1021/acsenergylett.8b00515
Ding R, Li X, Shi W, Xu Q, Wang L, Jiang H, Yang Z, Liu E (2016) Mesoporous Ni-P nanocatalysts for alkaline urea electrooxidation. Electrochim Acta 222:455–462. https://doi.org/10.1016/j.electacta.2016.10.198
Liu H, Zhu S, Cui Z, Li Z, Wu S, Liang Y (2020) Ni2P nanoflakes for high-performing urea oxidation reaction: linking active sites to UOR mechanism. Nanoscale 13:1759–1769. https://doi.org/10.1039/D0NR08025J
Liu D, Liu T, Zhang L, Qu F, Du G, Asiri AM, Sun X (2017) High-performance urea electrolysis towards less energy-intensive electrochemical hydrogen production using a bifunctional catalyst electrode. J Mater Chem A 5(7):3208–3213. https://doi.org/10.1039/C6TA11127K
Wang G, Ye K, Shao J, Zhang Y, Zhu K, Cheng K, Yan J, Wang G, Cao D (2018) Porous Ni2P nanoflower supported on nickel foam as an efficient three-dimensional electrode for urea electro-oxidation in alkaline medium. Int J Hydrogen Energ 43(19):9316–9325. https://doi.org/10.1016/j.ijhydene.2018.03.221
Xu X, Du P, Guo T, Zhao B, Wang H, Huang M (2020) In situ grown Ni phosphate@Ni12P5 nanorod arrays as a unique core-shell architecture: competitive bifunctional electrocatalysts for urea electrolysis at large current densities. ACS Sustain Chem Eng 8(19):7463–7471. https://doi.org/10.1021/acssuschemeng.0c01814
Tang C, Zhao ZL, Chen J, Li B, Chen L, Li CM (2017) Se-Ni(OH)2-shelled vertically oriented NiSe nanowires as a superior electrocatalyst toward urea oxidation reaction of fuel cells. Electrochim Acta 248:243–249. https://doi.org/10.1016/j.electacta.2017.06.159
Wang X, Wang J, Sun X, Wei S, Cui L, Yang W, Liu J (2018) Hierarchical coral-like NiMoS nanohybrids as highly efficient bifunctional electrocatalysts for overall urea electrolysis. Nano Res 11(2):988–996. https://doi.org/10.1007/s12274-017-1711-3
Hu S, Feng C, Wang S, Liu J, Wu H, Zhang L, Zhang J (2019) Ni3N/NF as bifunctional catalysts for both hydrogen generation and urea decomposition. ACS Appl Mater Interf 11(14):13168–13175. https://doi.org/10.1021/acsami.8b19052
Li J, Yao C, Kong X, Li Z, Jiang M, Zhang F, Lei X (2019) Boosting hydrogen production by electrooxidation of urea over 3D hierarchical Ni4N/Cu3N nanotube arrays. ACS Sustain Chem Eng 7(15):13278–13285. https://doi.org/10.1021/acssuschemeng.9b02510
Yan L, Sun Y, Hu E, Ning J, Zhong Y, Zhang Z, Hu Y (2019) Facile in-situ growth of Ni2P/Fe2P nanohybrids on Ni foam for highly efficient urea electrolysis. J Colloid Interf Sci 541:279–286. https://doi.org/10.1016/j.jcis.2019.01.096
Liu MM, Jiao YL, Zhan SH, Wang HT (2020) Ni3S2 nanowires supported on Ni foam as efficient bifunctional electrocatalyst for urea-assisted electrolytic hydrogen production. Catal Today 355:596–601. https://doi.org/10.1016/j.cattod.2019.05.032
Liu Z, Zhang CZ, Liu H, Feng LG (2020) Efficient synergism of NiSe2 nanoparticle/NiO nanosheet for energy-relevant water and urea electrocatalysis. Appl Catal B-Environ. https://doi.org/10.1016/j.apcatb.2020.119165
Liu H, Liu Z, Feng L (2019) Bonding state synergy of the NiF2/Ni2P hybrid with the co-existence of covalent and ionic bonds and the application of this hybrid as a robust catalyst for the energy-relevant electrooxidation of water and urea. Nanoscale 11(34):16017–16025. https://doi.org/10.1039/C9NR05204F
Chen N, Du Y-X, Zhang G, Lu W-T, Cao F-F (2021) Amorphous nickel sulfoselenide for efficient electrochemical urea-assisted hydrogen production in alkaline media. Nano Energ 81:105605. https://doi.org/10.1016/j.nanoen.2020.105605
Zhu D, Guo C, Liu J, Wang L, Du Y, Qiao S-Z (2017) Two-dimensional metal–organic frameworks with high oxidation states for efficient electrocatalytic urea oxidation. Chem Commun 53(79):10906–10909. https://doi.org/10.1039/C7CC06378D
Yuan M, Wang R, Sun Z, Lin L, Yang H, Li H, Nan C, Sun G, Ma S (2019) Morphology-controlled synthesis of Ni-MOFs with highly enhanced electrocatalytic performance for urea oxidation. Inorg Chem 58(17):11449–11457. https://doi.org/10.1021/acs.inorgchem.9b01124
Maruthapandian V, Kumaraguru S, Mohan S, Saraswathy V, Muralidharan S (2018) An insight on the electrocatalytic mechanistic study of pristine Ni MOF (BTC) in alkaline medium for enhanced OER and UOR. ChemElectroChem 5(19):2795–2807. https://doi.org/10.1002/celc.201800802
Zhu DD, Guo CX, Liu JL, Wang L, Dub Y, Qiao SZ (2017) Two-dimensional metal-organic frameworks with high oxidation states for efficient electrocatalytic urea oxidation. Chem Commun 53(79):10906–10909. https://doi.org/10.1039/c7cc06378d
Cheng Y, Xiao X, Guo X, Yao H, Pang H (2020) Synthesis of “Quasi-Ce-MOF” electrocatalysts for enhanced urea oxidation reaction performance. ACS Sustain Chem Eng 8(23):8675–8680. https://doi.org/10.1021/acssuschemeng.0c01800
de Torresi SIC, Provazi K, Malta M, Torresi RM (2001) Effect of additives in the stabilization of the α phase of Ni(OH)2 electrodes. J Electrochem Soc 148(10):A1179–A1184. https://doi.org/10.1149/1.1403731
Provazi K, Giz MJD, Dall’Antonia L, de Torresi SC (2001) The effect of Cd Co, and Zn as additives on nickel hydroxide opto-electrochemical behavior. J Power Sources 102(1–2):224–232. https://doi.org/10.1016/S0378-7753(01)00819-9
Folquer M, Vilche J, Arvia AJ (1980) Electrochemical reactions at multiple interfaces the nickel hydroxide electrode formed by precipitation on a platinum surface. J Electrochem Soc 127(12):2634–2640. https://doi.org/10.1016/S0378-7753(01)00819-9
Sun CB, Guo MW, Siwal SS, Zhang QB (2020) Efficient hydrogen production via urea electrolysis with cobalt doped nickel hydroxide-riched hybrid films: cobalt doping effect and mechanism aspect. J Catal 381:454–461. https://doi.org/10.1016/j.jcat.2019.11.034
Yan W, Wang D, Botte GG (2012) Electrochemical decomposition of urea with Ni-based catalysts. Appl Catal B-Environ 127:221–226. https://doi.org/10.1016/j.apcatb.2012.08.022
Wang SL, Yang XD, Liu Z, Yang DW, Feng LG (2020) Efficient nanointerface hybridization in a nickel/cobalt oxide nanorod bundle structure for urea electrolysis. Nanoscale 12(19):10827–10833. https://doi.org/10.1039/d0nr01386b
Rezaee S, Shahrokhian S (2020) 3D ternary NixCo2-xP/C nanoflower/nanourchin arrays grown on HCNs: a highly efficient bi-functional electrocatalyst for boosting hydrogen production via the urea electro-oxidation reaction. Nanoscale 12(30):16123–16135. https://doi.org/10.1039/D0NR04616G
Adhikari S, Kwon Y, Kim D-H (2020) Three-dimensional core–shell structured NiCo2O4@ CoS/Ni-Foam electrocatalyst for oxygen evolution reaction and electrocatalytic oxidation of urea. Chem Eng J 402:126192. https://doi.org/10.1016/j.cej.2020.126192
Yang H, Yuan M, Sun Z, Wang D, Lin L, Li H, Sun G (2020) In-situ construction of Mn2+ doped Ni3S2 electrode with highly enhanced urea oxidation reaction performance. ACS Sustain Chem Eng 8(22):8348–8355. https://doi.org/10.1021/acssuschemeng.0c02160
Yu Z-Y, Lang C-C, Gao M-R, Chen Y, Fu Q-Q, Duan Y, Yu S-H (2018) Ni–Mo–O nanorod-derived composite catalysts for efficient alkaline water-to-hydrogen conversion via urea electrolysis. Energ Environ Sci 11(7):1890–1897. https://doi.org/10.1039/c8ee00521d
Tong Y, Chen P, Zhang M, Zhou T, Zhang L, Chu W, Wu C, Xie Y (2017) Oxygen vacancies confined in nickel molybdenum oxide porous nanosheets for promoted electrocatalytic urea oxidation. ACS Catal 8(1):1–7. https://doi.org/10.1021/acscatal.7b03177
Han W-K, Li X-P, Lu L-N, Ouyang T, Xiao K, Liu Z-Q (2020) Partial S substitution activates NiMoO4 for efficient and stable electrocatalytic urea oxidation. Chem Commun 56(75):11038–11041. https://doi.org/10.1039/D0CC03177A
Hu KL, Jeong S, Elumalai G, Kukunuri S, Fujita J, Ito Y (2020) Phase-dependent reactivity of nickel molybdates for electrocatalytic urea oxidation. ACS Appl Energ Mater 3(8):7535–7542. https://doi.org/10.1021/acsaem.0c00968
Yang D, Yang L, Zhong L, Yu X, Feng L (2019) Urea electro-oxidation efficiently catalyzed by nickel-molybdenum oxide nanorods. Electrochim Acta 295:524–531. https://doi.org/10.1016/j.electacta.2018.10.190
Xu Q, Qian G, Yin S, Yu C, Chen W, Yu T, Luo L, Xia Y, Tsiakaras P (2020) Design and synthesis of highly performing bifunctional Ni-NiO-MoNi hybrid catalysts for enhanced urea oxidation and hydrogen evolution reactions. ACS Sustain Chem Eng 8(18):7174–7181. https://doi.org/10.1021/acssuschemeng.0c01637
Yang M, Jiang Y, Qu M, Qin Y, Wang Y, Shen W, He R, Su W, Li M (2020) Strong electronic couple engineering of transition metal phosphides-oxides heterostructures as multifunctional electrocatalyst for hydrogen production. Appl Catal B-Environ 269:118803. https://doi.org/10.1016/j.apcatb.2020.118803
Meng L, Li L, Wang J, Fu S, Zhang Y, Li J, Xue C, Wei Y, Li G (2020) Valence-engineered MoNi4/MoOx@ NF as a Bi-functional electrocatalyst compelling for urea-assisted water splitting reaction. Acta Electrochim. https://doi.org/10.1016/j.electacta.2020.136382
Zhang J-Y, He T, Wang M, Qi R, Yan Y, Dong Z, Liu H, Wang H, Xia BY (2019) Energy-saving hydrogen production coupling urea oxidation over a bifunctional nickel-molybdenum nanotube array. Nano Energ 60:894–902
Forslund RP, Mefford JT, Hardin WG, Alexander CT, Johnston KP, Stevenson KJ (2016) Nanostructured LaNiO3 perovskite electrocatalyst for enhanced urea oxidation. ACS Catal 6(8):5044–5051. https://doi.org/10.1021/acscatal.6b00487
Forslund RP, Alexander CT, Abakumov AM, Johnston KP, Stevenson KJ (2019) Enhanced electrocatalytic activities by substitutional tuning of nickel-based Ruddlesden-popper catalysts for the oxidation of urea and small alcohols. ACS Catal 9(3):2664–2673. https://doi.org/10.1021/acscatal.8b04103
Yun TG, Heo Y, Bae HB, Chung S-Y (2021) Elucidating intrinsic contribution of d-orbital states to oxygen evolution electrocatalysis in oxides. Nat Commun 12(1):1–11. https://doi.org/10.1038/s41467-021-21055-0
Kim Y-T, Lopes PP, Park S-A, Lee A-Y, Lim J, Lee H, Back S, Jung Y, Danilovic N, Stamenkovic V (2017) Balancing activity, stability and conductivity of nanoporous core-shell iridium/iridium oxide oxygen evolution catalysts. Nat Commun 8(1):1–8. https://doi.org/10.1038/s41467-017-01734-7
Chung DY, Lopes PP, Martins PFBD, He H, Kawaguchi T, Zapol P, You H, Tripkovic D, Strmcnik D, Zhu Y (2020) Dynamic stability of active sites in hydr (oxy) oxides for the oxygen evolution reaction. Nat Energy 5(3):222–230. https://doi.org/10.1038/s41560-020-0576-y
Li JT, Chu D, Dong H, Baker DR, Jiang RZ (2020) Boosted oxygen evolution reactivity by igniting double exchange interaction in spinel oxides. J Am Chem Soc 142(1):50–54. https://doi.org/10.1021/jacs.9b10882
Ding R, Qi L, Jia M, Wang H (2014) Facile synthesis of mesoporous spinel NiCo2O4 nanostructures as highly efficient electrocatalysts for urea electro-oxidation. Nanoscale 6(3):1369–1376. https://doi.org/10.1039/C3NR05359H
Wang D, Vijapur SH, Wang Y, Botte GG (2017) NiCo2O4 nanosheets grown on current collectors as binder-free electrodes for hydrogen production via urea electrolysis. Int J Hydrogen Energ 42(7):3987–3993. https://doi.org/10.1016/j.ijhydene.2016.11.048
Periyasamy S, Subramanian P, Levi E, Aurbach D, Gedanken A, Schechter A (2016) Exceptionally active and stable spinel nickel manganese oxide electrocatalysts for urea oxidation reaction. ACS Appl Mater Interf 8(19):12176–12185. https://doi.org/10.1021/acsami.6b02491
Sha LN, Ye K, Yin JL, Zhu K, Cheng K, Yan J, Wang GL, Cao DX (2020) In situ grown 3D hierarchical MnCo2O4.5@Ni(OH)2 nanosheet arrays on Ni foam for efficient electrocatalytic urea oxidation. Chem Eng J https://doi.org/10.1016/j.cej.2019.122603
Tran TQN, Yoon SW, Park BJ, Yoon HH (2018) CeO2-modified LaNi0.6Fe0.4O3 perovskite and MWCNT nanocomposite for electrocatalytic oxidation and detection of urea. J Electroanal Chem 818:76–83. https://doi.org/10.1016/j.jelechem.2018.04.003
Galal A, Atta NF, Hefnawy MA (2020) Lanthanum nickel oxide nano-perovskite decorated carbon nanotubes/poly (aniline) composite for effective electrochemical oxidation of urea. J Electroanal Chem. https://doi.org/10.1016/j.jelechem.2020.114009
Wang Q, O’Hare D (2012) Recent advances in the synthesis and application of layered double hydroxide (LDH) nanosheets. Chem Rev 112(7):4124–4155. https://doi.org/10.1021/cr200434v
Feng Y, Wang X, Huang J, Dong P, Ji J, Li J, Cao L, Feng L, Jin P, Wang C (2020) Decorating CoNi layered double hydroxides nanosheet arrays with fullerene quantum dot anchored on Ni foam for efficient electrocatalytic water splitting and urea electrolysis. Chem Eng J 390:124525. https://doi.org/10.1016/j.cej.2020.124525
Sun H, Zhang W, Li J-G, Li Z, Ao X, Xue K-H, Ostrikov KK, Tang J, Wang C (2020) Rh-engineered ultrathin NiFe-LDH nanosheets enable highly-efficient overall water splitting and urea electrolysis. Appl Catal B-Environ 284:119740. https://doi.org/10.1016/j.apcatb.2020.119740
Khalafallah D, Li XY, Zhi MJ, Hong ZL (2020) 3D hierarchical NiCo layered double hydroxide nanosheet arrays decorated with noble metal nanoparticles for enhanced urea electrocatalysis. ChemElectroChem 7(1):163–174. https://doi.org/10.1002/celc.201901423
Vidotti M, Silva M, Salvador R, de Torresi SC, Dall’Antonia L (2008) Electrocatalytic oxidation of urea by nanostructured nickel/cobalt hydroxide electrodes. Electrochim Acta 53(11):4030–4034. https://doi.org/10.1016/j.electacta.2007.11.029
Yan W, Wang D, Botte GG (2012) Nickel and cobalt bimetallic hydroxide catalysts for urea electro-oxidation. Electrochim Acta 61:25–30. https://doi.org/10.1016/j.electacta.2011.11.044
Wang G, Wen Z (2018) Self-supported bimetallic Ni-Co compound electrodes for urea-and neutralization energy-assisted electrolytic hydrogen production. Nanoscale 10(45):21087–21095
Nadeema A, Kashyap V, Gururaj R, Kurungot S (2019) [MoS4]2–-intercalated NiCo-layered double hydroxide nanospikes: an efficiently synergized material for urine to direct H2 generation. ACS Appl Mater Interf 11(29):25917–25927. https://doi.org/10.1021/acsami.9b06545
Zhang WH, Tang YH, Yu LM, Yu XY (2020) Activating the alkaline hydrogen evolution performance of Mo-incorporated Ni(OH)2 by plasma-induced heterostructure. Appl Catal B-Environ. https://doi.org/10.1016/j.apcatb.2019.118154
Xu W, Du D, Lan R, Humphreys J, Wu Z, Tao S (2017) Highly active Ni–Fe double hydroxides as anode catalysts for electrooxidation of urea. New J Chem 41(10):4190–4196. https://doi.org/10.1039/C6NJ04060H
Babar P, Lokhande A, Karade V, Lee IJ, Lee D, Pawar S, Kim JH (2019) Trifunctional layered electrodeposited nickel iron hydroxide electrocatalyst with enhanced performance towards the oxidation of water, urea and hydrazine. J Colloid Interf Sci 557:10–17. https://doi.org/10.1016/j.jcis.2019.09.012
Chakrabarty S, Offen-Polak I, Burshtein TY, Farber EM, Kornblum L, Eisenberg D (2020) Urea oxidation electrocatalysis on nickel hydroxide: the role of disorder. J Solid State Electr. https://doi.org/10.1007/s10008-020-04744-6
Xie JF, Liu WW, Zhang XD, Guo YQ, Gao L, Lei FC, Tang B, Xie Y (2019) Constructing hierarchical wire-on-sheet nanoarrays in phase-regulated cerium-doped nickel hydroxide for promoted urea electro-oxidation. ACS Mater Lett 1(1):103–110. https://doi.org/10.1021/acsmaterialslett.9b00124
Wu M-S, Lin G-W, Yang R-S (2014) Hydrothermal growth of vertically-aligned ordered mesoporous nickel oxide nanosheets on three-dimensional nickel framework for electrocatalytic oxidation of urea in alkaline medium. J Power Sources 272:711–718. https://doi.org/10.1016/j.jpowsour.2014.09.009
Wu M-S, Jao C-Y, Chuang F-Y, Chen F-Y (2017) Carbon-encapsulated nickel-iron nanoparticles supported on nickel foam as a catalyst electrode for urea electrolysis. Electrochim Acta 227:210–216. https://doi.org/10.1016/j.electacta.2017.01.035
Liang Y, Liu Q, Asiri AM, Sun X (2015) Enhanced electrooxidation of urea using NiMoO4 xH2O nanosheet arrays on Ni foam as anode. Electrochim Acta 153:456–460. https://doi.org/10.1016/j.electacta.2014.11.193
Das G, Tesfaye RM, Won Y, Yoon HH (2017) NiO–Fe2O3 based graphene aerogel as urea electrooxidation catalyst. Electrochim Acta 237:171–176. https://doi.org/10.1016/j.electacta.2017.03.197
Wang L, Li M, Huang Z, Li Y, Qi S, Yi C, Yang B (2014) Ni–WC/C nanocluster catalysts for urea electrooxidation. J Power Sources 264:282–289. https://doi.org/10.1016/j.jpowsour.2014.04.104
Hameed RA, Medany SS (2017) Enhanced electrocatalytic activity of NiO nanoparticles supported on graphite planes towards urea electro-oxidation in NaOH solution. Int J Hydrogen Energ 42(38):24117–24130. https://doi.org/10.1016/j.ijhydene.2017.07.236
Hameed RA, Medany SS (2018) Influence of support material on the electrocatalytic activity of nickel oxide nanoparticles for urea electro-oxidation reaction. J Colloid Interf Sci 513:536–548. https://doi.org/10.1016/j.jcis.2017.11.032
Cao Z, Mao H, Guo X, Sun D, Sun Z, Wang B, Zhang Y, Song X-M (2018) Hierarchical Ni (OH)2/polypyrrole/graphene oxide nanosheets as excellent electrocatalysts for the oxidation of urea. ACS Sustain Chem Eng 6(11):15570–15581. https://doi.org/10.1021/acssuschemeng.8b04027
Shi W, Sun XJ, Ding R, Ying DF, Huang YF, Huang YX, Tan CN, Jia ZY, Liu EH (2020) Trimetallic NiCoMo/graphene multifunctional electrocatalysts with moderate structural/electronic effects for highly efficient alkaline urea oxidation reaction. Chem Commun 56(48):6503–6506. https://doi.org/10.1039/d0cc02132f
Muthuchamy N, Jang S, Park JC, Park S, Park KH (2019) Bimetallic NiPd nanoparticle-incorporated ordered mesoporous carbon as highly efficient electrocatalysts for hydrogen production via overall urea electrolysis. ACS Sustain Chem Eng 7(18):15526–15536. https://doi.org/10.1021/acssuschemeng.9b03275
Zhang JF, Xing F, Zhang HJ, Huang Y (2020) Ultrafine NiFe clusters anchored on N-doped carbon as bifunctional electrocatalysts for efficient water and urea oxidation. Dalton T 49(40):13962–13969. https://doi.org/10.1039/d0dt02459g
Ye K, Zhang D, Guo F, Cheng K, Wang G, Cao D (2015) Highly porous nickel @ carbon sponge as a novel type of three-dimensional anode with low cost for high catalytic performance of urea electro-oxidation in alkaline medium. J Power Sources 283:408–415. https://doi.org/10.1016/j.jpowsour.2015.02.149
Lu S, Hummel M, Gu Z, Wang Y, KeWang K, Pathak R, Zhou YZ, Jia H, Qi X, Xu B (2021) Highly efficient urea oxidation via nesting nano nickel oxide in eggshell membrane-derived carbon. ACS Sustain Chem Eng 9(4):1703–1713. https://doi.org/10.1021/acssuschemeng.0c07614
Wang L, Ren L, Wang X, Feng X, Zhou J, Wang B (2018) Multivariate MOF-Templated pomegranate-like Ni/C as efficient bifunctional electrocatalyst for hydrogen evolution and urea oxidation. ACS Appl Mater Interf 10(5):4750–4756. https://doi.org/10.1021/acsami.7b18650
Baker DR, Lundgren CA (2019) Expansion of the urea electrocatalytic oxidation window by adsorbed nickel ions. J Appl Electrochem 49(9):883–893. https://doi.org/10.1007/s10800-019-01328-9
Wang L, Du T, Cheng J, Xie X, Yang B, Li M (2015) Enhanced activity of urea electrooxidation on nickel catalysts supported on tungsten carbides/carbon nanotubes. J Power Sources 280:550–554. https://doi.org/10.1016/j.jpowsour.2015.01.141
Wang L, Liu Z, Zhu S, Shao M, Yang B, Chen JG (2018) Tungsten carbide and cobalt modified nickel nanoparticles supported on multiwall carbon nanotubes as highly efficient electrocatalysts for urea oxidation in alkaline electrolyte. ACS Appl Mater Interf 10(48):41338–41343. https://doi.org/10.1021/acsami.8b14397
Wang L, Zhu S, Marinkovic N, Kattel S, Shao M, Yang B, Chen JG (2018) Insight into the synergistic effect between nickel and tungsten carbide for catalyzing urea electrooxidation in alkaline electrolyte. Appl Catal B-Environ 232:365–370. https://doi.org/10.1016/j.apcatb.2018.03.064
Fan J, Dou Y, Jiang R, Du K, Deng B, Wang D (2020) Electro-synthesis of tungsten carbide containing catalysts in molten salt for efficiently electrolytic hydrogen generation assisted by urea oxidation. Int J Hydrogen Energy 28:14932–14943. https://doi.org/10.1016/j.ijhydene
Li C, Liu Y, Zhuo Z, Ju H, Li D, Guo Y, Wu X, Li H, Zhai T (2018) Local charge distribution engineered by Schottky heterojunctions toward urea electrolysis. Adv Energy Mater 8(27):1801775. https://doi.org/10.1016/j.ijhydene.2020.07.193
Wang C, Lu HL, Mao ZY, Yan CL, Shen GZ, Wang XF (2020) Bimetal Schottky heterojunction boosting energy-saving hydrogen production from alkaline water via urea electrocatalysis. Funct Mater Adv. https://doi.org/10.1002/adfm.202000556
Ji XY, Zhang YX, Ma Z, Qiu YF (2020) Oxygen vacancy-rich Ni/NiO@NC nanosheets with Schottky heterointerface for efficient urea oxidation reaction. Chemsuschem 13(18):5004–5014. https://doi.org/10.1002/cssc.202001185
Sha L, Liu T, Ye K, Zhu K, Yan J, Yin J, Wang G, Cao D (2020) A heterogeneous interface on NiS@Ni3S2/NiMoO4 heterostructures for efficient urea electrolysis. J Mater Chem A 8(35):18055–18063. https://doi.org/10.1039/D0TA04944A
Ji ZJ, Liu J, Deng Y, Zhang ST, Zhang Z, Du PY, Zhao YL, Lu XQ (2020) Accurate synergy effect of Ni-Sn dual active sites enhances electrocatalytic oxidation of urea for hydrogen evolution in alkaline medium. J Mater Chem A 8(29):14680–14689. https://doi.org/10.1039/d0ta05160h
Dong Z, Lin F, Yao Y, Jiao L (2019) Crystalline Ni (OH)2/amorphous NiMoOx mixed-catalyst with Pt-Like performance for hydrogen production. Adv Energy Mater 9(46):1902703. https://doi.org/10.1002/aenm.201902703
Zhang B, Wang L, Zhu Y, Wen Y, Li S, Cui C, Ni F, Liu Y, Lin H, Li Y (2021) Regulating the local charge distribution of Ni active sites for urea oxidation reaction. Angewan Chem Int Ed 13:1–7. https://doi.org/10.1002/ange.202100610
Zhang Q, Liu B, Li L, Ji Y, Wang C, Zhang L, Su Z (2021) Maximized Schottky effect: the ultrafine V2O3/Ni heterojunctions repeatedly arranging on monolayer nanosheets for efficient and stable water-to-hydrogen conversion. Small. https://doi.org/10.1002/smll.202005769
Chen S, Duan J, Vasileff A, Qiao SZ (2016) Size fractionation of two-dimensional sub-nanometer thin manganese dioxide crystals towards superior urea electrocatalytic conversion. Angewan Chem Int Ed 55(11):3804–3808. https://doi.org/10.1002/anie.201600387
Sun Y, Duan J, Zhu J, Chen S, Antonietti M (2018) Metal-cluster-directed surface charge manipulation of two-dimensional nanomaterials for efficient urea electrocatalytic conversion. ACS Appl Nano Mater 1(12):6649–6655. https://doi.org/10.1021/acsanm.8b01470
Liu T, Liu D, Qu F, Wang D, Zhang L, Ge R, Hao S, Ma Y, Du G, Asiri AM (2017) Enhanced electrocatalysis for energy-efficient hydrogen production over CoP catalyst with nonelectroactive Zn as a promoter. Adv Energy Mater 7(15):1700020. https://doi.org/10.1002/aenm.201700020
Wang ZL, Hu YM, Liu WJ, Xu L, Guan ML, Zhao Y, Bao J, Li HM (2020) Manganese-modulated cobalt-based layered double hydroxide grown on nickel foam with 1D–2D-3D heterostructure for highly efficient oxygen evolution reaction and urea oxidation reaction. Chem Eur J 26(42):9382–9388. https://doi.org/10.1002/chem.202001055
Du XQ, Huang CR, Zhang XS (2019) Synthesis of CoMoO4/Co9S8 network arrays on nickel foam as efficient urea oxidation and hydrogen evolution catalyst. Int J Hydrogen Energ 44(36):19595–19602. https://doi.org/10.1016/j.ijhydene.2019.06.012
Du X, Huang C, Zhang X (2019) Co3O4 arrays with tailored morphology as robust water oxidation and urea splitting catalyst. J Alloys Comp 809:151821. https://doi.org/10.1016/j.jallcom.2019.151821
Li Y, Wang H, Wang R, He B, Gong Y (2018) 3D self-supported FeOP film on nickel foam as a highly active bifunctional electrocatalyst for urea-assisted overall water splitting. Mater Res Bullet 100:72–75. https://doi.org/10.1016/j.materresbull.2017.12.001
Wei S, Wang X, Wang J, Sun X, Cui L, Yang W, Zheng Y, Liu J (2017) CoS2 nanoneedle array on Ti mesh: a stable and efficient bifunctional electrocatalyst for urea-assisted electrolytic hydrogen production. Electrochim Acta 246:776–782. https://doi.org/10.1016/j.electacta.2017.06.068
Jiang Y, Gao S, Liu J, Xu G, Jia Q, Chen F, Song X (2020) Ti-Mesh supported porous CoS 2 nanosheet self-interconnected networks with high oxidation states for efficient hydrogen production via urea electrolysis. Nanoscale 12:11573–11581. https://doi.org/10.1039/D0NR02058C
Cai F, Liao L, Zhao Y, Li D, Zeng J, Fang Y, Zhou H (2021) Large-current-stable bifunctional nanoporous Fe-rich nitride electrocatalysts for highly efficient overall water and urea splitting. J Mater Chem A. https://doi.org/10.1039/D1TA00144B
Wang G, Chen J, Li Y, Jia J, Cai P, Wen Z (2018) Energy-efficient electrolytic hydrogen production assisted by coupling urea oxidation with a pH-gradient concentration cell. Chem Commun 54(21):2603–2606. https://doi.org/10.1039/C7CC09653D
Schranck A, Marks R, Yates E, Doudrick K (2018) Effect of urine compounds on the electrochemical oxidation of urea using a nickel cobaltite catalyst: an electroanalytical and spectroscopic investigation. Environ Sci Technol 52(15):8638–8648. https://doi.org/10.1021/acs.est.8b01743
Nangan S, Ding YC, Alhakemy AZ, Liu YJ, Wen ZH (2021) Hybrid alkali-acid urea-nitrate fuel cell for degrading nitrogen-rich wastewater. Appl Catal B-Environ. https://doi.org/10.1016/j.apcatb.2021.119892
Xu W, Zhang H, Li G, Wu Z (2016) A urine/Cr(VI) fuel cell-electrical power from processing heavy metal and human urine. J Electroanal Chem 764:38–44
Wang GM, Ling YC, Lu XH, Wang HY, Qian F, Tong YX, Li Y (2012) Solar driven hydrogen releasing from urea and human urine. Energy Environ Sci 5(8):8215–8219. https://doi.org/10.1039/c2ee22087c
Wang ZL, Song JH (2006) Piezoelectric nanogenerators based on zinc oxide nanowire arrays. Science 312(5771):242–246. https://doi.org/10.1126/science.1124005
Xu S, Qin Y, Xu C, Wei YG, Yang RS, Wang ZL (2010) Self-powered nanowire devices. Nat Nanotechnol 5(5):366–373. https://doi.org/10.1038/Nnano.2010.46
Singh RK, Schechter A (2018) Electrochemical investigation of urea oxidation reaction on β-Ni(OH)2 and Ni/Ni(OH)2. Electrochim Acta 278:405–411. https://doi.org/10.1016/j.electacta.2018.05.049
Wu M-S, Chen F-Y, Lai Y-H, Sie Y-J (2017) Electrocatalytic oxidation of urea in alkaline solution using nickel/nickel oxide nanoparticles derived from nickel-organic framework. Electrochim Acta 258:167–174. https://doi.org/10.1016/j.electacta.2017.10.113
Yue Z, Zhu W, Li Y, Wei Z, Hu N, Suo Y, Wang J (2018) Surface engineering of a nickel oxide-nickel hybrid nanoarray as a versatile catalyst for both superior water and urea oxidation. Inorg Chem 57(8):4693–4698. https://doi.org/10.1021/acs.inorgchem.8b00411
Yan W, Wang D, Diaz LA, Botte GG (2014) Nickel nanowires as effective catalysts for urea electro-oxidation. Electrochim Acta 134:266–271. https://doi.org/10.1016/j.electacta.2014.03.134
Yue Z, Yao S, Li Y, Zhu W, Zhang W, Wang R, Wang J, Huang L, Zhao D, Wang J (2018) Surface engineering of hierarchical Ni(OH)2 nanosheet@nanowire configuration toward superior urea electrolysis. Electrochim Acta 268:211–217. https://doi.org/10.1016/j.electacta.2018.02.059
Glass DE, Galvan V, Prakash GS (2017) The effect of annealing temperature on nickel on reduced graphene oxide catalysts on urea electrooxidation. Electrochim Acta 253:489–497. https://doi.org/10.1016/j.electacta.2017.09.064
Yang X, Xu W, Zhang H, Wu Z (2017) NixCo3-xO4 nanowire arrays grown on carbon fiber cloth as efficient electrocatalysts for urea oxidation. Energy Procedia 142:1414–1420. https://doi.org/10.1016/j.egypro.2017.12.528
Zhu W, Ren M, Hu N, Zhang W, Luo Z, Wang R, Wang J, Huang L, Suo Y, Wang J (2018) Traditional NiCo2S4 phase with porous nanosheets array topology on carbon cloth: a flexible, versatile and fabulous electrocatalyst for overall water and urea electrolysis. ACS Sustain Chem Eng 6(4):5011–5020. https://doi.org/10.1021/acssuschemeng.7b04663
Alajami M, Yassin MA, Ghouri ZK, Al-Meer S, Barakat NA (2018) Influence of bimetallic nanoparticles composition and synthesis temperature on the electrocatalytic activity of NiMn-incorporated carbon nanofibers toward urea oxidation. Int J Hydrogen Energ 43(11):5561–5575. https://doi.org/10.1016/j.ijhydene.2018.01.163
Shi W, Ding R, Li X, Xu Q, Liu E (2017) Enhanced performance and electrocatalytic kinetics of Ni-Mo/graphene nanocatalysts towards alkaline urea oxidation reaction. Electrochim Acta 242:247–259. https://doi.org/10.1016/j.electacta.2017.05.002
Zhang J-Y, Tian X, He T, Zaman S, Miao M, Yan Y, Qi K, Dong Z, Liu H, Xia BY (2018) In situ formation of Ni3Se4 nanorod arrays as versatile electrocatalysts for electrochemical oxidation reactions in hybrid water electrolysis. J Mater Chem A 6(32):15653–15658. https://doi.org/10.1039/C8TA06361C
Deng J, Chen S, Yao N, Wang Q, Li J, Wei Z (2020) Integrating H2 generation with sewage disposal by an efficient anti-poisoning bifunctional electrocatalyst. Appl Catal B-Environ 277:119175. https://doi.org/10.1016/j.apcatb.2020.119175
Li F, Chen JX, Zhang DF, Fu WF, Chen Y, Wen ZH, Lv XJ (2018) Heteroporous MoS2/Ni3S2 towards superior electrocatalytic overall urea splitting. Chem Commun 54(41):5181–5184. https://doi.org/10.1039/c8cc01404c
Wu FC, Ou G, Yang J, Li HN, Gao YX, Chen FM, Wang Y, Shi YM (2019) Bifunctional nickel oxide-based nanosheets for highly efficient overall urea splitting. Chem Commun 55(46):6555–6558. https://doi.org/10.1039/c9cc02507c
Cheng Y, Liao F, Dong H, Wei H, Geng H, Shao M (2020) Engineering CoN/Ni(OH)2 heterostructures with improved intrinsic interfacial charge transfer toward simultaneous hydrogen generation and urea-rich wastewater purification. J Power Sources 480:229151. https://doi.org/10.1016/j.jpowsour.2020.229151
Sha L, Yin J, Ye K, Wang G, Zhu K, Cheng K, Yan J, Wang G, Cao D Construction of self-supported leaf thorn-like nickel-cobalt bimetal phosphides. https://doi.org/10.1039/C9TA00481E
Sun Y, Wang S, Ning J, Zhang Z, Zhong Y, Hu Y (2020) A one-pot “shielding-to-etching” strategy to synthesize amorphous MoS2 modified CoS/Co0.85Se heterostructured nanotube arrays for boosted energy-saving H2 generation. Nanoscale 12 (2) 991–1001. https://doi.org/10.1039/C9NR08812A
Wang HW, Li YJ, Wang R, He BB, Gong YS (2018) Metal-organic-framework template-derived hierarchical porous CoP arrays for energy-saving overall water splitting. Electrochim Acta 284:504–512. https://doi.org/10.1016/j.electacta.2018.07.175
Acknowledgements
This work was supported by grants from the National Key Research and Development Program of China (No. 2019YFC1906700), the National Natural Science Foundation of China (No. 219611322025, 21876049, 91834301), the China Postdoctoral Science Foundation (No. 2019M661412, 2019M661409, 2020T130190), and the Strategic Priority Research Program of the Chinese Academy of Sciences (No. XDA23010400). Liaoning Revitalization Talents Program (No. XLYC1807245) and Dalian High-Level Talent Innovation Program (No. 2017RQ085).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, J., Li, J., Gong, M. et al. Catalyst Design and Progresses for Urea Oxidation Electrolysis in Alkaline Media. Top Catal 64, 532–558 (2021). https://doi.org/10.1007/s11244-021-01453-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11244-021-01453-w