Abstract
Introduction
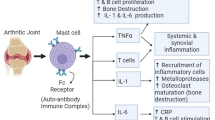
Rheumatoid arthritis (RA) is a chronic inflammatory autoimmune disease with an unclear etiology causing severe inflammation, joint pain, and destruction that increases the chance of disability over time. Dysregulation of various immune signaling cascades regulates the formation of synovial hyperplasia and pannus formation. Imbalance in cytokine levels, predominantly proinflammatory cytokines like TNF-α, IL-1, IL-6, IL-17, and IL-12p70 profoundly influences the disease's pathogenesis. Even though various strategies are adopted to treat arthritis, their side effects and cost limit their usage. This review discusses the multiple pathways involved in the pathogenesis of rheumatoid arthritis, provides a systematic analysis of various phytochemicals, and discusses their potential molecular targets in RA treatment.
Methods
The literature mining was done from scientific databases such as PubMed, Europe PMC, Web of Science, Scopus, etc. The terminologies used for literature mining were Rheumatoid arthritis, phytochemicals, cell signaling pathways, molecular mechanism, etc.
Results
NF-κB, MAPKs, and JAK–STAT are the key pathways potentially targeted for RA treatment. However, specific susceptible pathways and potential targets remain unexplored. Besides, the phytochemicals remain an immense source to be exploited for the effective treatment of RA, overcoming the demerits of the conventional strategies. Various in vitro and in vivo findings suggest that polyphenols and flavonoids effectively treat RA conditions overcoming the demerits, such as limitations in usage and toxicity. The phytochemicals should be explored in par with the pathological mechanisms with all the available targets to determine their therapeutic efficacy. Through the established therapeutic efficacy, phytochemicals can help developing therapeutics that are safe and efficacious for RA treatment.







Similar content being viewed by others
References
Guo Q, Wang Y, Xu D, Nossent J, Pavlos NJ, Xu J. Rheumatoid arthritis: pathological mechanisms and modern pharmacologic therapies. Bone Res. 2018;6:15.
McInnes IB, Buckley CD, Isaacs JD. Cytokines in rheumatoid arthritis—shaping the immunological landscape. Nat Rev Rheumatol. 2016;12:63.
Shao X, Hudson M, Colmegna I, Greenwood CMT, Fritzler MJ, Awadalla P, et al. Rheumatoid arthritis-relevant DNA methylation changes identified in ACPA-positive asymptomatic individuals using methylome capture sequencing. Clin Epigenetics. 2019;11:110.
Maibom-Thomsen SL, Trier NH, Holm BE, Hansen KB, Rasmussen MI, Chailyan A, et al. Immunoglobulin G structure and rheumatoid factor epitopes. PLoS ONE. 2019;14:e0217624–e0217624.
Alunno A, Carubbi F, Giacomelli R, Gerli R. Cytokines in the pathogenesis of rheumatoid arthritis: new players and therapeutic targets. BMC Rheumatol. 2017;1:1–13.
Tanaka T, Narazaki M, Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol. 2014;6:a016295.
Zhou J, Yu Y, Yang X, Wang Y, Song Y, Wang Q, et al. Berberine attenuates arthritis in adjuvant-induced arthritic rats associated with regulating polarization of macrophages through AMPK/NF-кB pathway. Eur J Pharmacol. 2019;852:179–88.
Chemin K, Gerstner C, Malmström V. Effector functions of CD4+ T cells at the site of local autoimmune inflammation-lessons from rheumatoid arthritis. Front Immunol. 2019. https://doi.org/10.3389/fimmu.2019.00353.
Fang Q, Zhou C, Nandakumar KS. Molecular and cellular pathways contributing to joint damage in rheumatoid arthritis. Mediat Inflamm. 2020;2020:3830212.
Cooles FAH, Isaacs JD, Anderson AE. Treg cells in rheumatoid arthritis: An update. Curr Rheumatol Rep. 2013. https://doi.org/10.1007/s11926-013-0352-0.
De S, Manna A, Kundu S, De Sarkar S, Chatterjee U, Sen T, et al. Allylpyrocatechol attenuates collagen-induced arthritis via attenuation of oxidative stress secondary to modulation of the MAPK, JAK/STAT, and Nrf2/HO-1 pathways. J Pharmacol Exp Ther. 2017;360:249.
McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med. 2011;365:2205–19.
Li G, Qin Y, Du P. Andrographolide inhibits the migration, invasion and matrix metalloproteinase expression of rheumatoid arthritis fibroblast-like synoviocytes via inhibition of HIF-1α signaling. Life Sci. 2015;136:67–72.
Choy E. Understanding the dynamics: pathways involved in the pathogenesis of rheumatoid arthritis. Rheumatology. 2012;51:v3-11.
Lipsky PE, van der Heijde DM, St Clair EW, Furst DE, Breedveld FC, Kalden JR, et al. Infliximab and methotrexate in the treatment of rheumatoid arthritis. Anti-tumor necrosis factor trial in rheumatoid arthritis with Concomitant Therapy Study Group. N Engl J Med. 2000;343:1594–602.
Hutami IR, Tanaka E, Izawa T. Crosstalk between Fas and S1P(1) signaling via NF-kB in osteoclasts controls bone destruction in the TMJ due to rheumatoid arthritis. Jpn Dent Sci Rev. 2019;55:12–9.
Choi M-C, Jo J, Park J, Kang HK, Park Y. NF-κB signaling pathways in osteoarthritic cartilage destruction. Cells. 2019;8:734.
Sarmiento Salinas FL, Santillán Benítez JG, Hernández Navarro MD, Mendieta ZH. NF-κB1/IKKε gene expression and clinical activity in patients with rheumatoid arthritis. Lab Med. 2017;49:11–7.
Noort AR, Tak PP, Tas SW. Non-canonical NF-κB signaling in rheumatoid arthritis: Dr Jekyll and Mr Hyde? Arthritis Res Ther. 2015;17:15.
Korb A, Tohidast-Akrad M, Cetin E, Axmann R, Smolen J, Schett G. Differential tissue expression and activation of p38 MAPK alpha, beta, gamma, and delta isoforms in rheumatoid arthritis. Arthritis Rheum US. 2006;54:2745–56.
Thalhamer T, McGrath MA, Harnett MM. MAPKs and their relevance to arthritis and inflammation. Rheumatology. 2008;47:409–14.
Clark AR, Dean JLE. The p38 MAPK pathway in rheumatoid arthritis: a sideways look. Open Rheumatol J. 2012;6:209–19.
Li X, Udagawa N, Itoh K, Suda K, Murase Y, Nishihara T, et al. p38 MAPK-mediated signals are required for inducing osteoclast differentiation but not for osteoclast function. Endocrinology. 2002;143:3105–13.
Kitanaka T, Nakano R, Kitanaka N, Kimura T, Okabayashi K, Narita T, et al. JNK activation is essential for activation of MEK/ERK signaling in IL-1β-induced COX-2 expression in synovial fibroblasts. Sci Rep. 2017;7:39914.
Hu H, Jin H, Yu L, Qu S. Inhibition of ERK pathway decreases the synovial hyperplasia and angiogenesis of rheumatoid arthritis rats. Eur J Inflamm. 2018;16:205873921879453.
Lu N, Malemud JC. Extracellular signal-regulated kinase: a regulator of cell growth, inflammation, chondrocyte and bone cell receptor-mediated gene expression. Int J Mol Sci. 2019;20:3972.
Singh K, Deshpande P, Pryshchep S, Colmegna I, Liarski V, Weyand CM, et al. ERK-Dependent T cell receptor threshold calibration in rheumatoid arthritis. J Immunol. 2009;183:8258–67.
Shang W, Zhao L-J, Dong X-L, Zhao Z-M, Li J, Zhang B-B, et al. Curcumin inhibits osteoclastogenic potential in PBMCs from rheumatoid arthritis patients via the suppression of MAPK/RANK/c-Fos/NFATc1 signaling pathways. Mol Med Rep. 2016;14:3620–6.
Han Z, Boyle DL, Aupperle KR, Bennett B, Manning AM, Firestein GS. Jun N-terminal kinase in rheumatoid arthritis. J Pharmacol Exp Ther. 1999;291:124–30.
Kato M. New insights into IFN-γ in rheumatoid arthritis: role in the era of JAK inhibitors. Immunol Med. 2020;43:72–8.
Ciobanu Alexandra D, Poenariu Sabin I, Crînguș L-I, Vreju Ananu F, Turcu-Stiolica A, Tica Adrian A, et al. JAK/STAT pathway in pathology of rheumatoid arthritis (review). Exp Ther Med. 2020;20:3498–503.
Xu L, Zhang L, Zhang H, Yang Z, Qi L, Wang Y, et al. The participation of fibroblast growth factor 23 (FGF23) in the progression of osteoporosis via JAK/STAT pathway. J Cell Biochem. 2018;119:3819–28.
Nicol LSC, Thornton P, Hatcher JP, Glover CP, Webster CI, Burrell M, et al. Central inhibition of granulocyte-macrophage colony-stimulating factor is analgesic in experimental neuropathic pain. Pain. 2018. https://doi.org/10.1097/j.pain.0000000000001130.
Malemud CJ. The discovery of novel experimental therapies for inflammatory arthritis. Mediat Inflamm. 2009. https://doi.org/10.1177/1759720X18776224.
Malemud CJ. The role of the JAK/STAT signal pathway in rheumatoid arthritis. Ther Adv Musculoskelet Dis. 2018;10:117–27.
Tanaka S. Regulation of bone destruction in rheumatoid arthritis through RANKL-RANK pathways. World J Orthop. 2013;4:1–6.
Bi H, Chen X, Gao S, Yu X, Xiao J, Zhang B, et al. Key triggers of osteoclast-related diseases and available strategies for targeted therapies: a review. Front Med. 2017;4:234.
Tobeiha M, Moghadasian MH, Amin N, Jafarnejad S. RANKL/RANK/OPG pathway: a mechanism involved in exercise-induced bone remodeling. Biomed Res Int. 2020;2020:6910312.
Papadaki M, Rinotas V, Violitzi F, Thireou T, Panayotou G, Samiotaki M, et al. New Insights for RANKL as a Proinflammatory Modulator in Modeled Inflammatory Arthritis. Front Immunol. 2019. https://doi.org/10.1038/s41419-019-1413-8.
Yang Y, Wang H, Kouadir M, Song H, Shi F. Recent advances in the mechanisms of NLRP3 inflammasome activation and its inhibitors. Cell Death Dis. 2019;10:128.
Swanson KV, Deng M, Ting JP-Y. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat Rev Immunol. 2019;19:477–89.
Es N. NLRP3 inflammasome: a novel therapeutic target in arthritis. J Arthritis. 2018;7:1000118.
Sun K, Luo J, Guo J, Yao X, Jing X, Guo F. The PI3K/AKT/mTOR signaling pathway in osteoarthritis: a narrative review. Osteoarthr Cartil. 2020;28:400–9.
Liu W, Yu W-M, Zhang J, Chan RJ, Loh ML, Zhang Z, et al. Inhibition of the Gab2/PI3K/mTOR signaling ameliorates myeloid malignancy caused by Ptpn11 (Shp2) gain-of-function mutations. Leukemia. 2017;31:1415–22.
Cheng Z, Sun W, Ni X, Xu H, Wang Y. GAB2 inhibits chondrocyte apoptosis through PI3K-AKT signaling in osteoarthritis. Int J Clin Exp Pathol. 2020;13:616–23.
Panda S, Sikdar M, Biswas S, Sharma R, Kar A. Allylpyrocatechol, isolated from betel leaf ameliorates thyrotoxicosis in rats by altering thyroid peroxidase and thyrotropin receptors. Sci Rep. 2019;9:12276.
De S, Kundu S, Chatterjee U, Chattopadhyay S, Chatterjee M. Allylpyrocatechol attenuates methotrexate-induced hepatotoxicity in a collagen-induced model of arthritis. Free Radic Res. 2018;52:698–711.
Kundu S, Bala A, Ghosh P, Mukhopadhyay D, Mitra A, Sarkar A, et al. Attenuation of oxidative stress by Allylpyrocatechol in synovial cellular infiltrate of patients with Rheumatoid Arthritis. Free Radic Res. 2011;45:518–26.
Li Z, Tan J, Wang L, Li Q. Andrographolide benefits rheumatoid arthritis via inhibiting MAPK pathways. Inflammation. 2017;40:1599–605.
Chiou W, Chen C, Lin J. Mechanisms of suppression of inducible nitric oxide synthase (iNOS ) expression in RAW 264.7 cells by andrographolide. Br J Pharmacol. 2014;129:1553–60.
Yan J, Chen Y, He C, Yang Z, Lü C, Chen X. Andrographolide induces cell cycle arrest and apoptosis in human rheumatoid arthritis fibroblast-like synoviocytes. Cell Biol Toxicol. 2012;28:47–56.
Tangyuenyong S, Viriyakhasem N, Peansukmanee S, Kongtawelert P, Ongchai S. Andrographolide exerts chondroprotective activity in equine cartilage explant and suppresses interleukin-1 β -induced MMP-2 expression in equine chondrocyte culture. Int Sch Res Not. 2014;2014:1–8.
Zhai ZJ, Li HW, Liu GW, Qu XH, Tian B, Yan W, et al. Andrographolide suppresses RANKL-induced osteoclastogenesis in vitro and prevents inflammatory bone loss in vivo. Br J Pharmacol. 2014;171:663–75.
Carretta MD, Alarcón P, Jara E, Solis L, Hancke JL, Concha II, et al. Andrographolide reduces IL-2 production in T-cells by interfering with NFAT and MAPK activation. Eur J Pharmacol. 2009;602:413–21.
Ding Q, Ji X, Cheng Y, Yu Y, Qi Y, Wang X. Inhibition of matrix metalloproteinases and inducible nitric oxide synthase by andrographolide in human osteoarthritic chondrocytes. Mod Rheumatol. 2013;23:1124–32.
Li Y, He S, Tang J, Ding N, Chu X, Cheng L, et al. Andrographolide inhibits inflammatory cytokines secretion in LPS-stimulated RAW2647 cells through suppression of NF-κB/MAPK signaling pathway. Evid Based Compl Altern Med. 2017. https://doi.org/10.1155/2017/8248142.
Lee W-R, Chung C-L, Hsiao C-J, Chou Y-C, Hsueh P-J, Yang P-C, et al. Suppression of matrix metalloproteinase-9 expression by andrographolide in human monocytic THP-1 cells via inhibition of NF-κB activation. Phytomedicine. 2012;19:270–7.
Li X, Han Y, Zhou Q, Jie H, He Y, Han J, et al. Apigenin, a potent suppressor of dendritic cell maturation and migration, protects against collagen-induced arthritis. J Cell Mol Med. 2016;20:170–80.
Shin G-C, Kim C, Lee J-M, Cho W-S, Lee S-G, Jeong M, et al. Apigenin-induced apoptosis is mediated by reactive oxygen species and activation of ERK1/2 in rheumatoid fibroblast-like synoviocytes. Chem Biol Interact. 2009;182:29–36.
Sun Q, Jiang S, Yang K, Zheng J, Zhang L, Xu W. Apigenin enhances the cytotoxic effects of tumor necrosis factor-related apoptosis-inducing ligand in human rheumatoid arthritis fibroblast-like synoviocytes. Mol Biol Rep. 2012;39:5529–35.
Xu L, Zhang L, Bertucci AM, Pope RM, Datta SK. Apigenin, a dietary flavonoid, sensitizes human T cells for activation-induced cell death by inhibiting PKB/Akt and NF-κB activation pathway. Immunol Lett. 2008;121:74–83.
Hu Z, Jiao Q, Ding J, Liu F, Liu R, Shan L, et al. Berberine induces dendritic cell apoptosis and has therapeutic potential for rheumatoid arthritis. Arthritis Rheum. 2011;63:949–59.
Wang X, Jiang S, Sun Q. Effects of berberine on human rheumatoid arthritis fibroblast-like synoviocytes. Exp Biol Med. 2011;236:859–66.
Ganova P, Belenska-Todorova L, Doncheva T, Ivanovska N. Berberine prevents bone and cartilage destruction and influences cell senescence in experimental arthritis. J Adv Med Pharm Sci. 2017;15:1–8.
Wang Z, Chen Z, Yang S, Wang Y, Huang Z, Gao J, et al. Berberine ameliorates collagen-induced arthritis in rats associated with anti-inflammatory and anti-angiogenic effects. Inflammation. 2014;37:1789–98.
Wang W, Sun W, Jin L. Caffeic acid alleviates inflammatory response in rheumatoid arthritis fibroblast-like synoviocytes by inhibiting phosphorylation of IκB kinase α/β and IκBα. Int Immunopharmacol. 2017;48:61–6.
Fikry EM, Gad AM, Eid AH, Arab HH. Caffeic acid and ellagic acid ameliorate adjuvant-induced arthritis in rats via targeting inflammatory signals, chitinase-3-like protein-1 and angiogenesis. Biomed Pharmacother. 2019;110:878–86.
Cascão R, Vidal B, Jalmari Finnilä MA, Lopes IP, Teixeira RL, Saarakkala S, et al. Effect of celastrol on bone structure and mechanics in arthritic rats. RMD Open. 2017;3:e000438.
Cascão R, Vidal B, Lopes IP, Paisana E, Rino J, Moita LF, et al. Decrease of CD68 synovial macrophages in celastrol treated arthritic rats. PLoS ONE. 2015;10:e0142448.
Wong VKW, Qiu C, Xu S, Law BYK, Zeng W, Wang H, et al. Ca2+ signalling plays a role in celastrol-mediated suppression of synovial fibroblasts of rheumatoid arthritis patients and experimental arthritis in rats. Br J Pharmacol. 2019;176:2922–44.
Fang Z, He D, Yu B, Liu F, Zuo J, Li Y, et al. High-throughput study of the effects of celastrol on activated fibroblast-like synoviocytes from patients with rheumatoid arthritis. Genes (Basel). 2017;8:221.
Yuan K, Huang G, Zhang S, Zhu Q, Yu R, Sheng H, et al. Celastrol alleviates arthritis by modulating the inflammatory activities of neutrophils. J Tradit Chin Med Sci. 2017;4:50–8.
Wang Y, Zhou C, Gao H, Li C, Li D, Liu P, et al. Therapeutic effect of Cryptotanshinone on experimental rheumatoid arthritis through downregulating p300 mediated-STAT3 acetylation. Biochem Pharmacol. 2017;138:119–29.
Wang Y, Wang S, Li Y, Jiang J, Zhou C, Li C, et al. Therapeutic effect of Cryptotanshinone on collagen-induced arthritis in rats via inhibiting nuclear factor kappa B signaling pathway. Transl Res. 2015;165:704–16.
Tang S, Shen X-Y, Huang H-Q, Xu S-W, Yu Y, Zhou C-H, et al. Cryptotanshinone suppressed inflammatory cytokines secretion in RAW264.7 macrophages through inhibition of the NF-κB and MAPK signaling pathways. Inflammation. 2011;34:111–8.
Dai Q, Zhou D, Xu L, Song X. Curcumin alleviates rheumatoid arthritis-induced inflammation and synovial hyperplasia by targeting mTOR pathway in rats. Drug Des Devel Ther. 2018;12:4095–105.
Huang G, Xu Z, Huang Y, Duan X, Gong W, Zhang Y, et al. Curcumin protects against collagen-induced arthritis via suppression of BAFF production. J Clin Immunol. 2013;33:550–7.
Park C, Moon D-OO, Choi I-WW, Choi BT, Nam T-JJ, Rhu C-HH, et al. Curcumin induces apoptosis and inhibits prostaglandin E2 production in synovial fibroblasts of patients with rheumatoid arthritis. Int J Mol Med. 2007;20:365–72.
Shakibaei M, Schulze-Tanzil G, John T, Mobasheri A. Curcumin protects human chondrocytes from IL-l1beta-induced inhibition of collagen type II and beta1-integrin expression and activation of caspase-3: an immunomorphological study. Ann Anat. 2005;187:487–97.
Mun SH, Kim HS, Kim JW, Ko NY, Kim DK, Lee BY, et al. Oral administration of curcumin suppresses production of matrix metalloproteinase (MMP)-1 and MMP-3 to ameliorate collagen-induced arthritis: inhibition of the PKCδ/JNK/c-Jun pathway. J Pharmacol Sci. 2009;111:13–21.
Moon D-O, Kim M-O, Choi YH, Park Y-M, Kim G-Y. Curcumin attenuates inflammatory response in IL-1beta-induced human synovial fibroblasts and collagen-induced arthritis in mouse model. Int Immunopharmacol. 2010;10:605–10.
Huang G, Yang Y, Xu Z, Zhou P, Gong W, Li Y, et al. Downregulation of B lymphocyte stimulator expression by curcumin in B lymphocyte via suppressing nuclear translocation of NF-kappaB. Eur J Pharmacol. 2011;650:451–7.
Mathy-Hartert M, Jacquemond-Collet I, Priem F, Sanchez C, Lambert C, Henrotin Y. Curcumin inhibits pro-inflammatory mediators and metalloproteinase-3 production by chondrocytes. Inflamm Res. 2009;58:899.
Schulze-Tanzil G, Mobasheri A, Sendzik J, John T, Shakibaei M. Effects of curcumin (diferuloylmethane) on nuclear factor kappaB signaling in interleukin-1beta-stimulated chondrocytes. Ann N Y Acad Sci. 2004;1030:578–86.
Byun E-H, Omura T, Yamada K, Tachibana H. Green tea polyphenol epigallocatechin-3-gallate inhibits TLR2 signaling induced by peptidoglycan through the polyphenol sensing molecule 67-kDa laminin receptor. FEBS Lett. 2011;585:814–20.
Lee J-H, Jin H, Shim H-E, Kim H-N, Ha H, Lee ZH. Epigallocatechin-3-gallate inhibits osteoclastogenesis by down-regulating c-Fos expression and suppressing the nuclear factor-kappaB signal. Mol Pharmacol. 2010;77:17–25.
Lee S-Y, Jung YO, Ryu J-G, Oh H-J, Son H-J, Lee SH, et al. Epigallocatechin-3-gallate ameliorates autoimmune arthritis by reciprocal regulation of T helper-17 regulatory T cells and inhibition of osteoclastogenesis by inhibiting STAT3 signaling. J Leukoc Biol. 2016;100:559–68.
Morinobu A, Biao W, Tanaka S, Horiuchi M, Jun L, Tsuji G, et al. (-)-Epigallocatechin-3-gallate suppresses osteoclast differentiation and ameliorates experimental arthritis in mice. Arthritis Rheum. 2008;58:2012–8.
Singh R, Ahmed S, Malemud CJ, Goldberg VM, Haqqi TM. Epigallocatechin-3-gallate selectively inhibits interleukin-1beta-induced activation of mitogen activated protein kinase subgroup c-Jun N-terminal kinase in human osteoarthritis chondrocytes. J Orthop Res. 2003;21:102–9.
Huang G-S, Tseng C-Y, Lee C-H, Su S-L, Lee H-S. Effects of (-)-epigallocatechin-3-gallate on cyclooxygenase 2, PGE(2), and IL-8 expression induced by IL-1beta in human synovial fibroblasts. Rheumatol Int. 2010;30:1197–203.
Ahmed S, Pakozdi A, Koch AE. Regulation of interleukin-1beta-induced chemokine production and matrix metalloproteinase 2 activation by epigallocatechin-3-gallate in rheumatoid arthritis synovial fibroblasts. Arthritis Rheum. 2006;54:2393–401.
Yun H-J, Yoo W-H, Han M-K, Lee Y-R, Kim J-S, Lee S-I. Epigallocatechin-3-gallate suppresses TNF-α -induced production of MMP-1 and -3 in rheumatoid arthritis synovial fibroblasts. Rheumatol Int. 2008;29:23–9.
Singh R, Ahmed S, Islam N, Goldberg VM, Haqqi TM. Epigallocatechin-3-gallate inhibits interleukin-1beta-induced expression of nitric oxide synthase and production of nitric oxide in human chondrocytes: suppression of nuclear factor kappaB activation by degradation of the inhibitor of nuclear factor kappa. Arthritis Rheum. 2002;46:2079–86.
Singh AK, Umar S, Riegsecker S, Chourasia M, Ahmed S. Regulation of transforming growth factor beta-activated kinase activation by epigallocatechin-3-gallate in rheumatoid arthritis synovial fibroblasts: suppression of K(63)-linked autoubiquitination of tumor necrosis factor receptor-associated Factor 6. Arthritis Rheumatol (Hoboken, NJ). 2016;68:347–58.
Lin S-KK, Chang H-HH, Chen Y-JJ, Wang C-CC, Galson DL, Hong C-YY, et al. Epigallocatechin-3-gallate diminishes CCL2 expression in human osteoblastic cells via up-regulation of phosphatidylinositol 3-kinase/Akt/Raf-1 interaction: A potential therapeutic benefit for arthritis. Arthritis Rheum. 2008;58:3145–56.
Huh J-E, Jung I-T, Choi J, Baek Y-H, Lee J-D, Park D-S, et al. The natural flavonoid galangin inhibits osteoclastic bone destruction and osteoclastogenesis by suppressing NF-kappaB in collagen-induced arthritis and bone marrow-derived macrophages. Eur J Pharmacol. 2013;698:57–66.
Fu Q, Gao Y, Zhao H, Wang Z, Wang J. Galangin protects human rheumatoid arthritis fibroblast-like synoviocytes via suppression of the NF-κB/NLRP3 pathway. Mol Med Rep. 2018;18:3619–24.
Zhang Y, Dong J, He P, Li W, Zhang Q, Li N, et al. Genistein inhibit cytokines or growth factor-induced proliferation and transformation phenotype in fibroblast-like synoviocytes of rheumatoid arthritis. Inflammation. 2012;35:377–87.
Li JJ, Li JJ, Yue Y, Hu Y, Cheng W, Liu R, et al. Genistein suppresses tumor necrosis factor α-induced inflammation via modulating reactive oxygen species/Akt/nuclear factor κB and adenosine monophosphate-activated protein kinase signal pathways in human synoviocyte MH7A cells. Drug Des Devel Ther. 2014;8:315–23.
Liu F-C, Wang C-C, Lu J-W, Lee C-H, Chen S-C, Ho Y-J, et al. Chondroprotective effects of genistein against osteoarthritis induced joint inflammation. Nutrients. 2019;11:1180.
Yoon HY, Lee EG, Lee H, Cho IJ, Choi YJ, Sung MS, et al. Kaempferol inhibits IL-1β-induced proliferation of rheumatoid arthritis synovial fibroblasts and the production of COX-2, PGE2 and MMPs. Int J Mol Med. 2013;32:971–7.
Pan D, Li N, Liu Y, Xu Q, Liu Q, You Y, et al. Kaempferol inhibits the migration and invasion of rheumatoid arthritis fibroblast-like synoviocytes by blocking activation of the MAPK pathway. Int Immunopharmacol. 2018;55:174–82.
Zhuang Z, Ye G, Huang B. Kaempferol alleviates the interleukin-1β-induced inflammation in rat osteoarthritis chondrocytes via suppression of NF-κB. Med Sci Monit. 2017;23:3925–31.
Ansari M, Neha KH. Quercetin alleviate oxidative stress and inflammation through upregulation of antioxidant machinery and down- regulation of COX2 and NF-κB expression in collagen induced rheumatoid arthritis. Int J Drug Dev Res. 2014;6:215–30.
Guazelli CFS, Staurengo-Ferrari L, Zarpelon AC, Pinho-Ribeiro FA, Ruiz-Miyazawa KW, Vicentini FTMC, et al. Quercetin attenuates zymosan-induced arthritis in mice. Biomed Pharmacother. 2018;102:175–84.
Xiao P, Hao Y, Zhu X, Wu X. p53 contributes to quercetin-induced apoptosis in human rheumatoid arthritis fibroblast-like synoviocytes. Inflammation. 2013;36:272–8.
Haleagrahara N, Miranda-Hernandez S, Alim MA, Hayes L, Bird G, Ketheesan N. Therapeutic effect of quercetin in collagen-induced arthritis. Biomed Pharmacother. 2017;90:38–46.
Yang Y, Zhang X, Xu M, Wu X, Zhao F, Zhao C. Quercetin attenuates collagen-induced arthritis by restoration of Th17/Treg balance and activation of Heme Oxygenase 1-mediated anti-inflammatory effect. Int Immunopharmacol. 2018;54:153–62.
Kim H-R, Kim B-M, Won J-Y, Lee K-A, Ko HM, Kang YS, et al. Quercetin, a plant polyphenol, has potential for the prevention of bone destruction in rheumatoid arthritis. J Med Food. 2018;22:152–61.
Yamaguchi M, Weitzmann MN. Quercetin, a potent suppressor of NF-kappaB and Smad activation in osteoblasts. Int J Mol Med. 2011;28:521–5.
Javadi F, Ahmadzadeh A, Eghtesadi S, Aryaeian N, Zabihiyeganeh M, Rahimi Foroushani A, et al. The effect of quercetin on inflammatory factors and clinical symptoms in women with rheumatoid arthritis: a double-blind, randomized controlled trial. J Am Coll Nutr. 2017;36:9–15.
Corrêa MG, Pires PR, Ribeiro FV, Pimentel SP, Cirano FR, Napimoga MH, et al. Systemic treatment with resveratrol reduces the progression of experimental periodontitis and arthritis in rats. PLoS ONE. 2018;13:e0204414.
Xuzhu G, Komai-Koma M, Leung BP, Howe HS, McSharry C, McInnes IB, et al. Resveratrol modulates murine collagen-induced arthritis by inhibiting Th17 and B-cell function. Ann Rheum Dis. 2012;71:129–35.
Oz B, Yildirim A, Yolbas S, Celik ZB, Etem EO, Deniz G, et al. Resveratrol inhibits Src tyrosine kinase, STAT3, and Wnt signaling pathway in collagen induced arthritis model. BioFactors. 2019;45:69–74.
Riveiro-Naveira RR, Valcárcel-Ares MN, Almonte-Becerril M, Vaamonde-García C, Loureiro J, Hermida-Carballo L, et al. Resveratrol lowers synovial hyperplasia, inflammatory markers and oxidative damage in an acute antigen-induced arthritis model. Rheumatology. 2016;55:1889–900.
Lomholt S, Mellemkjaer A, Iversen MB, Pedersen SB, Kragstrup TW. Resveratrol displays anti-inflammatory properties in an ex vivo model of immune mediated inflammatory arthritis. BMC Rheumatol. 2018;2:27.
Tian J, Chen JW, Gao JS, Li L, Xie X. Resveratrol inhibits TNF-α-induced IL-1β, MMP-3 production in human rheumatoid arthritis fibroblast-like synoviocytes via modulation of PI3kinase/Akt pathway. Rheumatol Int. 2013;33:1829–35.
Nakayama H, Yaguchi T, Yoshiya S, Nishizaki T. Resveratrol induces apoptosis MH7A human rheumatoid arthritis synovial cells in a sirtuin 1-dependent manner. Rheumatol Int. 2012;32:151–7.
Zhu X, Liu Q, Wang M, Liang M, Yang X, Xu X, et al. Activation of sirt1 by resveratrol inhibits TNF-α induced inflammation in fibroblasts. PLoS ONE. 2011;6:e27081.
Liu C, Zhang Y, Kong X, Zhu L, Pang J, Xu Y, et al. Triptolide prevents bone destruction in the collagen-induced arthritis model of rheumatoid arthritis by targeting RANKL/RANK/OPG signal pathway. Evid Based Compl Altern Med. 2013;2013:626038.
Kong X, Zhang Y, Liu C, Guo W, Li X, Su X, et al. Anti-angiogenic effect of triptolide in rheumatoid arthritis by targeting angiogenic cascade. PLoS ONE. 2013;8:e77513.
Gong Y, Huang X, Wang D, Li M, Liu Z. Triptolide protects bone against destruction by targeting RANKL-mediated ERK/AKT signalling pathway in the collagen-induced rheumatoid arthritis. Biomed Res. 2017;28:4111–6.
Wang S, Liu Z, Wang J, Wang Y, Liu J, Ji X, et al. The triptolide-induced apoptosis of osteoclast precursor by degradation of cIAP2 and treatment of rheumatoid arthritis of TNF-transgenic mice. Phyther Res. 2019;33:342–9.
Wang S, Zuo S, Liu Z, Ji X, Yao Z, Wang X. Study on the efficacy and mechanism of triptolide on treating TNF transgenic mice with rheumatoid arthritis. Biomed Pharmacother. 2018;106:813–20.
Shenghao T, Yonghong H, Keqin Z, Mingmin Z, Xianyang L, Weichen Z. Effects of triptolide on the expression and activity of NF-KB in synovium of collagen-induced arthritis rats. J Huazhong Univ Sci Technol. 2005;25:543–5.
Lin N, Liu C, Xiao C, Jia H, Imada K, Wu H, et al. Triptolide, a diterpenoid triepoxide, suppresses inflammation and cartilage destruction in collagen-induced arthritis mice. Biochem Pharmacol. 2007;73:136–46.
Rifaii M. Andrographolide ameliorate rheumatoid arthritis by promoting the development of regulatory T Cells. J Trop Life Sci. 2010;1:5–8.
Cascão R, Vidal B, Raquel H, Neves-Costa A, Figueiredo N, Gupta V, et al. Effective treatment of rat adjuvant-induced arthritis by celastrol. Autoimmun Rev. 2012;11:856–62.
Moon D-O, Kim M-O, Choi YH, Park Y-M, Kim G-Y. Curcumin attenuates inflammatory response in IL-1β-induced human synovial fibroblasts and collagen-induced arthritis in mouse model. Int Immunopharmacol. 2010;10:605–10.
Akhtar N, Haqqi TM. Epigallocatechin-3-gallate suppresses the global interleukin-1beta-induced inflammatory response in human chondrocytes. Arthritis Res Ther. 2011;13:R93.
Huang G-S, Tseng C-Y, Lee C-H, Su S-L, Lee H-S. Effects of (−)-epigallocatechin-3-gallate on cyclooxygenase 2, PGE2, and IL-8 expression induced by IL-1β in human synovial fibroblasts. Rheumatol Int. 2010;30:1197–203.
Zou Y, Hu W. Investigation of gene expression profiles in a rat adjuvant arthritis model suggests an effective role of triptolide via PI3K-AKT signaling. Exp Ther Med. 2019. https://doi.org/10.3892/etm.2019.7425.
Xiao C, Zhou J, He Y, Jia H, Zhao L, Zhao N, et al. Effects of triptolide from Radix Tripterygium wilfordii (Leigongteng) on cartilage cytokines and transcription factor NF-κB: a study on induced arthritis in rats. Chin Med. 2009;4:13.
Lu Y, Wang W-J, Leng J-H, Cheng L-F, Feng L, Yao H-P. Inhibitory effect of triptolide on interleukin-18 and its receptor in rheumatoid arthritis synovial fibroblasts. Inflamm Res. 2008;57:260–5.
Fan D, He X, Bian Y, Guo Q, Zheng K, Zhao Y, et al. Triptolide modulates TREM-1 signal pathway to inhibit the inflammatory response in rheumatoid arthritis. Int J Mol Sci. 2016;57:260–5.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sivasakthi, P., Sanmuga Priya, E. & Senthamil Selvan, P. Molecular insights into phytochemicals exhibiting anti-arthritic activity: systematic review. Inflamm. Res. 70, 665–685 (2021). https://doi.org/10.1007/s00011-021-01471-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-021-01471-0