Abstract
Understanding of relationship between structure and property of thermally activated delayed fluorescence (TADF) materials is essential for developing efficient TADF materials. To investigate the substituent effect of electron donors on luminescent properties of TADF materials, a series of single crystals based on triphenylamine (TPA)/anthraquinone (AQ) hybrids namely 1-TPA-AQ, 2-TPA-AQ, 1,8-2TPA-AQ, 2,6-2TPA-AQ have been prepared in this work. Interestingly, it is demonstrated that the substituent site of TPA unit has an important impact on the conformation of AQ for the first time. The planarity of the AQ units in these four molecules is in the order 2,6-2TPA-AQ > 2-TPA-AQ ≈ 1-TPA-AQ > 1,8-2TPA-AQ, which is associated with the molecule symmetry. In addition, intermolecular interactions in these crystals are also investigated. In α-substituted AQ derivatives (1-TPA-AQ and 1,8-2TPA-AQ) typical π–π intermolecular interactions may account for their normal TADF but weak emission in solid state. In contrast, β-substituted AQ derivatives (2-TPA-AQ and 2,6-2TPA-AQ) display weak intermolecular interactions, which are beneficial to restricting molecular motions and to suppressing the non-radiative decay, resulting in efficient TADF and aggregation-induced emission properties simultaneously.
Graphic Abstract
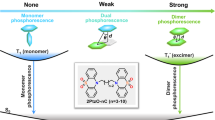
It is demonstrated that the substituent site of triphenylamine unit on anthraquinone (AQ) group has an important impact on the conformation of AQ.




Similar content being viewed by others
References
Petra G, Romana CK, Maja V, Boris S (2014) Crystal sructures and emission properties of the BF2 complex 1-phenyl-3-(3,5-dimethoxyphenyl)-propane-1,3-dione: multiple chromisms, aggregation- or crystallization-induced emission, and the self-assembly effect. J Am Chem Soc 136:7383–7394
Wang K, Zhang H, Chen S, Yang G, Zhang J, Tian W, Su Z, Wang Y (2014) Organic polymorphs: one-compound-based crystals with molecular-conformation- and packing-dependent luminescent properties. Adv Mater 26:6168–6173
Wang K, Zheng CJ, Liu W, Liang K, Shi YZ, Tao SL, Lee CS, Ou XM, Zhang XH (2017) Avoiding energy loss on TADF emitters: controlling the dual conformations of D-A structure molecules based on the pseudoplanar segments. Adv Mater 29:1701476
Huang B, Chen WC, Li Z, Zhang J, Zhao W, Feng Y, Tang BZ, Lee CS (2018) Manipulation of molecular aggregation states to realize polymorphism, AIE, MCL, and TADF in a single molecule. Angew Chem Int Ed 57:12473–12477
Yang J, Zhen X, Wang B, Gao X, Ren Z, Wang J, Xie Y, Li J, Peng Q, Pu K, Li Z (2018) The influence of the molecular packing on the room temperature phosphorescence of purely organic luminogens. Nat Commun 9:840
Uoyama H, Goushi K, Shizu K, Nomura H, Adachi C (2012) Highly efficient organic light-emitting diodes from delayed fluorescence. Nature 492:234–240
Tao Y, Yuan K, Chen T, Xu P, Li H, Chen R, Zheng C, Zhang L, Huang W (2014) Thermally activated delayed fluorescence materials towards the breakthrough of organoelectronics. Adv Mater 26:7931–7958
Zhang QS, Li B, Huang SP, Nomura H, Tanaka H, Adachi C (2014) Efficient blue organic light- emitting diodes employing thermally activated delayed fluorescence. Nat Photon 8:326–332
Hirata S, SakaiY, Masui K, Tanaka H, Lee SY, Nomura H, Nakamura N, Yasumatsu M, Nakanotani H, Zhang QS, Shizu K, Miyazaki H, Adachi C (2015) Highly efficient blue electroluminescence based on thermally activated delayed fluorescence. Nat Mater 14:330–336
Yang Z, Mao Z, Xie Z, Zhang Y, Liu S, Zhao J, Xu J, Chi Z, Aldred MP (2017) Recent advances in organic thermally activated delayed fluorescence materials. Chem Soc Rev 46:915–1016
Li W, Cai X, Li B, Gan L, He Y, Liu K, Chen D, Wu Y, Su SJ (2019) Adamantane-substituted acridine donor for blue sual fluorescence and efficient organic light-emitting diodes. Angew Chem Int Ed 58:582–586
Kim BS, Lee JY (2014) Engineering of mixed host for high external quantum efficiency above 25 % in green thermally activated delayed fluorescence device. Adv Funct Mater 24:3970–3977
Huang B, Jiang W, Tang J, Ban X, Xu H, Yang W, Sun Y (2014) Thermally activated delayed fluorescence materials based on 3,6-ditert-butyl-9-[(phenylsulfonyl)phenyl]-9H-carbazoles. Dyes Pigm 111:135–114
Lee CW, Lee JY (2015) Systematic control of photophysical properties of host materials for high quantum efficiency above 25 % in green thermally activated delayed fluorescent devices. ACS Appl Mater Interfaces 7:2899–2904
Xie GZ, Li XL, Chen DJ, Wang ZH, Cai XY, Chen DC, Li YC, Liu KK, Cao Y, Su SJ (2016) Evaporation- and solution-process-feasible highly efficient thianthrene-9,9′,10,10′-tetraoxide-based thermally activated delayed fluorescence emitters with reduced efficiency roll-off. Adv Mater 28:181–187
Li Y, Li XL, Chen D, Cai X, Xie G, He Z, Wu YC, Lien A, Cao Y, Su SJ (2016) Design strategy of blue and yellow thermally activated delayed fluorescence emitters and their all-fluorescence white OLEDs with external quantum efficiency beyond 20 %. Adv Funct Mater 26:6904–6912
Zhang Q, Kuwabara H, Potscavage WJ Jr, Huang S, Hatae Y, Shibata T, Adachi C (2014) Anthraquinone-based intramolecular charge-transfer compounds:computational molecular design, thermally activated delayed fluorescence, and highly efficient red electroluminescence. J Am Chem Soc 136:18070–18081
Huang B, Ji Y, Li Z, Zhou N, Jiang W, Feng Y, Lin B, Sun Y (2017) Simple aggregation-induced delayed fluorescence materials based on anthraquinone derivatives for highly efficient solution-processed red OLEDs. J Lumin 187:414–420
Zhang J, Chen R, Zhu Z, Adachi C, Zhang X, Lee CS (2015) Highly stable near-infrared fluorescent organic nanoparticles with a large stokes shift for noninvasive long-term cellular imaging. ACS Appl Mater Interfaces 7:26266–26274
Huang B, Li ZJ, Hu D, Wu WJ, Zhou N, Feng Y (2018) Red-emitting delayed fluorescence materials based on anthraquinone and triphenylamine. Fine Chemicals 35:764–768
CCDC 1822788 (1-TPA-AQ), 1822826 (2-TPA-AQ), 1822827 (1,8-2TPA-AQ) and 1822828 (2,6-2TPA-AQ) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GAH, Nakatsuji M, Caricato X, Li HPF, Hratchian A, Izmaylov J, Bloino G, Zheng JL, Sonnenberg M, Hada M, Ehara K, Toyota R, Fukuda J, Hasegawa M, Ishida T, Nakajima Y, Honda O, Kitao H, Nakai T, Vreven JA, Montgomery JE Jr, Peralta F, Ogliaro M, Bearpark JJ, Heyd E, Brothers KN, Kudin VN, Staroverov T, Keith R, Kobayashi J, Normand K, Raghavachari A, Rendell JC, Burant SS, Iyengar J, Tomasi M, Cossi N, Rega JM, Millam M, Klene JE, Knox JB, Cross V, Bakken C, Adamo J, Jaramillo R, Gomperts RE, Stratmann O, Yazyev AJ, Austin R, Cammi C, Pomelli JW, Ochterski RL, Martin K, Morokuma VG, Zakrzewski GA, Voth P, Salvador JJ, Dannenberg S, Dapprich AD, Daniels O, Farkas JB, Foresman JV, Ortiz J, Cioslowski J, Fox D J (2010) Gaussian 09 Revision C.01, Gaussian Inc, Wallingford CT
Lee SY, Yasuda T, Yang YS, Zhang Q, Adachi C (2014) Luminous butterflies: efficient exciton harvesting by benzophenone derivatives for full-color delayed fluorescence OLEDs. Angew Chem Int Ed 53:6402–6406
Li Y, Tan T, Wang S, Xiao Yin, Li X (2017) Highly solvatochromic fluorescence of anthraquinone dyes based on triphenylamines. Dyes Pigm 144:262–270
Dong Y, Xu B, Zhang J, Tan X, Wang L, Chen J, Lv H, Wen S, Li B, Ye L, Zou B, Tian W (2012) Piezochromic luminescence based on the molecular aggregation of 9,10-bis((E)-2-(pyrid-2-yl)vinyl)anthracene. Angew Chem Int Ed 51:10782–10785
Xie Z, Yu T, Chen J, Ubba E, Wang L, Mao Z, Su T, Zhang Y, Aldred M P, Chi Z (2018) Weak interactions but potent effect: tunable mechanoluminescence by adjusting intermolecular C-H⋯π interactions. Chem Sci 9:5787–5794
Liang K, Zheng C, Wang K, Liu W, Guo Z, Li Y, Zhang X (2016) Theoretical investigation of the singlet-triplet splittings for carbazole-based thermally activated delayed fluorescence emitters. Phys Chem Chem Phys 18:26623–26629
Acknowledgements
This work was supported Qing Lan Project of Jiangsu Province, the Natural Science Foundation of the Jiangsu Higher Education Institutions of China (No. 19KJB150006) and China Postdoctoral Science Foundation Funded Project (No. 2020M681464).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts to declare.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Huang, B. Crystallographic and Computational Investigations of Triphenylamine/Anthraquinone Hybrids. J Chem Crystallogr 52, 53–61 (2022). https://doi.org/10.1007/s10870-021-00890-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10870-021-00890-5