Abstract
Key message
Melolontha hippocastani is a major pest in European mixed and broadleaf forests. Investigating its oviposition behavior, we observed that a dense shrub layer reduces the density of egg clusters and eggs in the soil. Conversely, canopy openness and a large proportion of oak appear as favoring conditions.
Context
Melolontha hippocastani is a major pest in European mixed and broadleaf forests. In north-eastern France, an epidemic phase has been observed since 2007, characterized by a high mortality rate of seedlings and young trees, and by massive swarming flights every 4 years.
Aims
We investigated the oviposition behavior of M. hippocastani in the northern Vosges Mountains.
Methods
We set up study plots in the infected area after adults had emerged and the females had laid their eggs. We excavated pits and counted the eggs and egg clusters they contained. We also carried out dendrometric surveys.
Results
A dense shrub layer had a negative effect on the density of egg clusters and the number of eggs in the soil, while canopy openness and the proportion of oak basal area had positive effects.
Conclusion
We hypothesized that a dense shrub layer could create a barrier for females. On the other hand, an open canopy may improve conditions for the larvae in the soil, just as a high proportion of oak trees in the surrounding area may provide a good food source for both larvae and adults. We suggest several research orientations and propose guidelines for forest management in view of our results.
Similar content being viewed by others
1 Introduction
Cockchafers (Coleoptera, Scarabaeidae) are well known for the significant damage they cause to plants and for the resulting economic losses in agriculture, horticulture and forestry (Wagenhoff et al. 2014). The forest cockchafer, Melolontha hippocastani Fabr., is a major pest in mixed and broadleaf forests in Europe. Outbreaks have been described in the Czech Republic (Švestka 2010; Švestka and Drapela 2009), Germany (Wagenhoff et al. 2014) and Poland (Niemczyk et al. 2017). As a natural disturbance, M. hippocastani outbreaks can either incite or contribute to forest dieback (Cours 2019a; Manion 1981; Nageleisen et al. 2015). In north-eastern France (Vosges Mountains), forest cockchafer populations have been at epidemic levels since 2007 (Nageleisen et al. 2015), and high larval densities have been recorded (11.4 ± 0.7 3rd-instar larvae/m2 in 2018 (Cours 2019a) and 6.0 ± 0.3 2nd and 3rd-instar larvae/m2 in 2014 (Nageleisen et al. 2015)). Empirically, a density above five (2nd-instar) or two (3rd-instar)/m2 of M. hippocastani larvae induces a high mortality risk for forest plantations (Späth and Schanowski 2007). High larval densities are associated with tree regeneration mortality and, in the longer term, dieback in adult trees (Nageleisen et al. 2015; Woreta and Sukovata 2014).
Damages result from the feeding activity of both larvae and adults. Larvae are rhizophagous and can consume a large proportion of the root system of seedlings and young trees, leading to mortality and ultimately hindering forest regeneration (Serre 2017; Woreta and Sukovata 2014). Adults are phyllophagous and feed on the leaves of broadleaf species (mainly Quercus spp.; (Nageleisen and Cours 2020; Wagenhoff et al. 2014)). Populations of M. hippocastani are highly synchronized and follow 4-year cycles of massive swarming flights. This can result in severe tree crown defoliation, which can in turn significantly reduce radial growth (Billamboz 2014; Huber 1982; Wagenhoff et al. 2014).
Cockchafer population control has been mainly achieved by spraying tree canopies with pesticide during the adults’ maturation feeding and by spraying pesticide on the infested soil around seedlings to control the larvae (Woreta 2016; Woreta 2015). Currently, in order to reduce the adverse consequences of pesticides such as their persistence in environment and pest resistance (Holmes and MacQuarrie 2016), the application of insecticides is increasingly restricted in European countries (Directive 2009./128/EC of the European Parliament and of the Council). A better understanding of the pest’s ecology may make it possible to develop more sustainable and efficient population control strategies. Understanding the factors underpinning oviposition preference is therefore of central interest (Minkenberg et al. 1992).
After the adult females deposit their eggs in the soil, the larvae spend 36 months underground feeding on plant roots. Their growth phase is divided into three distinct instars (Fig. 2) (Švestka and Drapela 2009; Woreta and Sukovata 2014). They pupate in summer, the year before swarming, and spend their last winter as adults (Wagenhoff et al. 2014). The following year, in late April or early May depending on the temperature, the adult insects emerge from the soil and begin feeding on tree foliage (Švestka and Drapela 2009; Wagenhoff et al. 2014). About 2 weeks after emergence, oviposition flights are observed. Females land on the ground at dusk and burrow into the soil to lay eggs in clusters (Fig. 2; Wagenhoff et al. 2014). Sandy soils are highly preferred since they facilitate the females’ digging and the larvae’s movement. Sandy soils also allow volatile compounds from host to spread, which facilitates the larvae’s orientation (Eilers et al. 2016; Johnson and Gregory 2006; Weissteiner et al. 2012). Moreover, sandy soils are often characterized by a low pH, which has been shown to inhibit the development of Beauveria spp. (Hypocreales: Cordycipitaceae), one of the main fungal parasites of Melolontha spp. in natural conditions (Niemczyk et al. 2019). Females lay an average of 24 eggs during their first egg-laying phase and 15 during their second egg-laying phase (Wagenhoff et al. 2014).
Soil properties as well as other environmental factors appear to influence oviposition behavior, leading to an uneven distribution of M. hippocastani larvae in the forest (Nageleisen et al. 2015; Niemczyk et al. 2017; Švestka and Drapela 2009). Oviposition preference is an important aspect of female behavior because it has a great influence on the fitness of a species: the female insect’s choice of an egg-laying site is generally one of the last decisions taken to ensure her progeny’s survival (Cury et al. 2019). Several theories have been proposed to explain the rationale governing egg-laying site selection by insects.
(i) The “optimal oviposition theory” (a.k.a. the preference-performance hypothesis; Jaenike 1978) assumes that the choice of an egg-laying site is determined by a search for maximized offspring development and survival (Bonebrake et al. 2010; Jaenike 1978). It has been shown that weather conditions can influence the egg-laying site decision taken by M. hippocastani females: in cold and wet conditions, they mainly lay in open sites while they lay in shady sites when conditions are hot and dry (Švestka and Drapela 2009). These changes in oviposition behavior could be linked to the search for the best environment for progeny, as in the “optimal oviposition theory”. Another survey found that canopy openness could be a significant predictor for larval density in the soil (Niemczyk et al. 2017). A closed-environment oviposition site could be chosen to avoid desiccation, as during dry, warm weather. For example, Hylobius abietis (L.) females choose to deposit their eggs either in soil or in root bark depending on the risk of desiccation (Nordlander et al. 1997). In contrast, in context of low temperatures, M. hippocastani female could avoid a very closed environment like dense shrubby to ensure insolation of the oviposition site and thus higher soil temperatures for better larval development (Švestka and Drapela 2009). Other observations of flying swarms indicate that dense shrubby clumps might hamper the females’ flight and prevent egg laying (Cours et al. 2019; Niemczyk et al. 2017). Furthermore, preferred egg-laying sites might be close to oaks as their roots provide suitable growth and feeding habitat for the larvae (Woreta and Sukovata 2014).
(ii) A second hypothesis, the “optimal foraging theory” predicts that the egg-laying site will be close to the adult’s food source (Scheirs and De Bruyn 2002). Oak leaves are the best food for both the survival and fertility of adult M. hippocastani (Woreta et al. 2018; Woreta et al. 2016; Woreta and Sukovata 2010). According to the “optimal foraging theory”, egg-laying sites would logically be close to a maturation feeding site.
(iii) A third hypothesis, partly related to the second hypothesis, the “associational resistance hypothesis” predicts that the choice of habitat for phytophagous insects and, by extension, egg-laying sites, could be disturbed by the presence of non-host species (Castagneyrol et al. 2014; Castagneyrol et al. 2013; Jactel et al. 2021). High plant diversity generally correlates with high pressure from natural enemies on insect herbivores while simultaneously diluting host species concentration (Castagneyrol et al. 2013; Plath et al. 2012). As a result, an area with a high density of oak trees would concentrate food resources for both M. hippocastani adults and larvae, resulting in a population concentration in the forest landscape, while tree species mixture could hinder M. hippocastani herbivory and, thus, egg-cluster density (Jactel et al. 2021). However, M. hippocastani is a polyphagous species (with a strong preference for oak species) (Wagenhoff et al. 2014), and therefore mixing forest tree could have only restricted effects on its population dynamic (Jactel et al. 2021; Jactel and Brockerhoff 2007).
These diverging oviposition behaviors are related to the “parent-offspring conflict”, and a trade-off between advantages to the adult and advantages to the larvae could ultimately determine the choice of egg-laying sites (Scheirs and De Bruyn 2002).
We therefore tested the following three hypotheses:
-
(i)
A dense shrub layer negatively influences M. hippocastani oviposition in the soil.
-
(ii)
Canopy openness positively influences egg-laying.
-
(iii)
Increased oak proportion stimulates oviposition.
2 Material and methods
2.1 Study area
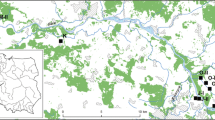
We installed 106 study plots in and around the Ingwiller Forest (Fig. 1; north-eastern France; 48°54′26.9″N 7°25′13.4″E), which is the site of the initial M. hippocastani outbreak in the Vosges Mountains (Nageleisen et al. 2015). This region has a continental climate with long, severe winters and short, hot summers. The average annual temperature varies between 6 and 10 °C. Rainfall is generally well distributed throughout the year, and the annual average is 924 mm. The Ingwiller Forest is composed of 36% Scots pine (Pinus sylvestris L.), 31% common beech (Fagus sylvatica) and 13% oak (Quercus spp.).
In this region, M. hippocastani emergence flights take place every 4 years, from April to June. Therefore, we conducted our study from June 11th to July 11th 2019, during the adult emergence period in the 4-year biological cycle. The mass swarming period included several phases of low and high temperatures: high temperatures in the second half of April triggered the emergence of the adults, followed by low temperatures in May when oviposition occurred, and finally, rising temperatures in June (Appendix Fig. 6).
2.2 Field experiment
To select our plots, we targeted three environmental gradients: (i) a gradient of oak proportion in the stand; (ii) a gradient of canopy openness; and (iii) the presence or absence of a dense shrub layer. Based on information from forest management documents, we carried out a first selection to retain mature forests with a majority of trees larger than 40 cm (diameter at breast height). The selected stands were all managed as even-aged high forests. From this first selection, we used information from forest management documents to select 40 forest plots along a theoretical gradient of oak proportion (Quercus spp.) (Appendix Fig. 7). We selected these plots so that they were scattered throughout the territory but still remained within the area infested by M. hippocastani (Fig. 1). In each of these forest plots, we set up two sub-plots: one in the middle of the most open area and a second one in the middle of the most shaded area (Fig. 1 & Appendix Fig. 7). For both of these two sub-plots, the soil was bare of shrubby vegetation. If canopy closure was homogeneous across the forest plot, we used a randomly selected upstream point in the plot and set up sub-plot on the nearest bare ground. Finally, when there was a dense clump of shrub vegetation on the plot, we installed a third sub-plot (second in case of homogeneous canopy cover) in the middle of the clump. Shrub-layer clumps were less than 5 m in height, with a radius of at least 5 m, and dense vegetation cover (> 90% of soil cover). They were mainly composed of young common beech (Fagus sylvatica; 12 plots) or Norway spruce seedlings (Picea abies (L.) H. Karst; 2 plots). Our objective was to compare M. hippocastani oviposition behavior over the largest possible gradient of environmental conditions in a same geographical area. In the end, we installed 106 sub-plots in the study area (including 14 located in shrub clumps).
In the center of each of these sub-plots, we excavated two 50 × 50 × 40 cm pits in a north-south direction 1 m apart from each other. We counted the number of egg clusters and the total number of eggs in each pit (Fig. 2) and averaged them to the sub-plot.
We applied a relascope sampling protocol to measure the basal area (BA) of each living tree species. Relascopic sampling is characterized by a variable sampling radius and the use of a dendrometer linked to a basal area factor (f = 1 in the present study) (Bitterlich 1984; Piqué et al. 2011). In relascopic sampling, the probability that each tree is included in the plot is proportional to its diameter (Piqué et al. 2011). Therefore, we calculated total plot BA, species BA and relative BA for each living tree species. In the center of each study plot, we also took a hemispherical image to measure canopy openness. We used the camera from an iPhone 6S with a circular fisheye lens to create a 180° image. The photographs were taken horizontally at a height of 1 m above the ground. We manually changed the camera settings for each photograph to adapt to the light conditions throughout the sampling day. We ran the images through the Gap Light Analyser software (Frazer et al. 1999) to separate the sky pixels from the canopy pixels. The analysis provided the canopy opening as the percentage of sky visible through the forest canopy.
We did not record any edaphic variables since soil structure and texture were homogeneous in our study area (Nageleisen et al. 2015); all the plots had brown sandy soil, which is globally favorable for the development of M. hippocastani larvae (Nageleisen et al. 2015).
2.3 Data analysis
The statistical analysis was performed with the R free software (R Core Team 2020). Our objective was to describe egg and egg-cluster density in the soil. Firstly, we calculated Moran’s I statistic to detect spatial autocorrelation with the “moransI” function from lctools R-package (Kalogirou 2020); we did not detect any spatial autocorrelation in our data (Morans.I = 0.044, P = 0.56). We then calculated the Shannon diversity index with the basal areas for Quercus spp., F. sylvatica and P. sylvestris.
Since we did not find any stands with a dense shrub layer under a very closed canopy, we first analyzed the effect of a dense shrub layer on a subset of plots including sub-plot with a dense shrub layer. In addition, since F. sylvatica leaves can be a food resource for M. hippocastani adults, we analyzed the effect of shrub-layer species composition on the density of eggs and egg clusters. We carried out two analyses: first, with the full subset of data; and second, in order to test shrub-layer composition, without the plots whose shrub layer was composed of P. abies (2 plots) (Table 1).
In a second step, we analyzed the effect of other factors on a subset that excluded the 14 plots with a dense shrub layer. We selected certain non-colinear predictors from our data sub-set and computed a variance inflection factor (VIF ≤ 3.62; Log canopy openness + relative proportion of oak BA + total BA + relative proportion of beech BA + relative proportion of Scots pine BA + Shannon diversity index). We included the relative proportion of common beech and Scots pine BA as well as the Shannon diversity index in order to test the mixture effect. We implemented a negative binomial error distribution because our response variables were countable and over-dispersed. Furthermore, in order to include the nesting effect of the sub-plots, we preferred to use mixed models rather than an ANOVA, and added “plot” as random variable. As a result, we built a generalized linear mixed model with a “glmmTMB” function (glmmTMB R-package; Magnusson et al. 2020). We applied a global model using the non-colinear predictors to our data sub-set and selected the best model with a second-order Akaike information criterion (AICc) from the “dredge” function in the MuMin R-package (Bartoń 2020). The classification of the best models is presented in the Appendix section (Tables 3 and 4). Finally, we implemented a classification and regression tree (CART) analysis (Breiman et al. 1984) to examine the relationships between egg and egg-cluster density and environmental factors with the “rpart” function from the rpart R-package (Therneau and Atkinson 2019). We used a regression tree method (“method = anova” in the rpart function) since our response variables were continuous. We also split our CART analysis in two: we first considered only the effect of the presence of a dense shrub layer on the subset of plots including sub-plot with a dense shrub layer; second, for the data subset with no dense shrub layer only, we analyzed the effects of the non-collinear predictors (canopy openness + relative proportion of oak BA + total BA + relative proportion of beech BA + relative proportion of Scots pine BA + Shannon diversity index) on egg and egg-cluster density. For the second regression tree, we limited the number of final partitions in order to reduce the LOOVC cross-validation error. Data are available in Knowledge Network for Biocomplexity (KNB) (Cours 2019b).
3 Results
Overall, we observed an average of 151 ± 14 (standard error (SE)) M. hippocastani eggs/m2 and an average of 6.7 ± 0.6 (SE) egg clusters/m2. This gives an average of 22.3 ± 0.5 (SE) eggs per cluster. Egg clusters were generally located at an average depth of 15–20 cm. Egg number per cluster was generally constant over all the study plots. Therefore, the results of the egg-density analysis and the egg-cluster density analysis are nearly the same. We therefore only show figures for egg clusters since the data are less over-dispersed.
The first analysis (on the subset of plots including sub-plot with a dense shrub layer; see Methods) revealed that a dense shrub layer negatively affected egg and egg-cluster densities (Tables 1 & 2; Figs. 3 & 5). Egg and egg-cluster densities were four times as high in plots without a shrub layer than in plots with a dense shrub layer (egg-cluster density: P < 0.01, egg density: P < 0.05; Fig. 3). The second analysis conducted to select the best model with the lowest AICc based on the subset of plots without any dense shrub layer (see Methods) highlighted two significant variables that strongly influenced egg-cluster and total egg densities in the soil. Canopy openness and relative proportion of oak BA both had positive effects (Table 2; Figs. 4 & 5). These two variables appeared in the five best models in our AICc-based selection and were always significant, contrary to the other environmental variables we used in the global model for egg and egg-cluster density (Appendix Tables 3 and 4).
A Mean (± se) Melolontha hippocastani egg-cluster density and B mean (± se) egg density according to the presence-absence of a dense shrub layer. Estimates (± se) indicate coefficients and “P <”, P values from univariable generalized (negative binomial) linear models (egg-cluster and egg density ~ dense shrub presence). “n =” indicates the number of study plots in each category
Melolontha hippocastani egg-cluster density plotted against relative proportion of oak basal area (A) and canopy openness (B) in the study plots. The black line is the regression curve from the negative binomial model (with a log-transformation of x for canopy openness) and the gray area indicates the 95% confident interval
Multivariate regression trees obtained by the classification and regression trees (CART) method for our data set for the prediction of mean egg-cluster density (“Nb egg-laying”). The grey scale in the lower boxes (terminal leaf nodes) shows differences in the average Melolontha hippocastani laying level from the lowest (light gray = 1.9 egg-clusters) to the highest (dark gray = 16 egg-clusters). “n =” indicates the number of study plots, and percentages indicate plot proportion
Egg and egg-cluster densities were twice as high in forest stands with at least 50% oak BA as they were in forest stands without oak (egg-cluster density: P < 0.001, egg density: P < 0.05; Fig. 4A). Furthermore, the more open the forest canopy, the higher the egg and egg-cluster densities (Figs. 4B & 5). Concerning regression trees, first tree (shrub layer effect) showed an accuracy rate of 23%, while second tree had 39% of accuracy rate (calculated with predicted values). We observed a minimal average egg-cluster density in the regression tree for the study plots with a dense shrub layer (1.9 egg clusters/m2). For the plots without a shrub layer, we observed two analysis paths: relative proportion of oak basal area (threshold value: 42%) followed by canopy openness (threshold value: 23%; Fig. 5).
4 Discussion
4.1 A dense shrub layer acts as a barrier
Our results reveal that a dense shrub layer is the most critical determinant of egg density and egg-cluster density in soils. Indeed, the presence of a dense shrub layer induced the most significant effect with the highest magnitude (Table 1). Melolontha hippocastani egg cluster and egg density were four times higher without a shrub layer than with one (Figs. 3 & 5); overall, despite the potentially greater root resource under shrubs, M. hippocastani females did not lay eggs under patches of dense shrubby vegetation.
Our field observations suggest that a dense vegetation layer could be an impenetrable physical barrier for adults. Unlike the members of the subfamily Cetoniidae (Coleoptera, Scarabaeidae), which close their elytra during flight, M. hippocastani can only partially close their elytra, and this makes their flight imprecise, thus reducing their capacity to pass through dense vegetation (Frantsevich 2010). In addition, female insects generally use long-range sensing such as olfaction and vision to find an egg-laying site (Cury et al. 2019) so that shrubs could have a repellent effect or open areas could have an attractive effect on M. hippocastani through volatile compounds (olfactory barrier or attraction) or through soil camouflage (visual barrier) (Dulaurent et al. 2012). Foraging Melolontha hippocastani seem to rely mainly on olfactory cues (Ruther et al. 2001), whereas the closely related species M. melolontha is also very sensitive to light at 520 nm, which characterizes tree canopy color at dusk (Hegedüs et al. 2006).
Forests affected by M. hippocastani outbreaks often harbor dense game animal populations. For instance, the forests in north-eastern France are often described as having an overabundance of wild ungulates (ONF 2017). The resulting imbalance is defined by the failure of forest regeneration due to damages caused by high ungulate populations (Bradshaw and Waller 2016). Wild ungulates generally regulate the shrub layer, thus favoring ruderal herbaceous species (Boulanger et al. 2018). Therefore, we hypothesize that the overabundance of wild ungulates could have favored the current outbreak of M. hippocastani in north-eastern France through the reduction of shrub-layer cover.
In addition, biotic interactions related to the density of the shrub layer could also influence the female M. hippocastani’s choice of egg-laying site. Niemczyk et al. (2017) proposed that a higher ground cover with shrubby vegetation increases soil moisture and thus creates a more suitable habitat for entomopathogenic organisms such as Beauveria brongniartii, a highly host-specific pathogenic fungus of Melolontha spp. larvae (Niemczyk et al. 2019). Moreover, the cold weather prevailing in May during most of the oviposition period (Appendix Fig. 6) may have influenced oviposition site choice outside of the densely vegetated areas, as Švestka and Drapela (2009) observed, and in accordance with the optimal oviposition theory.
4.2 Canopy openness in the forest as a factor of choice for egg-laying sites
In our study, canopy openness had a positive influence on the choice of egg-laying site (Table 2; Figs. 4B & 5). Niemczyk et al. (2017) had already observed higher larval occurrence in forest environments near open areas, like forest-meadow ecotones. Open areas in the forest could benefit M. hippocastani foraging through light polarization by tree canopies at dusk (Hegedüs et al. 2006), which could make canopies more attractive to adults. As a consequence, according to the optimal foraging theory, females may preferentially choose open areas as egg-laying sites simply because they usually feed nearby (Scheirs and De Bruyn 2002). Nevertheless, open areas could also provide better microclimate conditions for larval survival and development, mostly during cold and wet weather conditions, which would be in accordance with the optimal oviposition theory (Scheirs and De Bruyn 2002; Švestka and Drapela 2009). The optimal oviposition theory is consistent with the cold wet weather in May 2019 during most of the oviposition period: open areas could ensure higher insolation and therefore higher soil temperatures, thereby enhancing larva development (Švestka and Drapela 2009).
We identified two factors that opened the forest canopy in recent decades and may have partially favored M. hippocastani outbreaks. Many forests in north-eastern France were heavily impacted by the Lothar windstorm (1999), notably the forests in our study area (Appendix Fig. 8; Colin 2003). The resulting windfalls may have created favorable conditions for M. hippocastani development. Additionally, a general rule of thumb in forest management is to open stands up in order to improve resistance and resilience in a context of climate change (Legay and Mortier 2006). These two factors combined could have created a larger area of interesting potential egg-laying sites, thus promoting M. hippocastani outbreaks in north-eastern France. In addition, severe defoliation of oaks by M. hippocastani adults could increase canopy openness and thus improve the area’s potential egg-laying sites. Overall, it seems impossible to conclude that one theory bests the other in explaining the site preference for open areas we found in 2019. Further, more precisely structured research should be conducted to better describe the influence of weather and site openness on the oviposition behavior of M. hippocastani females.
4.3 Egg-laying sites occur close to main adult food sources and/or to abundant resources for larval development
As expected, a high proportion of oak, as reflected by basal area, around the center of the plots (and pits) promoted egg-laying (Table 2; Figs. 4A & 5) regardless of the proportion of other tree species (Appendix Tables 3 and 4). Oak leaves are the best food source for M. hippocastani adults and improve both their survival and fertility (Woreta et al. 2018; Woreta et al. 2016; Woreta and Sukovata 2010). This is consistent with field observations reporting a higher consumption of young oak leaves than leaves of other species, although high secondary consumption was also observed on Fagus sylvatica (Wagenhoff et al. 2014). Moreover, M. hippocastani swarming is generally synchronized with oak budburst, when fresh young leaves with a higher nutritional value are available (Wagenhoff et al. 2014).
Therefore, our results suggest that female M. hippocastani lay eggs in the vicinity of their main food source, as expected in the optimal foraging theory (Scheirs and De Bruyn 2002). Female reproductive success is determined by the number of eggs laid and offspring survival rate (Minkenberg et al. 1992). By laying near oaks, females could avoid spending too much energy looking for egg-laying sites and ensure a higher number of eggs laid. In addition, M. hippocastani larvae generally survive and develop well when they feed on oak roots (Woreta and Sukovata 2014). Therefore, laying eggs in the vicinity of oaks may result in better subsequent development and survival of the offspring, which would be in accordance with the optimal oviposition theory (Scheirs and De Bruyn 2002). Finally, both theories, optimal foraging and optimal oviposition, could be described within the framework of associational resistance and the resource concentration hypothesis: a high concentration of the host species induces better habitat, foraging and oviposition sites for the associated herbivore species than a low host-species concentration (dilution of the host species among non-host species) (Castagneyrol et al. 2013). Therefore, reducing the proportion of oak in stands in favor of other, phylogenetically distant tree species could result in less susceptibility of stands to M. hippocastani (Jactel et al. 2021; Jactel and Brockerhoff 2007). However, as the species has a polyphagous (non-host-specific) diet, mixing oak with other phylogenetically related species (such as F. sylvatica) may have no effect on the population dynamics of M. hippocastani (Jactel et al. 2021; Jactel and Brockerhoff 2007).
5 Implications for forest management and future research
The present study highlights several factors involved in the oviposition behavior of M. hippocastani that concern forest management; notably, we highlight the sensitivity of the early-succession stand phase to M. hippocastani overpopulation. This phase is characterized by canopy opening (through regeneration cuts or natural disturbances; Swanson et al. 2011) and seedling recruitment, two important factors in oviposition behavior (Tables 1 & 2, Figs. 3, 4 & 5). We also suggest some caution in the regeneration phases (late and early-succession phases) through the limitation of silvicultural interventions. In addition, we emphasize the need to establish special monitoring of stands with a high proportion of oaks, an open canopy and the absence of a shrub layer (Fig. 5) within and nearby outbreak areas. We show that, in a context of forest management adapted to climate change (Legay and Mortier 2006), it is very important to closely study the interactions between canopy opening due to new silvicultural rules and wild ungulate densities. In addition, it would be interesting to study the choice of egg-laying sites in an outbreak area across several silvicultural systems (e.g. close-to-nature silviculture of uneven-aged stands and even-aged high-forest stands).
Future research should focus on the interactions between factors affecting forest dynamics and M. hippocastani populations: wild ungulates, natural and anthropogenic disturbances (droughts, storms, outbreaks of other secondary pests (e.g. Jacquet et al. 2012 for processionary moth; Sallé et al. 2020, Sallé et al. 2014 for bark and wood boring species), forest harvesting). Though we introduced questions concerning the avoidance by female M. hippocastani of soils infected with the fungus Beauveria spp; more research should be carried out on predation, competition and parasite interactions. The conditions for selecting the egg-laying site should also be studied: is the strategy implemented by M. hippocastani females closer to the “optimal foraging theory” or the “optimal oviposition theory” (Scheirs and De Bruyn 2002)? In parallel, how weather conditions influence oviposition behavior, especially in a context of global warming, should be further explored. According to optimal oviposition theory, studies could be carried out on the relationship between egg abundance and the amount of roots in the soil. Further research should investigate possible links between recent M. hippocastani outbreaks and anthropogenic climate change. As an example, dendroarchaeology has highlighted that past phases of Melolontha outbreaks were influenced by warming temperatures (Billamboz 2014).
Data availability
The datasets generated during and/or analyzed during the current study are available in the Knowledge Network for Biocomplexity (KNB) repository, https://doi.org/10.5063/7D2SJ6.
References
Bartoń, K, (2020). MuMIn: Multi-model inference.
Billamboz A (2014) Dendroarchaeology and cockchafers north of the Alps: regional patterns of a middle frequency signal in oak tree-ring series. Environ Archaeol 19:114–123. https://doi.org/10.1179/1461410313Z.00000000055
Bitterlich, W., (1984). The relascope idea. Relative measurements in forestry. The relascope idea. Relative measurements in forestry.
Bonebrake TC, Boggs CL, McNally JM, Ranganathan J, Ehrlich PR (2010) Oviposition behavior and offspring performance in herbivorous insects: consequences of climatic and habitat heterogeneity. Oikos 119:927–934. https://doi.org/10.1111/j.1600-0706.2009.17759.x
Boulanger V, Dupouey J-L, Archaux F, Badeau V, Baltzinger C, Chevalier R, Corcket E, Dumas Y, Forgeard F, Mårell A, Montpied P, Paillet Y, Picard J-F, Saïd S, Ulrich E (2018) Ungulates increase forest plant species richness to the benefit of non-forest specialists. Glob Chang Biol 24:e485–e495. https://doi.org/10.1111/gcb.13899
Bradshaw L, Waller DM (2016) Impacts of white-tailed deer on regional patterns of forest tree recruitment. For Ecol Manag 375:1–11. https://doi.org/10.1016/j.foreco.2016.05.019
Breiman L, Friedman J, Stone CJ, Olshen RA (1984) Classification and regression trees, new ed. Chapman and Hall/CRC, Boca Raton
Castagneyrol B, Giffard B, Péré C, Jactel H (2013) Plant apparency, an overlooked driver of associational resistance to insect herbivory. J Ecol 101:418–429. https://doi.org/10.1111/1365-2745.12055
Castagneyrol B, Régolini M, Jactel H (2014) Tree species composition rather than diversity triggers associational resistance to the pine processionary moth. Basic and Applied Ecology 15:516–523. https://doi.org/10.1016/j.baae.2014.06.008
Colin, G, (2003). Les forêts françaises après les tempêtes de décembre 1999—Bas-Rhin. Inventaire Forestier National.
Core Team R (2020) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria
Cours, J, (2019a). Study of ecological niche of forest cockchafer (Melolontha hippocastani Fabr. 1801) in Northern Vosges forests (Mémoire d’ingénieur). Office national des forêts—AgroParisTech, Nancy.
Cours, J, (2019b). Melolontha hippocastani—study of female oviposition behavior, North-East France. [dataset], V1, KNB repository, https://doi.org/10.5063/7D2SJ6.
Cours J, Nageleisen L-M, Touffait R (2019) Gestion forestière intégrée des insectes ravageurs: exemple par l’étude de la niche écologique du hanneton forestier (Melolontha hippocastani Fabr. 1801). Revue Forestière Française 71:553–567. https://doi.org/10.4267/2042/70886
Cury KM, Prud’homme B, Gompel N (2019) A short guide to insect oviposition: when, where and how to lay an egg. J Neurogenet 33:75–89. https://doi.org/10.1080/01677063.2019.1586898
Dulaurent A-M, Porté AJ, van Halder I, Vétillard F, Menassieu P, Jactel H (2012) Hide and seek in forests: colonization by the pine processionary moth is impeded by the presence of nonhost trees. Agric For Entomol 14:19–27. https://doi.org/10.1111/j.1461-9563.2011.00549.x
Eilers EJ, Veit D, Rillig MC, Hansson BS, Hilker M, Reinecke A (2016) Soil substrates affect responses of root feeding larvae to their hosts at multiple levels: orientation, locomotion and feeding. Basic and Applied Ecology 17:115–124. https://doi.org/10.1016/j.baae.2015.09.006
Frantsevich L (2010) Indirect closing of the elytra in a cockchafer, Melolontha hippocastani F. (Coleoptera: Scarabaeidae). J Exp Biol 213:1836–1843. https://doi.org/10.1242/jeb.041350
Frazer, GW, Canham, CD, Lertzman, KP, Sallaway, P, Marinakis, D, (1999). Gap Light Analyzer (GLA), Version 2.0: imaging software to extract canopy structure and gap light transmission indices from true-colour fisheye photographs, users manual and program documentation. Simon Fraser University, Burnaby, British Columbia, and the Institute of Ecosystem Studies, Millbrook, New York 36.
Hegedüs R, Horváth Á, Horváth G (2006) Why do dusk-active cockchafers detect polarization in the green? The polarization vision in Melolontha melolontha is tuned to the high polarized intensity of downwelling light under canopies during sunset. J Theor Biol 238:230–244. https://doi.org/10.1016/j.jtbi.2005.05.033
Holmes SB, MacQuarrie CJK (2016) Chemical control in forest pest management. The Canadian Entomologist 148:S270–S295. https://doi.org/10.4039/tce.2015.71
Huber F (1982) Effet de défolliaisons des chênes par les hannetons sur la structure du bois. Revue Forestiere Francaise 34:185–190
Jacquet J-S, Orazio C, Jactel H (2012) Defoliation by processionary moth significantly reduces tree growth: a quantitative review. Ann For Sci 69:857–866. https://doi.org/10.1007/s13595-012-0209-0
Jactel H, Brockerhoff EG (2007) Tree diversity reduces herbivory by forest insects. Ecol Lett 10:835–848. https://doi.org/10.1111/j.1461-0248.2007.01073.x
Jactel H, Moreira X, Castagneyrol B (2021) Tree diversity and forest resistance to insect pests: patterns, mechanisms, and prospects. Annu Rev Entomol 66:277–296. https://doi.org/10.1146/annurev-ento-041720-075234
Jaenike J (1978) On optimal oviposition behavior in phytophagous insects. Theor Popul Biol 14:350–356. https://doi.org/10.1016/0040-5809(78)90012-6
Johnson SN, Gregory PJ (2006) Chemically-mediated host-plant location and selection by root-feeding insects. Physiol Entomol 31:1–13. https://doi.org/10.1111/j.1365-3032.2005.00487.x
Kalogirou, S, (2020). lctools: local correlation, spatial inequalities, geographically weighted regression and other tools.
Legay, M, Mortier, F, (2006). La forêt face au changement climatique: adapter la gestion forestière, Les Dossiers forestiers. ONF ; INRA, Paris.
Magnusson, A, Skaug, H, Nielsen, A, Berg, C, Kristensen, K, Maechler, M, van Bentham, K, Bolker, B, Sadat, N, Lüdecke, D, Lenth, R, O’Brien, J, Brooks, M, (2020). glmmTMB: generalized linear mixed models using template model builder.
Manion, PD, |(1981). Tree disease concepts. Prentice-Hall.
Minkenberg OPJM, Tatar M, Rosenheim JA (1992) Egg load as a major source of variability in insect foraging and oviposition behavior. Oikos 65:134–142. https://doi.org/10.2307/3544896
Nageleisen L-M, Cours J (2020) Biologie des hannetons : bilan des connaissances. Rendez-vous Techniques ONF:13–17
Nageleisen L-M, Bélouard T, Meyer J (2015) Le Hanneton Forestier (Melolontha Hippocastani Fabricius 1801) En Phase Épidémique Dans Le Nord de l’Alsace. Revue Forestiere Francaise 67:353–366. https://doi.org/10.4267/2042/59290
Niemczyk M, Karwański M, Grzybowska U (2017) Effect of environmental factors on occurrence of cockchafers (Melolontha Spp.) in forest stands. Balt For 23:334–341
Niemczyk M, Sierpińska A, Tereba A, Sokołowski K, Przybylski P (2019) Natural occurrence of Beauveria spp. in outbreak areas of cockchafers (Melolontha spp.) in forest soils from Poland. BioControl 64:159–172. https://doi.org/10.1007/s10526-019-09927-3
Nordlander G, Nordenhem H, Bylund H (1997) Oviposition patterns of the pine weevil Hylobius abietis. Entomologia Experimentalis et Applicata 85:1–9. https://doi.org/10.1046/j.1570-7458.1997.00229.x
ONF (2017) Bilan patrimoniale des forêts domaniales hors DOM. ONF Office national des forêts, Paris
Piqué M, Obon B, Condés S, Saura S (2011) Comparison of relascope and fixed-radius plots for the estimation of forest stand variables in northeast Spain: an inventory simulation approach. Eur J Forest Res 130:851–859. https://doi.org/10.1007/s10342-010-0477-x
Plath M, Dorn S, Riedel J, Barrios H, Mody K (2012) Associational resistance and associational susceptibility: specialist herbivores show contrasting responses to tree stand diversification. Oecologia 169:477–487
Ruther J, Reinecke A, Tolasch T, Hilker M (2001) Make love not war: a common arthropod defence compound as sex pheromone in the forest cockchafer Melolontha hippocastani. Oecologia 128:44–47. https://doi.org/10.1007/s004420100634
Sallé A, Nageleisen L-M, Lieutier F (2014) Bark and wood boring insects involved in oak declines in Europe: current knowledge and future prospects in a context of climate change. For Ecol Manag 328:79–93. https://doi.org/10.1016/j.foreco.2014.05.027
Sallé A, Parmain G, Nusillard B, Pineau X, Brousse R, Fontaine-Guenel T, Ledet R, Vincent-Barbaroux C, Bouget C (2020) Forest decline differentially affects trophic guilds of canopy-dwelling beetles. Ann For Sci 77:86. https://doi.org/10.1007/s13595-020-00990-w
Scheirs J, De Bruyn L (2002) Integrating optimal foraging and optimal oviposition theory in plant–insect research. Oikos 96:187–191. https://doi.org/10.1034/j.1600-0706.2002.960121.x
Serre, T, (2017). Caractérisation écologique et stratégie d’évaluation de la biomasse de racines fines sur l’observatoire Hanneton, massif forestier des Vosges gréseuses—Objectif n°2 stratégie d’évaluation de la biomasse de racines fines sur l’observatoire Hanneton, massif forestier des Vosges gréseuses (Rapport de stage Master 1). AgroParisTech - Université de Lorraine—INRA, Nancy.
Späth, V, Schanowski, A, (2007). Maikäfer und Waldschutz. Zur Maikäferproblematik in der nordbadischen Rheinebene. Ministerium für Ernährung und Ländlichen Raum Baden-Württemberg, Drucknummer 55, 32.
Švestka M (2010) Changes in the abundance of Melolontha hippocastani Fabr. and Melolontha melolontha (L.) (Coleoptera: Scarabeidae) in the Czech Republic in the period 2003-2009. J For Sci 56:417–428
Švestka, M, Drapela, K, (2009). The effect of environmental conditions on the abundance of grubs of the cockchafer (Melolontha hippocastani F.). Journal of Forest Science—UZEI (Czech Republic).
Swanson ME, Franklin JF, Beschta RL, Crisafulli CM, DellaSala DA, Hutto RL, Lindenmayer DB, Swanson FJ (2011) The forgotten stage of forest succession: early-successional ecosystems on forest sites. Front Ecol Environ 9:117–125. https://doi.org/10.1890/090157
Therneau, T., Atkinson, B., (2019) port, B.R. (producer of the initial R., maintainer 1999-2017). rpart: Recursive Partitioning and Regression Trees.
Wagenhoff E, Blum R, Delb H (2014) Spring phenology of cockchafers, Melolontha Spp. (Colepoptera : Scarabaeidae), in forests of south-western Germany : results of a 3-year survey on adult emergence, swarming flights,and oogenesis from 2009 to 2011. J For Sci 60:154–165
Weissteiner S, Huetteroth W, Kollmann M, Weißbecker B, Romani R, Schachtner J, Schütz S (2012) Cockchafer larvae smell host root scents in soil. PLoS One 7:e45827. https://doi.org/10.1371/journal.pone.0045827
Woreta D (2015) Control of cockchafer Melolontha spp. grubs—a review of methods. Folia Forestalia Polonica 57:33–41. https://doi.org/10.1515/ffp-2015-0005
Woreta D (2016) Reduction of population numbers of Melolontha spp. adults—a review of methods. Folia Forestalia Polonica 58:87–95. https://doi.org/10.1515/ffp-2016-0010
Woreta D, Sukovata L (2010) Effect of food on development of the Melolonta hippocastani F. beetles (Coleoptera, Melolonthidae). Leśne Prace Badawcze 71:195–199
Woreta D, Sukovata L (2014) Survival and growth of the Melolontha spp. grubs on the roots of the main forest tree species. For Res Pap 75:375–383
Woreta D, Lipiński S, Wolski R (2016) Effects of food source quality on the adults of Melolontha melolontha and M. hippocastani. For Res Pap 77:14–23. https://doi.org/10.1515/frp-2016-0002
Woreta D, Wolski R, Lipiński S, Tkaczyk M (2018) Effects of food quality on Melolontha Spp. adults. Folia Forestalia Polonica 60:108–121. https://doi.org/10.2478/ffp-2018-0011
Acknowledgements
Firstly, we would like to thank the two reviewers who greatly improved the article with their comments. We are so grateful to Joseph Meyer, former correspondent-observer for the DSF who was almost the first to follow the Melolontha hippocastani outbreak in the Vosges mountains and expressed first major concerns about the phenomenon. He performed the first local observations on the biology and ecology of forest cockchafer. We are thankful to Benoît Cuillier and Benoît Donzé from ONF who both largely helped us for field logistic. We also thank Catherine Kern from ONF who helped us to map larvae density over the territory. We are also grateful to all territorial forest technician (TFT) who expressed their concerns and indicated forest plots infested by M. hippocastani larvae in field. We take opportunity of this insert to thank all the forest workers who helped us for a first study on the subject by digging pits and implementing the dendrometric protocols. Many thanks to Aurélien Sallé and Christophe Bouget for their discussion and constructive comments on this manuscript. We thank Vicki Moore for proofreading the manuscript.
Funding
This work was supported by a grant overseen by the French National Research Agency (ANR) as part of the “Investissements d’Avenir” program (ANR-11-LABX-0002-01, Lab of Excellence ARBRE, BENCHAFOR project). It was also largely supported by French National Forest Office (ONF).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Handling Editor: Erwin Dreyer
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributions of the co-authors
Conceptualization: J. Cours, L.M. Nageleisen; Methodology: J. Cours, L.M. Nageleisen; Software: J. Cours, V. Boulanger; Formal analysis: J. Cours; Data curation: J. Cours; Writing—original draft: J. Cours; Writing—review & editing: J. Cours, L.M. Nageleisen, R. Touffait, H. Schmuck, S. Brault, N. Bréda, C. Richter, F.X. Saintonge, V. Boulanger; Visualization: J. Cours; Supervision: L.M. Nageleisen, R. Touffait, V. Boulanger; Funding acquisition: L.M. Nageleisen, R. Touffait, N. Bréda, C. Richter, V. Boulanger.
This paper is part of the Topical Collection on Entomological issues during forest diebacks
Appendix
Appendix
Map of the forest damage caused by the Lothar windstorm in 1999 in north-eastern France. The study area is circled in red. Legend: light green = undamaged forests, light red = forests 10–50% damage, dark red = forests with more than 50% damage. Figure translated from Colin (2003)
Rights and permissions
About this article
Cite this article
Cours, J., Nageleisen, LM., Touffait, R. et al. Oviposition preference of the forest cockchafer (Melolontha hippocastani Fabr. 1801) at the stand scale depends on oak proportion, canopy openness and ground accessibility. Annals of Forest Science 78, 53 (2021). https://doi.org/10.1007/s13595-021-01066-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13595-021-01066-z