Abstract
Introduction
Leucocytozoon spp. causes a vector-borne disease that is nonpathogenic in domestic and wild birds. To date, there was no report of leucocytozoonosis in raptors from Thailand.
Methods
This study was carried out to perform morphological and molecular analyses of Leucocytozoon in 400 raptors at a rehabilitation center at Kasetsart University, Thailand during a 7-year period. The nested PCR was used to amplify the cytochrome b gene of Leucocytozoon with primers HaemNF1 and HaemNR3 as the primary reaction.
Results
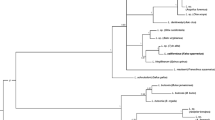
The light microscopic examination revealed Leucocytozoon gametocytes in five raptors; three diurnal raptors [two Crested Goshawks (CGs, Accipiter trivirgatus) and one Eastern Imperial Eagle (EIE, Aquila heliaca)], and two nocturnal raptors (one Oriental Scops-Owl (OSO, Otus sunia,) and one Short-eared Owl, Asio flammeus) and two species were identified: Leucocytozoon danilewskyi in both owl species and L. californicus in two CGs. The PCR method revealed more infection rate (2.0%, 8/400) than the light microscopic method including one Barred Eagle-Owl (BEO, Bubo sumatranus), one Brown Hawk Owl (BHO, Ninox scutulata) and one OSO. A phylogeny revealed that sequences from one SEO and one OSO were clustered with L. danilewskyi and the three Leucocytozoon sequences from diurnal raptors were clustered with L. californicus. The other three sequences from a BHO, a BEO and an OSO were ambiguous.
Conclusion
This study combined morphological, morphometric and molecular phylogenetic analyses to identify L. danilewskyi in two species of owls, L. californicus in three diurnal raptors, and unknown species in three other owls, representing the first records of leucocytozoon infection in raptors from Thailand.



Similar content being viewed by others
References
Valkiūnas G (2005) Avian malaria parasites and other haemosporidia. CRC, Boca Raton
Forrester DJ, Greiner EC (2008) Leucocytozoonosis. In: Atkinson CT, Thomas NJ, Hunter DB (eds) Parasitic diseases of wild birds. Blackwell, Ames, pp 54–107
Valkiūnas G, Sehgal RN, Iezhova TA, Hull AC (2010) Identification of Leucocytozoon toddi group (Haemosporida: Leucocytozoidae), with remarks on the species taxonomy of leucocytozoids. J Parasitol 96:170–177. https://doi.org/10.1645/GE-2109.1
Nakamura K, Ogiso M, Shibahara T, Kasuga H, Isobe T (2001) Pathogenicity of Leucocytozoon caulleryi for specific pathogen-free laying hens. J Parasitol 87:1202–1204
Greiner EC (1976) Leucocytozoon maccluri sp. n. (Haemosphorida: Leucocytozoidae) from a Thailand thrush Zoothera marginata Blyth. J Parasitol 62:545–547
Tongkamsai S, Napoon W (2015) Parasitemia in natural infection of Leucocytozoon sabrazesi in chickens in eastern Thailand. Proceeding of 14th Chulalongkorn University Veterinary Conference 2015: Responsible for Lives. April 20–22, 2015, Bangkok, Thailand, p 119
Worasing R, Kongkeaw W, Tiptara A, Anant S (2001) Leucocytozoonosis with avian malaria in layer chicken and treatment. Proc. of 39th Kasetsart University Annual Conference, Bangkok, February 5–7, 2001, pp 557–563
Prasopsom P, Salakij C, Lertwatcharasarakul P, Pornpranom P (2020) Hematological and phylogenetic studies of Leucocytozoon spp. in backyard chickens and fighting cocks around Kamphaeng Saen, Thailand. Agr Nat Resour 54:595–602. https://doi.org/10.34044/j.anres.2020.54.6.04
Ishak HD, Dumbacher JP, Anderson NL, Keane JJ, Valkiūnas G, Haig SM, Tel LA, Sehgal RN (2008) Blood parasites in owls with conservation implications for the Spotted Owl (Strix occidentalis). PLoS ONE 3:e2304. https://doi.org/10.1371/journal.pone.0002304
Krone O, Waldenström J, Valkiūnas G, Lessow O, Müller K, Iezhova TA, Fickel J, Bensch S (2008) Haemosporidian blood parasites in European birds of prey and owls. J Parasitol 94:709–715. https://doi.org/10.1645/GE-1357.1
Walther E, Valkiūnas G, Wommack EA, Bowie RC, Iezhova TA, Sehgal RN (2016) Description and molecular characterization of a new Leucocytozoon parasite (Haemosporida: Leucocytozoidae), Leucocytozoon californicus sp. nov., found in American kestrels (Falco sparverius sparverius). Parasitol Res 115:1853–1862. https://doi.org/10.1007/s00436-016-4925-5
Pérez-Rodríguez A, de la Puente J, Onrubia A, Pérez-Tris J (2013) Molecular characterization of haemosporidian parasites from kites of the genus Milvus (Aves: Accipitridae). Int J Parasit 43:381–387. https://doi.org/10.1016/j.ijpara.2012.12.007
Hanel J, Doležalová J, Stehlíková S, Modrý D, Chudoba J, Synek P, Votýpka J (2016) Blood parasites in northern goshawk (Accipiter gentilis) with an emphasis to Leucocytozoon toddi. Parasitol Res 115:263–270. https://doi.org/10.1007/s00436-015-4743-1
Kocan AA, Kocan KM (1978) The fine structure of elongate gametocytes of Leucocytozoon ziemanni (Laveran). J Parasitol 64:1057–1059
Hellgren O, Waldenström J, Bensch S (2004) A new PCR assay for simultaneous studies of Leucocytozoon, Plasmodium, and Haemoproteus from avian blood. J Parasitol 90:797–802. https://doi.org/10.1645/GE-184R1
Valkiūnas G, Iezhova TA, Krizanauskiene A, Palinauskas V, Sehgal RN, Bensch S (2008) A comparative analysis of microscopy and PCR-based detection methods for blood parasites. J Parasitol 94:1395–1401. https://doi.org/10.1645/GE-2150.1
Waldenström J, Bensch S, Hasselquist D, Ostman O (2004) A new nested polymerase chain reaction method very efficient in detecting Plasmodium and Haemoproteus infections from avian blood. J Parasitol 90:191–194. https://doi.org/10.1645/GE-3221RN
Hellgren O, Krizanauskiene A, Valkiūnas G, Bensch S (2007) Diversity and phylogeny of mitochondrial cytochrome b lineages from six morphospecies of avian Haemoproteus (Haemosporida: Haemoproteidae). J Parasitol 93:889–896. https://doi.org/10.1645/GE-1051R1.1
Martinsen ES, Perkins SL, Schall JJ (2008) A three-genome phylogeny of malaria parasites (Plasmodium and closely related genera): evolution of life-history traits and host switches. Mol Phylogenet Evol 47:261–273. https://doi.org/10.1016/j.ympev.2007.11.012
Sehgal RN, Hull AC, Anderson NL, Valkiūnas G, Markovets MJ, Kawamura S, Tell LA (2006) Evidence for cryptic speciation of Leucocytozoon spp. (Haemosporida, Leucocytozoidae) in diurnal raptors. J Parasitol 92:375–379. https://doi.org/10.1645/GE-656R.1
Valkiūnas G, Iezhova TA, Loiseau C, Sehgal RNM (2009) Nested cytochrome b polymerase chain reaction diagnostics detect sporozoites of hemosporidian parasites in peripheral blood of natural infected birds. J Parasitol 95:1512–1515. https://doi.org/10.1645/GE-1570
Campbell TW, Ellis C (2007) Avian and exotic animal hematology and cytology. Blackwell, Ames
Salakij J, Lertwatcharasarakul P, Kasorndorkbua C, Salakij C (2012) Plasmodium circumflexum in a Shikra (Accipiter badius): phylogeny and ultra-structure of the haematozoa. Jpn J Vet Res 60:105–109. https://doi.org/10.14943/jjvr.60.2-3.105
Bennett GF, Earle RA, Peircs MA, Huchzermeyer FW, Squires-Parsons D (1991) Avian leucocytozoidae: the leucocytozoids of the Phasianidae sensu lato. J Nat Hist 25:1407–1428
Pornpanom P, Chagas CRF, Lertwatcharasarakul P, Kasorndorkbua C, Valkiūnas G, Salakij C (2019) Molecular prevalence and phylogenetic relationship of Haemoproteus and Plasmodium parasites of owls in Thailand: data from a rehabilitation centre. IJP Parasites Wildlife 9:248–257. https://doi.org/10.1016/j.ijppaw.2019.06.002
Pornpanom P, Salakij C, Lertwatcharasarakul P, Kasorndorkbua C, Santavakul M (2019) Morphological and molecular characterisation of avian trypanosomes in raptors from Thailand. Parasitol Res 118:2419–2429. https://doi.org/10.1007/s00436-019-06379-7
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser 41:95–98
Bensch S, Hellgren O, Perez-Tris J (2009) MalAvi: a public database of malaria parasites and related haemosporidians in avian hosts based on mitochondrial cytochrome b lineages. Mol Ecol Resour 9:1353–1358. https://doi.org/10.1111/j.1755-0998.2009.02692.x
Chagas CRF, Valkiūnas G, Guimarães LO, Monteiro EF, Guida FJ, Simões RF, Rodrigues PT, Luna EJA, Kirchgatter K (2017) Diversity and distribution of avian malaria and related haemosporidian parasitesin captive birds from a Brazilian megalopolis. Malar J 16:83. https://doi.org/10.1186/s12936-017-1729-8
Ivanova K, Zehtindjiev P, Mariaux J, Georgiev BB (2015) Genetic diversity of avian haemosporidians in Malaysia: Cytochrome b lineages of the genera Plasmodium and Haemoproteus (Haemosporida) from Selangor. Infect Genet Evol 31:33–39. https://doi.org/10.1016/j.meegid.2015.01.004
Xue HR, Yamaguchi N, Driscoll CA, Han Y, Bar-Gal GK, Zhuang Y, Mazak JH, Macdonald DW, O’Brien SJ, Luo SJ (2015) Genetic ancestry of the extinct Javan and Bali tigers. J Hered 106:247–257. https://doi.org/10.1093/jhered/esv002
Kumar S, Stecher G, Tamura K (2016) MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 33:1870–1874. https://doi.org/10.1093/molbev/msw054
Ammersbach M, Beaufrere H, Gionet RA, Tully T (2015) Laboratory blood analysis in Strigiformes-Part I: hematologic reference intervals and agreement between manual blood cell counting techniques. Vet Clin Pathol 44:94–108. https://doi.org/10.1111/vcp.12229
Silva-Iturriza A, Ketmaier V, Tiedemann R (2012) Prevalence of avian haemosporidian parasites and their host fidelity in the central Philippine islands. Parasitol Int 61:650–657. https://doi.org/10.1016/j.parint.2012.07.003
Svobodová M, Weidinger K, Peške L, Volf P, Votýpka J, Voříšek P (2015) Trypanosomes and haemosporidia in the buzzard (Buteo buteo) and sparrowhawk (Accipiter nisus): factors affecting the prevalence of parasites. Parasitol Res 114:551–560. https://doi.org/10.1007/s00436-014-4217-x
Ortego J, Cordero PJ (2009) PCR-based detection and genotyping of haematozoa (Protozoa) parasitizing eagle owls, Bubo bubo. Parasitol Res 104:467–470. https://doi.org/10.1007/s00436-008-1207-x
Bennett GF, Campbell AG (1975) Avian leucocytozoidae I. Morphometric variation in three species of Leucocytozoon and some taxonomic implications. Can J Zool 53:800–812
Valkiūnas G, Bensch S, Iezhova TA, Krizanauskiene A, Hellgren O, Bolshakov CV (2006) Nested cytochrome b polymerase chain reaction diagnostics underestimate mixed infections of avian blood haemosporidian parasites: microscopy is still essential. J Parasitol 92:418–422. https://doi.org/10.1645/GE-3547RN.1
Imura T, Sato Y, Ejiri H, Tamada A, Isawa H, Sawabe K, Omori S, Murata K, Yukawa M (2010) Molecular identification of blood source animals from black flies (Diptera: Simuliidae) collected in the Alpine regions of Japan. Parasitol Res 106:543–547. https://doi.org/10.1007/s00436-009-1667-7
Acknowledgements
This work was (partially) supported by the Faculty of Veterinary Medicine, Kasetsart University, and the Kasetsart University Research and Development Institute (grant number 32.60), Thailand.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
Approval was granted by the Institutional Laboratory Animal Care and Use Committee of Kasetsart University, Thailand under protocol number ACKU 01560 (Date 25 September, 2014) and ACKU59-VET-027 (Date 13 September, 2016). The national guideline for using raptors was approved every 5-year since 2012 to 2019 by the Department of National Parks, Wildlife and Plant Conservation, Ministry of Natural Resources and Environment, Thailand.
Supplementary Information
Below is the link to the electronic supplementary material.

11686_2021_403_MOESM1_ESM.jpg
Supplementary Fig. 1 General diagram for measurement of a the length (A-top) and the width (A-bottom) of macrogametocytes; b the gametocytes and host cell which are used for the description of species of L. toddi; width and length of nucleus of host cell (d and D); width and length of gametocyte (l and L); width and length of cytoplasmic process (r and R) (Valkiünas 2005). c Diagrams of roundish (1) and fusiform (2) morphs of the Leucocytozoidae to demonstrate the morphological parameters used and the method of measurement. (PMxD = maximum diameter of the parasite; PMiD = minimum diameter of the parasite; PNMxD = maximum diameter of the parasite nucleus; NMiD = minimum diameter of the parasite nucleus, HPCD = maximum diameter of host-parasite complex; HPCL = maximum length of host-parasite complex (fusiform morph); (Bennett et al. 1991) (JPG 692 KB)
Rights and permissions
About this article
Cite this article
Lertwatcharasarakul, P., Salakij, C., Prasopsom, P. et al. Molecular and Morphological Analyses of Leucocytozoon Parasites (Haemosporida: Leucocytozoidae) in Raptors From Thailand. Acta Parasit. 66, 1406–1416 (2021). https://doi.org/10.1007/s11686-021-00403-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11686-021-00403-6