Abstract
Learned fear can be generalized through both perceptual and conceptual information. This study investigated how perceptual and conceptual similarities influence this generalization process. Twenty-three healthy volunteers completed a fear-generalization test as brain activity was recorded in the form of event-related potentials (ERPs). Participants were exposed to a de novo fear acquisition paradigm with four categories of conditioned stimuli (CS): two conceptual cues (animals and furniture); and two perceptual cues (blue and purple shapes). Animals (C+) and purple shapes (P+) were paired with the unconditioned stimulus (US), whereas furniture (C−) and blue shapes (P−) never were. The generalized stimuli were thus blue animals (C+P+, determined danger), blue furniture (C−P+, perceptual danger), purple animals (C+P−, conceptual danger), and purple furniture (C−P−, determined safe). We found that perceptual cues elicited larger fear responses and shorter reaction times than did conceptual cues during fear acquisition. This suggests that a perceptually related pathway might evoke greater fear than a conceptually based route. During generalization, participants were more afraid of C+ exemplars than of C− exemplars. Furthermore, C+ trials elicited greater N400 amplitudes. Thus, participants appear able to use conceptually based cues to infer the value of the current stimuli. Additionally, compared with C+ exemplars, we found an enhanced late positive potential effect in response to C− exemplars, which seems to reflect a late inhibitory process and might index safety learning. These findings may offer new insights into the pathological mechanism of anxiety disorders.
Similar content being viewed by others
Introduction
Fear generalization is an evolutionarily adaptive mechanism in which individuals quickly respond to potential threats based on learned experience (Lange et al., 2017). However, when individuals exhibit excessive fear responses to similar but safe stimuli, fear generalization can be maladaptive, a phenomenon termed overgeneralization (Tsafrir et al., 2013). Previous studies have demonstrated that overgeneralization underlies the pathogenesis of emotional disorders, such as anxiety and posttraumatic stress disorder (PTSD) (Kaczkurkin et al., 2017; Lissek, 2012), and can severely affect an individual’s daily life, as per the phrase “once bitten, twice shy” (Lei et al., 2019a). Clinically, exposure therapy encourages patients to approach perceived threats in efforts to learn that the fearful stimuli are not actually associated with the anticipated danger (Raij et al., 2018). However, overgeneralization may increase excessive avoidance behavior and adversely affect the effects of exposure therapy (van Meurs et al., 2014).
Experimental models of fear conditioning are commonly composed of fear acquisition, generalization, extinction, and return of fear (Lonsdorf et al., 2017). In fear acquisition, a conditioned stimulus (CS) (such as a 500-Hz tone, CS+) is repeatedly presented with an unconditioned stimulus (US, such as an electric shock). The CS+ alone can subsequently elicit the fear response. In the generalization test, a series of generalized stimuli (GS, such as 200–1,000-Hz tones) would elicit fear (Norrholm et al., 2014). Thus, fear can be generalized via similarities between stimuli. Stimulus generalization has been hypothesized to be a categorization outcome based on perceptual (primary) or conceptual (secondary) similarity (Leventhal, 1968). Primary stimulus generalization thus occurs when the generalized stimuli are physically similar to the originally learned stimuli. Secondary stimulus generalization occurs when they are conceptually similar. Additionally, research on fear generalization is divided into two areas: perceptually based fear generalization; and category-based fear generalization (Dunsmoor & Murphy, 2015; Dunsmoor et al., 2015).
Perceptually based fear generalization typically employs simple sensory stimuli that vary in physical dimensions. The GS and CS will have physical features that are similar but differ in one aspect, such as light that differs in color (Raij et al., 2018), circles that differ in size (Hunt et al., 2017), or tones that differ in frequency (Resnik et al., 2011). In conceptually based fear generalization, the similarity between the GS and CS is conceptual. For instance, if the word “help” (CS) is paired with an electric shock (US) in the acquisition phase, the word “assist” (GS), which is synonymous with “help,” also would elicit fear responses in the generalization test (Boyle et al., 2015). Similarly, if the “animal” category appears with the US, presentation of different animals also can lead to fear responses during the generalization test (Dunsmoor & Murphy, 2015). Previous research on fear generalization has ignored conceptual factors in percept-based fear generalization. However, classifying fear generalization according to perceptual or conceptual similarities is difficult in real life, because both factors operate concurrently to promote the generalization of fear. For example, patients who are afraid of Tibetan mastiffs are not only afraid of physically similar dogs but also are afraid of a series of dog-like and dog chain-related stimuli (Bennett et al., 2015).
The fear response of patients with arachnophobia can be activated by specific perceptual cues or conceptual information related to spiders. In Peperkorn et al. (2014), patients with arachnophobia and healthy participants were randomly assigned to a fear-relevant perceptual cue condition, a fear-relevant information condition, or a congruent combination of both. They found that the combined cues elicited the greatest fear response, followed by the perceptual cues alone, and lastly the conceptual cues. Perceptual cues play a crucial role in the treatment of phobias (Phan et al., 2002). We examined whether the arachnophobia results applied to spatial phobias such as claustrophobia. Although both conditions are phobias (one of spiders; the other of an environment), they are essentially different (Hofmann et al., 2009; Loken et al., 2014). Shiban et al. (2016) found that, as in Peperkorn et al. (2014), individuals exhibited greater fear in response to perceptual cues alone than to conceptual cues alone. However, combining conceptual and perceptual cues did not result in a significant increase in fear response. Thus, for spatial phobias, simply presenting a perceptual cue can trigger a sufficiently strong fear response.
The weight that these two types of information have in the generalization process is not yet clear. Nor is how perceptual and conceptual cues work together to activate the brain’s fear network to promote the generalization of fear. One possibility is that participants are more inclined to make decisions based on conceptual characteristics, because they might focus on the internal characteristics of the stimuli. We tested this hypothesis by examining behavior and brain activity in the form of event-related potentials (ERPs). Several ERPs have already been associated with fear learning. For example, compared with neutral stimuli, threatening and emotional stimuli have been shown to elicit an enhanced early P1 amplitude (Gupta et al., 2019). Other related ERPs include early posterior negativity (EPN) and late positive potential (LPP). EPN is distributed over the parieto-temporo-occipital regions and typically emerges around 150–300 ms after stimulus onset (Schupp et al., 2006). In contrast, LPP is usually observed around 400 ms after stimulus onset with an occipitoparietal and central scalp distribution (Desatnik et al., 2017; Hajcak et al., 2010; Schupp et al., 2000). EPN and LPP are generally considered to reflect selective attentional orientation toward emotional stimuli (Schupp et al., 2004; Schupp et al., 2006). Studies have demonstrated that LPP is modulated by the degree to which observed stimuli are arousing (e.g., salient pictures; positive or negative) and that enhanced LPP might reflect downstream feedback from the amygdala to the visual cortical areas (de Rover et al., 2012). Studies of time dynamics in fear conditioning have demonstrated that compared with the CS−, the CS+ elicited enhanced EPN and LPP during the fear-association phase (Ferreira de Sá et al., 2019). The increased EPN indicates that newly learned fear can automatically capture attention, and the enhanced LPP suggests elaborative processing of salient stimuli. Since its discovery in 1980, the N400 component of electroencephalograms (EEGs) has become a hallmark of cognitive studies in the fields of language processing, object and facial recognition, actions, gestures, mathematics, and semantic and recognition memory, as well as a wide range of developmental or acquired disorders. Kutas and Hillyard (1980) examined N400 amplitudes using an oddball paradigm in which they presented a series of consistent statements (e.g., “I just shaved my beard”) interspersed with infrequently occurring inconsistent statements (e.g., “He planted beans in the car”). They found that inconsistent statements led to larger N400 amplitudes. In addition, emotional words have also been shown to elicit larger N400 amplitudes, indicating that N400 potentials can be modulated by the emotional content of stimuli (Kanske et al., 2011). However, P1, EPN, N400, and LPP responses have not been examined as a means to explore the fear-generalization process with concurrent perceptual and conceptual cues.
This study examined how perceptual and conceptual cues simultaneously affect the degree to which people feel that a stimulus is dangerous and evaluated the time course of this process. We used a novel paradigm to evaluate the influence that the threat and safety values of perceptual and conceptual information have on an individual’s fear response. The conditioning phase had a 2 × 2 experimental design: stimulus type (conceptual, perceptual) × conditioning type (CS+, CS−). Specifically, four types of stimuli were used. The conceptual cues were animals and furniture were with animals paired with the US (C+) and furniture never paired with the US (C−). The perceptual cues were randomly colored blue and purple shapes; purple paired with the US (P+), and blue never paired with the US (P−). We hypothesized that the US-expectancy ratings and the mean response time (RT) in the acquisition phase would be significantly greater for CS+ conditions than for CS− conditions. According to Peperkorn et al. (2014), we also predicted that US-expectancy ratings would be significantly larger for the C+ stimuli than for the P+ exemplars. From a learning perspective, category learning involves more elaborate processing; thus, we expected conceptual cues to have longer RTs than perceptual cues.
In the generalization phase, we measured fear responses using US expectancy, RT, and ERP magnitudes. The four kinds of CS (C+, C−, P+, and P−) were fully crossed to create four types of generalized stimuli: purple animals (C+P+, determined danger), purple furniture (C−P+, perceptual danger), blue animals (C+P−, conceptual danger), and blue furniture (C−P−, determined safe). Behaviorally, we hypothesized that individuals would be more inclined to infer the attributes of the stimulus based on conceptual cues. Specifically, the US-expectancy ratings and RTs for the C+P+ and C+P− conditions would be significantly greater than those for the C−P+ and C−P− conditions. In terms of brain activity, based on previous studies, we hypothesized that perceptually related threat cues would evoke an early attentional bias characterized by two ERP components: P1 and EPN. Additionally, we expected to see the largest N400 responses for the C+P− and C−P+ conditions, when stimulus attributes were inconsistent. N400 is a crucial EEG indicator that reflects the brain’s higher-order cognitive processes (Chwilla et al., 1995). We expected that conceptual threat cues (C+P+, C+P−) would elicit larger N400 and LPP potentials than would the C−P+ or C−P− cues. Finally, we expected LPP amplitude to be modulated by perceptually threatening characteristics when the conceptual information was a safety signal.
Materials and Methods
Participants
We performed a power analysis before data collection. The a priori calculation of statistical power (G*Power) suggested that the recruitment target of 24 participants would achieve a medium effect size of 0.25, with an alpha level of 0.05, and a 1−beta level of 0.80 (Faul et al., 2007; Hendrikx et al., 2021). The sample we recruited (N = 27) was large enough to detect an effect at the given significance level (α = 0.05). The number of repeated measures was 4, the assumed sphericity correction was 1, and the repeated-measures correlation for the power analyses was 0.5. One participant was excluded due to unsuccessful fear acquisition (i.e., the US-expectancy ratings for the CS+ were smaller than those for the CS−), and three other participants were excluded due to artefacts in the EEG signal. Thus, the current study included 23 volunteers (12 women) between the ages of 18 and 25 years. All participants were right−handed, had normal or corrected-to-normal eyesight, and were without neurological or psychological disorders. All participants provided written, informed consent and received monetary compensation. The investigation was approved by the Medicine Ethics Committee of Shenzhen University.
CS and GS
Animals and furniture were selected as target conceptual categories to ensure familiarity, as these two types of objects are common in daily life. We then selected images from a database (http://www.iconfont.cn) that corresponded to the 50 most frequently listed animals and altered the colors (20 black and white, 15 blue, and 15 purple). We did the same for the 50 most frequently listed furniture. Next, using an online questionnaire method (questionnaire star), 45 college students were asked to evaluate the valence and arousal of these stimuli on a scale of 1 to 9 (1: extremely unpleasant/extremely calm or relaxed; 9: extremely pleasant/extremely excited). We then selected 12 neutral black and white animals and furniture pictures as the CSs 10 each of neutral blue animals, blue furniture, purple animals, and purple furniture pictures as the GSs. Independent sample t-tests revealed no significant difference in valence between the furniture (M = 5.33, SD = 0.34) and animals (M = 5.24, SD = 0.52; p = 0.356) and no significant difference in arousal (furniture: M = 5.24, SD = 0.18; animals: M = 5.15, SD = 0.23; p = 0.09). All pictures of animals and furniture were different, and each CS or GS was a different basic-level exemplar of the categories (Keller & Dunsmoor, 2019). We generated the perceptual cues using Adobe Photoshop, and the shapes were all different (e.g., different shapes of clouds). Thus, all images were different during both the acquisition phase and the generalization phase.
US
Electric shock combined with fearful images served as the US. Fear images were selected from the fear picture system created by Lei et al. (2019b). We applied a calibration procedure to set the intensity of electrical stimulation according to each participant’s electrical fear thresholds. An Ag/AgCl electrode was attached to the left wrist of the participant and connected to a constant current stimulator (SXC−4A, Sanxia Technique Inc., China). Weak current stimulation was delivered to the wrist through the pair of electrodes. Participants received a series of electrical stimulations of different intensities (100-ms duration; starting at 250 μA and increasing in 50 μA steps) and were required to rate the intensity of each stimulation on a verbal analog scale, where 1 indicated not unpleasant/painful/annoying and 10 indicated very unpleasant/painful/annoying. The magnitude of the stimulation intensity was set to the level which obtained a rating of 7 from the participant, which corresponded to a feeling of aversiveness (very unpleasant, but not painful) (Haaker et al., 2013).
For the fearful pictures, we first asked 115 participants (54 women; mean age, 21.92 years; standard deviation [SD], 1.43) to provide as many fear-inducing nouns as possible (e.g., snake) through a free-association task. We then selected the 90 most frequently given nouns from the image database and classified them into three categories (animals, scenes, and objects) with 30 images in each category. Then, we enrolled 84 participants (39 men; mean age, 20.55 years; SD, 1.43) to rate each image in terms of fear, valence, and arousal on a 9-point scale. Finally, a total of 81 fear-evoking pictures were chosen. The mean ratings were 4.80 ± 1.06 (M ± SD) for fear, 3.57 ± 0.16 for valence, and 6.16 ± 0.58 for arousal. For the current study, we selected 20 stimuli among these 81 as the fearful USs.
Procedure
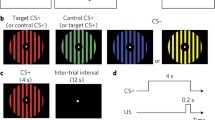
Experimental stimuli were presented by E-Prime (version 3.0), and the background of the computer during the experiment was gray. The experimental procedure comprised a fear-acquisition phase and generalization test (Fig. 1). Previous studies have demonstrated that giving explicit instructions to participants regarding threat association (i.e., the CS-US contingency) before the experiment leads to stronger fear acquisition and extinction and prevents overgeneralization (Duits et al., 2017). In the present study, we did not provide direct instructions to the participants. Before the fear-acquisition phase, participants were instructed to learn the association between the pictures and the US. In the acquisition phase, the computer screen randomly presented two types of pictures: “perceptual” or “conceptual.” The number of trials in the acquisition phase was 60 (30 perceptual and 30 conceptual). The perceptual trials included 15 blue images and 15 purple images, whereas the conceptual pictures included 15 animal images and 15 furniture images (all were different from each other). The CS+ (i.e., “animals, C+”; “purple, P+”) was paired with the US with an 80% reinforcement schedule (12/15), whereas the CS− (i.e., “furniture, C−”; “blue, P−”) was never paired with a US. Moreover, the assignment of CS+ and CS− was counterbalanced; i.e., “animals” and “purple” served as the CS+ for half of the participants, whereas “furniture” and “blue” were defined as the CS+ for the other 14 participants.
Experimental procedure for fear acquisition and the generalization test. (1) Acquisition phase: participants viewed conceptual and perceptual images for 3 s and were asked to use a 5-point scale to rate the possibility of receiving the US. The CS+ was followed by a 50-ms shock and a 1,000-ms fearful image (12 of 15 trials). The CS− was never paired with the US. The intertrial interval (ITI) was 1,200–1,500 ms. (2) Generalization test: four kinds of GS were pseudorandomly presented. Each category contained 10 different stimuli, which were each presented 5 times
The generalization test comprised four types of GS: purple animals (C+P+, determined danger), purple furniture (C−P+, perceptual danger), blue animals (C+P−, conceptual danger), and blue furniture (C−P−, determined safe). Each condition contained 10 different GSs, and each stimulus was presented 5 times. Hence, each condition included 50 trials. To prevent the extinction effect in the generalization process, the CS+ and CS− (i.e., the stimuli presented during acquisition, C+, C−, P+, and P−) were each presented 10 times, and the CS+ was followed by the US at an 80% reinforcement rate (Dunsmoor & Murphy, 2014). Thus, the total number of generalization trials was 240.
In both the conditioning and generalization phases, trials began when a fixation point appeared in the center of the screen, lasting 800–1,200 ms. After a blank screen was presented for a random duration between 400–600 ms, a stimulus was presented pseudorandomly with a 3,000-ms duration. The same stimulus did not occur consecutively. During the 3,000 ms, participants were asked to rate the possibility of receiving the US (the electric shock combined with the fearful picture) using a five−alternative forced−choice scale (a US expectancy of 1–5; 1, impossible; 3, moderate; and 5, very likely). They were instructed to press the corresponding number key with their right hand within 3,000 ms. When an original CS+ was presented during the acquisition and generalization phase, the electric shock and fearful image followed together (on 80% of trials) after the 3,000-ms period. The shock lasted 50 ms and the image lasted 1,000 ms. The intertrial interval (ITI) for both phases was 1,200–1,500 ms.
ERP recordings and data pre−processing
Continuous EEGs were recorded with a 64-channel Brain Products system (Brain Products GmbH, Munich, Germany; passband, 0.05–100 Hz; sampling rate, 500 Hz) using a standard 10-20 acquisition system EEG cap. The ground electrode was located on the medial frontal line, with the left and right mastoids as reference electrodes during recording. Vertical electrooculograms (vEOGs) were recorded via facial electrodes located above and below the left eye. Horizontal EOG (hEOG) electrodes were attached at the outer canthi of the eyes. The impedance was kept below 10 kΩ for all recordings. ERP data were analyzed using the EEGLAB Matlab toolbox (Delorme & Makeig, 2004) and were band-pass filtered at 0.1–20 Hz. Blinking and eye movements were corrected using independent component analysis. Activity above 100 μV or below −100 μV were removed by a semiautomatic procedure. The ERP analysis window ranged from 100 ms before stimulus onset to 1,000 ms after onset. The average number of trials included for each condition were as follows: C+P+, 49 (SD, 2.09; max, 50; min, 43); C−P+, 49 (SD, 1.81; max, 50; min, 43); C+P−, 49 (SD, 1.57; max, 50; min, 45); and C−P−, 48.83 (SD, 1.83; max, 50; min, 45).
Statistics
The acquisition phase had a 2 × 2 experimental design: stimulus type (conceptual, perceptual) × conditioned type (CS+, CS−). US-expectancy data and RTs in the acquisition phase were analyzed using stimulus type × conditioned type repeated-measures analyses of variance (ANOVAs). US-expectancy data and RTs in the generalization phase were calculated using perceptual type (P+, P−) × conceptual type (C+, C−) repeated measures ANOVAs. The ERP analysis window ranged from 100 ms before stimulus onset to 1,000 ms after onset. Based on previous studies and the grand-averaged ERP waveform, we scored P1 as the mean response between 125–165 ms (at electrode P3), EPN as the mean response between 260–280 ms (Schupp et al., 2004), N400 as the mean response between 380–480 ms (Cz, C1, C2, C3, C4), and LPP as the mean response between 500–630 ms (Fz, Cz) (Pavlov & Kotchoubey, 2019). Repeated measures ANOVAs were performed for Perceptual (P+, P−) and Conceptual (C+, C−) stimuli for the average P1, EPN, N400, and LPP amplitudes. Throughout our analysis, the effects were considered significant when P < 0.05.
Results
Behavioral Results
During the acquisition phase, the shock expectancy was 3.07 ± 0.17 (M ± SEM) for C+, 2.31 ± 0.18 for C−, 3.97 ± 0.15 for P+, and 2.39 ± 0.23 for P−. Repeated-measures ANOVA with the Stimulus Type (concept, perception) × Conditioning Type (CS+, CS−) as factors revealed a significant main effect of Stimulus Type (F1,22 = 13.51; p < 0.001; η2 = 0.34) and a significant main effect of Conditioning Type (F1,22 = 32.68; p < 0.001; η2 = 0.56), which demonstrated that participants successfully learned the contingency between the CS and the US. Furthermore, there was a significant interaction between these two factors (F1, 22 = 7.84; p = 0.010;, η2 = 0.23). Follow-up simple effects tests revealed that the shock expectation for P+ was significantly larger than that for C+ (Figure 2a).
The US-expectancy ratings (a) and mean response time (b) for fear acquisition and fear generalization (c, d). P, perceptual stimulus; C, conceptual stimulus; C+, conceptual CS+; C−, conceptual CS−; P+, perceptual CS+; C−, perceptual CS−. Means and SEM are given. ***p < 0 .001; **p < 0 .01; *p < 0 .05
During the acquisition phase, the response times were 1,475.65 ± 111.59 ms for C+, 1,397.05 ± 112.99 ms for C−, 1,285.13 ± 97.99 ms for P+, and 1,355.22 ± 133.32 ms for P−. Repeated-measures ANOVA with Stimulus Type (conceptual, perceptual) × Conditioning Type (CS+, CS−) as factors showed a significant main effect of Stimulus Type (F1,22 = 5.494; p = 0.027; η2 = 0.174), indicating that participants needed more time to evaluate the conceptual cues compared than they did the perceptual cues. The effect of Conditioning Type was not significant (F1,22 = 0.336; p = 0.567, η2 = 0.013). A significant interaction was found between the two factors (F1,22 = 6.003; p = 0.021, η2 = 0.188). Simple effects tests revealed that response times for the C+ was significantly longer than that for the C− (Figure 2b).
During the generalization test, shock expectancy was 1.75 ± 0.14 for C+P+, 1.57 ± 0.13 for C+P−, 1.58 ± 0.16 for C−P+, and 1.42 ± 0.12 for C−P−. Repeated-measures ANOVA with Perceptual Type (P+, P−) × Conceptual Type (C+, C−) as factors revealed a significant main effect of Conceptual Type (F1,22 = 8.70; p = 0.007, η2 = 0.25) but not Perceptual Type (F1,22 = 3.56; p = 0.071 , η2 = 0.12). No significant interaction was noted between the two factors (F1,22 = 0.26; p = 0.614, η2 = 0.01; Fig. 2c), suggesting that fear responses were greater for C+ stimuli than for C− stimuli. The response times for these four kinds of GS were 1,011.71 ± 75.14 ms for C+P+, 980.56 ± 72.12 ms for C+P−, 914.32 ± 60.83 ms for C−P+, and 900.99 ± 63.76 ms for C−P−. Repeated-measures ANOVA revealed a significant main effect of Conceptual Type (F1,22 = 9.52; p = 0.005, η2 = 0.26) but not for Perceptual Type (F1,22 = 1.60; p = 0.216, η2 = 0.06). No significant interaction was observed between the two factors (F1,22 = 0.62; p = 0.438, η2 = 0.022; Figure 2d) .
For an overview of fear conditioning, we plotted the participants’ learning courses. Statistical analysis was conducted using repeated-measures ANOVA with Stimulus Type (conceptual, perceptual) × Conditioning Type (CS+, CS−) × Time (1, 2, 3) as factors. The US expectancy showed a significant main effect of Time (F2,44 = 14.518; p < 0.001, η2 = 0.358) and a Conditioned Type (CS+, CS−) × Time interaction (F2,44 = 21.186, p < 0.001, η2 = 0.449), indicating a fear learning curve in the conditioning phase.
Considering the possibility of an extinction effect as the generalization test progressed, we analyzed the US-expectancy ratings across trials. Statistical analysis was conducted using repeated-measures ANOVA that included the Perceptual Type (P+, P−), Conceptual Type (C+, C−), and Time (1, 2, 3, 4, 5) as factors. We found a significant main effect of Time (F4,88 = 16.435; p < 0.001, η2 = 0.460) on US-expectancy rating but no significant Perceptual Type × Time, Conceptual Type × Time, or Perceptual Type × Conceptual Type × Time interaction, indicating general extinction during the generalization test (Figure 3). Pairwise comparison showed that US-expectancy ratings for C+P− in Block 1 and Block 2 were much higher than those for C−P− (p = 0.034, p = 0.009), and US-expectancy ratings for C−P+ in Block 1 and Block 4 were much higher than those for C−P− (p = 0.062, p = 0.047).
ERP waveform analysis for the fear-generalization test
P1
The P1 time window was between 125–165 ms (at electrode P3). Analysis showed that P1 amplitude did not significantly differ between CS+ and CS− conditions (no significant effect of Conceptual Type (F1,22 = 0.001; p = 0.975), perceptual type (F1,22 = 0.447; p = 0.511), or any interaction of the two (F1,22 = 0.880; p = 0.358; Figure 4).
P1 and EPN ERP results. Top: Grand average event-related potential (ERP) waveforms for the four kinds of generalized stimuli (GS) at electrode P3, 125–165 ms after stimulus onset for P1, and 260–280 ms after onset for EPN. Middle: Grand averaged ERP amplitudes for P1 and EPN. Bottom: Scalp topography of the grand average amplitudes for P1 and EPN
EPN
According to previous studies, the time window for EPN was set to 260–280 ms (at electrode P3) (Schupp et al., 2004). We found no significant effect for conceptual type (F1,22 = 0.008; p = 0.928), perceptual stimuli (F1,22 = 0.080; p = 0.780), or any interaction between the two (F1,22 = 0.192; p = 0.665; Figure 4).
N400
Analysis revealed a significant main effect of Conceptual Type (F1,22 = 5.91; p = 0.024, η2 = 0.21) but not for Perceptual Type (F1,22 = 0.03; p = 0.869, η2 = 0.01). It is worth noting that the interaction between these two factors showed was marginally significant (F1,22 = 3.96; p = 0.059, η2 = 0.15). Follow-up simple effects tests (Bonferroni-adjusted for multiple comparisons) indicated that the N400 amplitudes for the C−P+ and C+P− conditions were significantly larger than those for the C−P− condition (p = 0.035 and p = 0.008, respectively). Furthermore, N400 amplitudes did not statistically differ between C+P+ and C+P− conditions (p = 0.282, p > 0.05) or between C+P+ and C−P+ conditions (p = 0.361, p > 0.05; Figure 5).
N400 and LPP ERP results. (1) N400: Top. Grand average ERP waveforms for the four kinds of generalized stimuli (GS) at electrode C1 during the 380–480 ms after stimulus onset. Middle. Grand average ERP amplitudes during the 380–480 ms time window at Cz, C1, C2, C3, and C4 electrodes. Bottom. Scalp topography of the grand average amplitudes for N400. (2) LPP: Top. Grand average ERP waveforms for the four kinds of generalized stimuli (GS) at the CPz electrode during the 500–630 ms after stimulus onset. Middle. Grand average ERP amplitudes during the 500–630 ms time window at CPz electrodes. Bottom. Scalp topography of the grand average amplitude for LPP. ***p < 0.001; **p < 0.01; *p < 0.05
LPP
Analysis showed a significant main effect of Conceptual Type (F1,22 = 5.967; p = 0.023, η2 = 0.206) but not Perceptual Type (F1,22 = 0.244; p = 0.626, η2 = 0.010) or their interaction (F1,22 = 0.184; p = 0.672, η2 = 0.008 (Figure 5).
Discussion
This study evaluated the effects of perceptual and conceptual cues on fear acquisition and generalization. First, during the acquisition phase, expectations of the US for C+ and P+ stimuli were significantly greater than those for the C− and P− stimuli, indicating that participants successfully learned the association between the CS and the US (Davidson et al., 2018). Because P+ exemplars shared the same physical color (i.e., perceptually based) and C+ exemplars shared the same object category (i.e., conceptually based), this demonstrated that fear can be facilitated through different pathways (Bennett et al., 2015). Peperkorn et al. (2014) reported that both spider-related perceptual cues and conceptual information could elicit fear responses in healthy participants and patients with arachnophobia. Compared with presenting conceptual information alone, they found that perceptual cues alone could elicit a stronger fear response.
Our findings are similar: US-expectancy ratings for P+ stimuli were higher than those for C+ stimuli, suggesting that a perceptually based route might be evoke fear more than a conceptually based path. RTs for conceptual cues were longer than those for perceptual cues. Craddock et al. (2012) concluded that RTs could be used to evaluate associative strength; longer RTs indicate more uncertain signal value with conceptual cues (Lissek et al., 2008). The C+ versus C− comparison confirms this explanation. RTs in response to C+ stimuli were significantly longer than those for C− stimuli, which might be due to the probabilistic (80%) match between the C+ and the US, whereas C− stimuli were always presented alone. Thus, compared with C− stimuli that indicated safe information, the values of C+ stimuli were more uncertain. Another possible explanation is that having the same physical stimulus elements could influence the speed of processing differently than having different physical elements, and conceptually based fear learning involves higher-order cognitive processes.
In the subsequent generalization test, participants reported higher US-expectancy ratings for C+ exemplars (i.e., C+P+ and C+P−) than for C− exemplars (i.e., C−P+ and C−P−). This is an interesting finding considering that each GS was a different basic-level exemplar of animals or furniture and did not overlap the CS in terms of perceptual features. This result is consistent with that of a previous study, which showed that conditioned fear might generalize to a variety of stimuli because of their conceptual similarity to the CS (Dunsmoor et al., 2011). Additionally, the RTs for the conceptually based threat signals (C+P+, C+P−) were longer than those for conceptually related safety stimuli (C−P+, C−P−). In combination with the US-expectation scores, C+ stimuli were expected to be followed by the US but not always. It is possible that the longer RTs reflect a mismatch between what was expected (CS−US association) and what actually happened. However, we found no differences between P+ (C+P+, C−P+) and P− (C+P−, C−P−) in US expectation. We note that US-expectancy ratings for the C−P+ trials in Block 1 and Block 4 were higher than those for C−P− trials. This was probably due to the extinction of generalization as the number of generalization trials increased. In a study by Zbozinek and Craske (2018), participants were randomly exposed to three different extinction conditions: Extinction_CS+ (extinguished using the conditional stimulus), Extinction_Singular (extinguished using a single generalization stimulus), and Extinction_Variety (extinguished using various generalization stimuli). The results revealed that extinction with GSs could reduce fear to novel GS.
In contrast to what had been expected initially, there were no P1 or EPN component differences between the four conditions. Thus, the current results show that threat-related attentional bias occurs much later and that perceptually based threat signals do not modulate the early detection or vigilance process. One possible explanation for this result is the rather weak US used in the current research. Pavlov and Kotchoubey (2019) compared two different conditioning paradigms (aversive conditioning and name conditioning) using ERP. In aversive conditioning, the US was an aversive painful shock, whereas in name conditioning, the US was the name of the corresponding participant. The results revealed that when US conditioning is strong (i.e., aversive conditioning), learned fear induced P3a and LPP effects. Conversely, when it was weak (i.e., the name conditioning), conditioned stimuli were only able to enhance LPP. The aversive painful shock in that study was set to the level of “moderately painful, but not too strong.” However, in the current study, we set the shock-intensity level to “unpleasant, but not painful.” Furthermore, in classical fear conditioning paradigms, electric shock durations have typically ranged between 50 ms and 500 ms (Bos et al., 2012; Dou et al., 2020; Drexler et al., 2018; Ginat-Frolich et al., 2019; Lange et al., 2019; Torrents-Rodas et al., 2013). Considering the use of a compound US (shock with fearful image), we selected a 50-ms duration for the shock to ensure that it was safe and acceptable. Thus, compared with the US in previous research, the US used in the present study might not have been strong enough to modulate earlier, perceptual stages of processing.
N400 amplitude was higher for C+ stimuli (C+P+, C+P−) than for C− stimuli (C−P+, C−P−) and for C−P+ stimuli compared with C−P−astimuli. The enhanced N400 effect might be an electrophysiological index of fear generalization. Furthermore, when the conceptually related signal was safe, the threat value of the perceptual signal could elicit an attentional bias toward emotional stimuli. Additionally, N400 amplitude might be modulated by the inconsistent value of the stimuli. In an EEG study, Deveney and Pizzagalli (2008) employed electroencephalography to investigate the effect of cognitive reassessment strategies on information post-processing. They reported that two stimuli with different properties appearing at the same time triggered the N400 effect. Similar N400 modulations have been found in the current study: a higher amplitude N400 in response to C−P+ and C+P− stimuli compared with C−P− stimuli. In the C−P+ and C+P− conditions, the conceptual and perceptual signal values (threat vs. safety) were opposite, which led to inconsistency of information when the individual processed the stimulus.
We further asked whether fear-generalization effects can be indexed by LPP amplitude and how different signal values (safety vs. threat) of the different pathways (perceptual vs. conceptual) impact the later sustained attention processes. Contrary to what we expected, LPP amplitude was lower for trials with generalized C+ exemplars than for trials with generalized C− exemplars. Nelson et al. (2015) investigated the role of LPP in the process of fear generalization and reported that LPP was higher in response to CS+ stimuli than to GSs, but did not differ among GSs, which suggested that LPP may not be sensitive to the processing of fear generalization. It is worth noting that, unlike the CS+ (directly associated with the US) or GSs (20%, 40%, or 60% similarity to the CS+) that are used in classic fear generalization paradigms, the current research used compound value signals as the generalized stimuli. The test phase might thus have involved both fear and safety generalization. In a study by Seligowski et al. (2021), LPP latencies for CS+ and CS− conditions did not significantly differ, whereas LPP CS− latencies showed a significantly negatively startle response to the CS−. They concluded that LPP may be an ERP-based marker of safety-signal learning. In the current study, one possible explanation for this enhanced LPP effect for conceptually based safety GSs is that LPP might indicate a later inhibitory control process, which could be used to examine safety learning. Nevertheless, as evidence is weak, these interpretations need to be taken with caution, and further research is needed.
This study had several limitations. First, when an organism encounters an emergency, the autonomic nervous system (ANS) is stimulated, which leads to a series of physiological changes, such as accelerated heartbeat and sweat gland secretion (Cohen & Randall, 1984). The SCR is a valuable physiological tool for indexing human fear conditioning; fear can accurately be reflected by changes in skin conductance or sweating response, which avoids the bias of subjective reports (Glover et al., 2011). Future research can use EEG in conjunction with SCRs to investigate the simultaneous effect of perceptual and conceptual cues on human fear learning. Second, a previous study showed that the LPP component of EEGs is sensitive to fear learning (Baas et al., 2002); however, we did not record EEG data during the acquisition phase because the signal-to-noise ratio was insufficient for EEG. Previous fear-conditioning paradigms usually used one or only a few stimuli to serve as the CS, which allows for enough trials of the same condition to maximize the signal-to-noise ratio. However, it also could lead to habituation. In multi-CS conditioning, several different stimuli were paired with an aversive US (Steinberg et al. (2013), using a total of 208 face images as the CSs. Multiple CS conditional stimuli can be used in future studies to better utilize EEG and SCR data at the same time, to increase the number of experimental trials, and to reduce the number of repeated occurrences of each CS. In addition, the time interval required for EEG and SCR is different. To ensure the accuracy of data recording and control the time required for the experiment, different time intervals can be used to record different emotional indicators (some trials would use short intervals, and some would use long intervals). Finally, the methodological limitations of the study should be noted. Perceptual cues used in the acquisition phase were different meaningless shapes, which separated them from conceptual categories and led to a simple perceptually related color-aversive US association. In the generalization phase, the colors of the GSs were physically similar to those of the perceptually conditioned stimuli (P+; P−), whereas the GS categories (not physical characteristics) were the same as those of the conceptually conditioned stimuli (C+; C−). However, compared with perceptually based fear learning, conceptually based learning involved more stimuli; therefore, fear generalization might be influenced by category versus perception or one versus multiple stimuli. Moreover, the arousal ratings for the “black and white” category (M = 5.20; SD = 0.19) used in the present research was significantly greater than those for the “color” category (M = 5.05; SD = 0.17; p = 0.002). Future studies are necessary to investigate this by using more appropriate experimental stimuli, such as words, to clarify how physical and verbal stimulus characteristics might influence fear generalization.
Data availability
All data are fully available without restriction.
References
Baas, J.M., Kenemans, J.L., Böcker, K.B., & Verbaten, M.N. (2002). Threat-induced cortical processing and startle potentiation. Neuroreport, 13(1), 133-137.
Bennett, M., Vervoort, E., Boddez, Y., & Hermans, D. (2015). Perceptual and conceptual similarities facilitate the generalization of instructed fear. Journal of Behavior Therapy and Experimental Psychiatry, 48, 149-155.
Bos, M.G., Beckers, T., & Kindt, M. (2012). The effects of noradrenergic blockade on extinction in humans. Biological Psychology, 89(3), 598-605.
Boyle, S., Roche, B., Dymond, S., & Hermans, D. (2015). Generalisation of fear and avoidance along a semantic continuum. Cognition and Emotion, 30(2), 340-352.
Chwilla, D. J., Brown, C. M., & Hagoort, P. (1995). The N400 as a function of the level of processing. Psychophysiology, 32(3), 274–285.
Cohen, D.H., & Randall, D.C. (1984). Classical conditioning of cardiovascular responses. Annual Review of Physiology, 46(1), 187-197.
Craddock, P., Molet, M., & Miller, R.R. (2012). Reaction time as a measure of human associative learning. Behavioural Processes, 90(2), 189-197.
Davidson, P., Carlsson, I., Jönsson, P., Johansson, M. (2018). A more generalized fear response after a daytime nap. Neurobiology of Learning & Memory, 151, 18.
Deveney, C.M., & Pizzagalli, D.A. (2008). The cognitive consequences of emotion regulation: an ERP investigation. Psychophysiology, 45(3), 435-444.
Delorme, A., & Makeig, S. (2004). Eeglab: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. Journal of Neuroscience Methods, 134(1), 9-21.
de Rover, M., Brown, S.B., Boot, N., Hajcak, G., van Noorden, M.S., van der Wee, N.J., & Nieuwenhuis, S. (2012). Beta receptor-mediated modulation of the late positive potential in humans. Psychopharmacology, 219(4), 971-979.
Desatnik, A., Bel-Bahar, T., Nolte, T., Crowley, M., Fonagy, P., & Fearon, P. (2017). Emotion regulation in adolescents: An ERP study. Biological Psychology, 129, 52-61.
Dou, H., Lei, Y., Cheng, X., Wang, J., & Leppänen, P. (2020). Social exclusion influences conditioned fear acquisition and generalization: A mediating effect from the medial prefrontal cortex. NeuroImage, 218, 116735.
Drexler, S.M., Merz, C.J., & Wolf, O.T. (2018). Preextinction stress prevents context-related renewal of fear. Behavior Therapy, 49(6), 1008-1019.
Dunsmoor, J.E., & Murphy, G.L. (2015). Categories, concepts, and conditioning: how humans generalize fear. Trends in Cognitive Sciences, 19(2), 73-77.
Dunsmoor, J.E., & Murphy, G.L. (2014). Stimulus typicality determines how broadly fear is generalized. Psychological Science, 25(9), 1816-1821.
Dunsmoor, J.E., Murty, V.P., Davachi, L., Phelps, E.A. (2015). Emotional learning selectively and retroactively strengthens memories for related events. Nature, 520(7547), 345-348.
Dunsmoor, J.E., White, A.J., & LaBar, K.S. (2011). Conceptual similarity promotes generalization of higher order fear learning. Learning & Memory (Cold Spring Harbor, N.Y.), 18(3), 156-160.
Duits, P., Richter, J., Baas, J., Engelhard, I.M., Limberg-Thiesen, A., Heitland, I., Hamm, A.O., & Cath, D.C. (2017). Enhancing effects of contingency instructions on fear acquisition and extinction in anxiety disorders. Journal of Abnormal Psychology, 126(4), 378-391.
Faul, F., Erdfelder, E., Lang, A.G., & Buchner, A. (2007). G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods, 39(2), 175-191.
Ferreira de Sá, D.S., Michael, T., Wilhelm, F.H., & Peyk, P. (2019). Learning to see the threat: temporal dynamics of ERPs of motivated attention in fear conditioning. Social Cognitive and Affective Neuroscience, 14(2), 189-203.
Ginat-Frolich, R., Gendler, T., Marzan, D., Tsuk, Y., & Shechner, T. (2019). Reducing fear overgeneralization in children using a novel perceptual discrimination task. Behaviour Research and Therapy, 116, 131-139.
Glover, E.M., Phifer, J.E., Crain, D.F., Norrholm, S.D., Davis, M., Bradley, B., … Jovanovic, T. (2011). Tools for translational neuroscience: PTSD is associated with heightened fear responses using acoustic startle but not skin conductance measures. Depression and Anxiety, 28(12), 1058-1066.
Gupta, R.S., Kujawa, A., & Vago, D.R. (2019). The neural chronometry of threat-related attentional bias: Event-related potential (ERP) evidence for early and late stages of selective attentional processing. International Journal of Psychophysiology : Official Journal of the International Organization of Psychophysiology, 146, 20-42.
Haaker, J., Lonsdorf, T.B., Thanellou, A., Kalisch, R., (2013). Multimodal assessment of long-term memory recall and reinstatement in a combined cue and context fear conditioning and extinction paradigm in humans. PLoS ONE 8, e76179.
Hajcak, G., MacNamara, A., & Olvet, D.M. (2010). Event-related potentials, emotion, and emotion regulation: an integrative review. Developmental Neuropsychology, 35(2), 129-155. https://doi.org/10.1080/87565640903526504.
Hendrikx, L.J., Krypotos, A.M., & Engelhard, I.M. (2021). Enhancing extinction with response prevention via imagery-based counterconditioning: Results on conditioned avoidance and distress. Journal of Behavior Therapy and Experimental Psychiatry, 70, 101601.
Hunt, C., Cooper, S.E., Hartnell, M.P., Lissek, S. (2017). Distraction/suppression and distress endurance diminish the extent to which generalized conditioned fear is associated with maladaptive behavioral avoidance. Behaviour Research and Therapy, 96, 90-105.
Hofmann, S.G., Alpers, G.W., & Pauli, P. (2009). Phenomenology of panic and phobic disorders. In M. M. Antony, & M. B. Stein (Eds.), Oxford Handbook of Anxiety and Related Disorders (pp. 34e46). Oxford University Press.
Kaczkurkin, A.N., Burton, P.C., Chazin, S.M., Manbeck, A.B., Espensen-Sturges, T., Cooper, S.E., Sponheim, S.R., & Lissek, S. (2017). Neural substrates of overgeneralized conditioned fear in PTSD. The American Journal of Psychiatry, 174(2), 125-134.
Kanske, P., Plitschka, J., & Kotz, S.A. (2011). Attentional orienting towards emotion: P2 and N400 ERP effects. Neuropsychologia, 49(11), 3121-3129.
Keller, N.E., & Dunsmoor, J.E. (2019). The effects of aversive-to-appetitive counterconditioning on implicit and explicit fear memory. Learning & Memory (Cold Spring Harbor, N.Y.), 27(1), 12-19.
Kutas, M., & Hillyard, S.A. (1980). Reading senseless sentences: brain potentials reflect semantic incongruity. Science (New York, N.Y.), 207(4427), 203-205.
Lange, I., Goossens, L., Michielse S., Bakker, J., Lissek, S., Papalini, S.… Schruers, K.(2017). Behavioral pattern separation and its link to the neural mechanisms of fear generalization. Social Cognitive and Affective Neuroscience, 12(11): 1720-1729.
Lange, I., Goossens, L., Bakker, J., Michielse, S., Marcelis, M., Wichers, M., van Os, J., van Amelsvoort, T., & Schruers, K. (2019). Functional neuroimaging of associative learning and generalization in specific phobia. Progress in Neuro-psychopharmacology & Biological Psychiatry, 89, 275-285.
Leventhal D. S. (1968). Perceptual and conceptual categorization in stimulus generalization. Perceptual and motor skills, 27(1), 219–230.
Lissek, S., Biggs, A. L., Rabin, S. J., Cornwell, B. R., Alvarez, R. P., Pine, D. S., & Grillon, C. (2008). Generalization of conditioned fear-potentiated startle in humans: experimental validation and clinical relevance. Behaviour Research and Therapy, 46(5), 678-687.
Lissek, S. (2012). Toward an account of clinical anxiety predicated on basic, neurally mapped mechanisms of Pavlovian fear-learning: the case for conditioned overgeneralization. Depression and Anxiety, 29(4), 257-263.
Lonsdorf, T.B., Menz, M.M., Andreatta, M., Fullana, M.A., Golkar, A., Haaker, J., … Merz, C.J. (2017). Don't fear 'fear conditioning': methodological considerations for the design and analysis of studies on human fear acquisition, extinction, and return of fear. Neuroscience & Biobehavioral Reviews, 77, 247-285.
Loken, E.K., Hettema, J.M., Aggen, S.H., & Kendler, K.S. (2014). The structure of genetic and environmental risk factors for fears and phobias. Psychological Medicine, 44, 2375e2384.
Nelson, B.D., Weinberg, A., Pawluk, J., Gawlowska, M., & Proudfit, G.H. (2015). An event-related potential investigation of fear generalization and intolerance of uncertainty. Behavior Therapy, 46(5), 661-670.
Norrholm, S. D., Jovanovic, T., Briscione, M. A., Anderson, K. M., Kwon, C. K., Warren, V. T., Bosshardt, L., & Bradley, B. (2014). Generalization of fear-potentiated startle in the presence of auditory cues: a parametric analysis. Frontiers in behavioral neuroscience, 8, 361.
Pavlov, Y.G., & Kotchoubey, B. (2019). Classical conditioning in oddball paradigm: A comparison between aversive and name conditioning. Psychophysiology, 56(7), e13370.
Peperkorn, H.M., Alpers, G.W. , & Mühlberger, A. (2014). Triggers of fear: perceptual cues versus conceptual information in spider phobia. Journal of Clinical Psychology, 70(7), 704-714.
Phan, K.L., T. Wager, Taylor, S.F., & Liberzon, I. (2002). Functional neuroanatomy of emotion: A meta-analysis of emotion activation studies in PET and fMRI. NeuroImage, 16(2), 331-348.
Raij, T., Nummenmaa, A., Marin, M.F., Porter, D., Furtak, S., Setsompop, K., Milad, M.R. (2018). Prefrontal cortex stimulation enhances fear extinction memory in humans. Biological Psychiatry, 15(84), 129-137.
Resnik, J., Sobel, N., Paz, R. (2011). Auditory aversive learning increases discrimination thresholds. Nature Neuroscience, 14(6), 791-796.
Schupp, H.T., Cuthbert, B.N., Bradley, M.M., Cacioppo, J.T., Ito, T., & Lang, P.J. (2000). Affective picture processing: the late positive potential is modulated by motivational relevance. Psychophysiology, 37(2), 257-261.
Schupp, H.T., Flaisch, T., Stockburger, J., & Junghöfer, M. (2006). Emotion and attention: event-related brain potential studies. Progress in Brain Research, 156, 31-51.
Schupp, H.T., Ohman, A., Junghöfer, M., Weike, A. I., Stockburger, J., & Hamm, A.O. (2004). The facilitated processing of threatening faces: an ERP analysis. Emotion (Washington, D.C.), 4(2), 189-200.
Seligowski, A.V., Reffi, A.N., Phillips, K.A., Orcutt, H.K., Auerbach, R.P., Pizzagalli, D.A., & Ressler, K.J. (2021). Neurophysiological responses to safety signals and the role of cardiac vagal control. Behavioural Brain Research, 396, 112914.
Shiban, Y., Peperkorn, H., Alpers, G.W., Pauli, P., & Mühlberger, A. (2016). Influence of perceptual cues and conceptual information on the activation and reduction of claustrophobic fear. Journal of Behavior Therapy and Experimental Psychiatry, 51, 19-26.
Steinberg, C., Bröckelmann, A.K., Rehbein, M., Dobel, C., Junghöfer, M. (2013). Rapid and highly resolving associative affective learning: convergent electro- and magnetoencephalographic evidence from vision and audition. Biological Psychology, 92(3), 526-540.
Torrents-Rodas, D., Fullana, M.A., Bonillo, A., Caseras, X., Andión, O., & Torrubia, R. (2013). No effect of trait anxiety on differential fear conditioning or fear generalization. Biological Psychology, 92(2), 185-190.
Tsafrir, G., Carlson, J.M., Jiook, C., Greg, H., & Mujica-Parodi, L.R. (2013). Ventromedial prefrontal cortex reactivity is altered in generalized anxiety disorder during fear generalization. Depression & Anxiety, 30(3), 242-250.
van Meurs, B., Wiggert, N., Wicker, I., & Lissek, S. (2014). Maladaptive behavioral consequences of conditioned fear-generalization: A pronounced, yet sparsely studied, feature of anxiety pathology. Behaviour Research and Therapy, 57, 29-37.
Lei Y., Wang, J., Dou, H., Qiu, Y., & Li, H. (2019a). Influence of typicality in category−based fear generalization: Diverging evidence from the P2 and N400 effect, International Journal of Psychophysiology, 135, 12-20.
Lei, Y., Sun, X., & Dou, H. (2019b). Specifically inducing fear and disgust emotions by using separate stimuli: the development of fear and disgust picture systems. Journal of Psychological Science, 41(1), 1-6.
Zbozinek, T.D., & Craske, M.G. (2018). Pavlovian extinction of fear with the original conditional stimulus, a generalization stimulus, or multiple generalization stimuli. Behaviour Research and Therapy, 107, 64-75.
Acknowledgments
The authors thank Dr. Wikgren Jan, Faculty of Education and Psychology at University of Jyväskylä, for editing the manuscript about the readability and language usage.
Funding
This work was supported by the National Natural Science Foundation of China (NSFC, Grant Numbers, 31871130), the Guangdong Key Project in “Development of new tools for diagnosis and treatment of Autism” (2018B030335001), Shenzhen Peacock Plan (KQTD2015033016104926), Shenzhen-Hong Kong Institute of Brain Science-Shenzhen Fundamental Research Institutions (2019SHIBS0003; 2021SHIBS0003).
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: Yi Lei, Jinxia Wang, Haoran Dou
Performed the experiments: Jinxia Wang, Mei E, Qi Wu, Tao Xie
Analyzed the data: Jinxia Wang
Wrote the paper: Yi Lei, Jinxia Wang, Haoran Dou
Corresponding author
Ethics declarations
Ethics approval
The experimental protocol was established according to the ethical guidelines of the Helsinki Declaration and was approved the Ethical Committee of Shenzhen University.
Consent to participate
Written, informed consent was obtained from participants.
Consent for publication
The authors agree to publication in the Journal.
Conflicts of interest/Competing interests
The authors declare no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, J., E, M., Wu, Q. et al. Influence of Perceptual and Conceptual Information on Fear Generalization: A Behavioral and Event-Related Potential Study. Cogn Affect Behav Neurosci 21, 1054–1065 (2021). https://doi.org/10.3758/s13415-021-00912-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13415-021-00912-x