Abstract
It is known that the oxidative reactions of alcohols are sensitive to the characteristics of the catalyst, in such a way that the properties of the support are as important as those of the metallic phase. We consider the functionalization of support as a strategy to improve the catalytic performance in these reactions. We investigated the influence of the modification of the Si/Ti ratio of the TiO2@SBA-15 support (RSi/Ti = 75, 50 and 25) on the catalytic performance of the synthesized materials: Me/SBA-15, Me/TiO2@SBA-15 (Me = AuNPs or AuPdNPs). The techniques of XRD, adsorption, and desorption of N2, ICP-OES, XPS, SEM, and TEM were used for characterization. The structure of the SBA-15 was maintained in all supports and catalysts and a significant reduction in particle size was observed in the modified support S25 (AuPd/SBA-15: 18.96 ± 12.48 nm; AuPd/S25 (RSi/Ti = 25): 3.14 ± 0.85 nm). All Au and AuPd catalysts performed well, showing activities > 53% in 2.5 h. However, bimetallic catalysts achieved greater prominence, reaching activities of 20 to 90% and selectivity > 90% for benzaldehyde in 0.5 h. Among them, the AuPd/S25 catalyst stood out with significant activity and selectivity (90%), in addition to good stability in successive reuse experiments.
Graphic Abstract
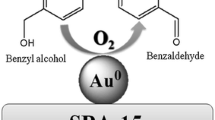
The TiO2@SBA-15 support allows the synthesis of potentially active AuPd catalysts in aerobic oxidation of benzyl alcohol under base-free conditions and solvents.









Similar content being viewed by others
References
Mallat T, Baiker A (2004) Oxidation of alcohols with molecular oxygen on solid catalysts. Chem Rev 104:3037–3058. https://doi.org/10.1021/cr0200116
Chen Y, Li W, Wang J et al (2016) Gold nanoparticle-modified TiO2/SBA-15 nanocomposites as active plasmonicphotocatalysts for the selective oxidation of aromatic alcohols. RSC Adv 6:70352–70363. https://doi.org/10.1039/c6ra11390g
Sheldon RA (2015) Recent advances in green catalytic oxidations of alcohols in aqueous media. Catal Today 247:4–13. https://doi.org/10.1016/j.cattod.2014.08.024
Anastas PT, Kirchhoff MM (2002) Origins, current status, and future challenges of green chemistry. AccChem Res 35:686–694. https://doi.org/10.1021/ar010065m
Sheldon RA (2000) Atom utilisation, E factors and the catalytic solution. ComptesRendusl’Academie des Sci - SerIIcChem 3:541–551. https://doi.org/10.1016/S1387-1609(00)01174-9
Anastas PT, Kirchhoff MM, Williamson TC (2001) Catalysis as a foundational pillar of green chemistry. ApplCatal A Gen 221:3–13. https://doi.org/10.1016/S0926-860X(01)00793-1
Arends IWCE, Sheldon RA (2010) Modern oxidation of alcohols using environmentally benign oxidants. Modern oxidation methods. Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, pp 147–185
Enache DI, Edwards JK, Landon P et al (2006) Solvent-free oxidation of primary alcohols to aldehydes using Au-Pd/TiO2 catalyst. Science 311(80):362–365. https://doi.org/10.1126/science.1120560
Haruta M (2002) Catalysis of gold nanoparticles deposited on metal oxides. CATTECH 6:102–115. https://doi.org/10.1023/A:1020181423055
Haruta M, Kobayashi T, Sano H, Yamada N (1987) Novel gold catalysts for the oxidation of carbon monoxide at a temperature far below 0 °C. Chem Lett 16:405–408. https://doi.org/10.1246/cl.1987.405
Prati L, Rossi M (1998) Gold on carbon as a new catalyst for selective liquid phase oxidation of diols. J Catal 176:552–560. https://doi.org/10.1006/jcat.1998.2078
Porta F, Prati L, Rossi M et al (2000) Metal sols as a useful tool for heterogeneous gold catalyst preparation: reinvestigation of a liquid phase oxidation. Catal Today 61:165–172. https://doi.org/10.1016/S0920-5861(00)00370-9
Prati L, Martra G (1999) New gold catalysts for liquid phase oxidation. Gold Bull 32:96–101. https://doi.org/10.1007/BF03216617
Davis SE, Ide MS, Davis RJ (2013) Selective oxidation of alcohols and aldehydes over supported metal nanoparticles. Green Chem 15:17–45. https://doi.org/10.1039/c2gc36441g
Leofanti G, Padovan M, Tozzola G, Venturelli B (1998) Surface area and pore texture of catalysts. Catal Today 41:207–219. https://doi.org/10.1016/S0920-5861(98)00050-9
Kumar A, Kumar VP, Srikanth A et al (2016) Vapor phase oxidation of benzyl alcohol over nano Au/SBA-15 catalysts: effect of preparation methods. Catal Lett 146:35–46. https://doi.org/10.1007/s10562-015-1656-7
Calzada LA, Castellanos R, García LA, Klimova TE (2019) TiO2, SnO2 and ZnO catalysts supported on mesoporous SBA-15 versus unsupported nanopowders in photocatalytic degradation of methylene blue. Microporous Mesoporous Mater 285:247–258. https://doi.org/10.1016/j.micromeso.2019.05.015
Liotta LF, Pantaleo G, Puleo F, Venezia AM (2012) Au/CeO2-SBA-15 catalysts for CO oxidation: effect of ceria loading on physic-chemical properties and catalytic performances. Catal Today 187:10–19. https://doi.org/10.1016/j.cattod.2012.01.001
Chaudhary V, Sharma S (2017) An overview of ordered mesoporous material SBA-15: synthesis, functionalization and application in oxidation reactions. J Porous Mater 24:741–749. https://doi.org/10.1007/s10934-016-0311-z
Peza-Ledesma CL, Escamilla-Perea L, Nava R et al (2010) Supported gold catalysts in SBA-15 modified with TiO2 for oxidation of carbon monoxide. ApplCatal A Gen 375:37–48. https://doi.org/10.1016/j.apcata.2009.12.009
Moreno-Martell A, Pawelec B, Nava R et al (2018) CO oxidation at 20 °C on Au catalysts supported on mesoporous silica: effects of support structural properties and modifiers. Materials (Basel). https://doi.org/10.3390/ma11060948
Schubert MM, Hackenberg S, Van Veen AC et al (2001) CO oxidation over supported gold catalysts -"Inert" and “active” support materials and their role for the oxygen supply during reaction. J Catal 197:113–122. https://doi.org/10.1006/jcat.2000.3069
Khawaji M, Chadwick D (2018) Au-Pd NPs immobilised on nanostructured ceria and Titania: impact of support morphology on the catalytic activity for selective oxidation. CatalSciTechnol 8:2529–2539. https://doi.org/10.1039/c7cy02329d
Zhao J, Huo Q, Melosh N et al (1998) Triblock copolymer syntheses of mesoporous silica with periodic 50 to 300 angstrom pores. Science 279(80):548–552. https://doi.org/10.1126/science.279.5350.548
Araújo MM, Silva LKR, Sczancoski JC et al (2016) Anatase TiO2nanocrystals anchored at inside of SBA-15 mesopores and their optical behavior. Appl Surf Sci 389:1137–1147. https://doi.org/10.1016/j.apsusc.2016.08.018
Lu X, Zhao G, Lu Y (2013) Propylene epoxidation with O2 and H2: a high-performance Au/TS-1 catalyst prepared via a deposition-precipitation method using urea. CatalSciTechnol 3:2906–2909. https://doi.org/10.1039/c3cy00339f
Delannoy L, Thrimurthulu G, Reddy PS et al (2014) Selective hydrogenation of butadiene over TiO2 supported copper, gold and gold-copper catalysts prepared by deposition-precipitation. PhysChemChemPhys 16:26514–26527. https://doi.org/10.1039/c4cp02141j
Zanella R, Delannoy L, Louis C (2005) Mechanism of deposition of gold precursors onto TiO2 during the preparation by cation adsorption and deposition-precipitation with NaOH and urea. ApplCatalA Gen 291:62–72. https://doi.org/10.1016/j.apcata.2005.02.045
Zanella R, Giorgio S, Henry CR, Louis C (2002) Alternative methods for the preparation of gold nanoparticles supported on TiO2. J PhysChem B 106:7634–7642. https://doi.org/10.1021/jp0144810
De Abreu WC, Garcia MAS, Nicolodi S et al (2018) Magnesium surface enrichment of CoFe2O4 magnetic nanoparticles immobilized with gold: reusable catalysts for green oxidation of benzyl alcohol. RSC Adv 8:3903–3909. https://doi.org/10.1039/c7ra13590d
Gualteros JAD, Garcia MAS, da Silva AGM et al (2019) Synthesis of highly dispersed gold nanoparticles on Al2O3, SiO2, and TiO2 for the solvent-free oxidation of benzyl alcohol under low metal loadings. J Mater Sci 54:238–251. https://doi.org/10.1007/s10853-018-2827-x
Bortolotti M, Lutterotti L, Lonardelli I (2009) ReX: a computer program for structural analysis using powder diffraction data. J ApplCrystallogr 42:538–539. https://doi.org/10.1107/S0021889809008309
Schindelin J, Arganda-Carreras I, Frise E et al (2012) Fiji: an open-source platform for biological-image analysis. Nat Methods 9:676–682. https://doi.org/10.1038/nmeth.2019
Wagner CD, Davis LE, Zeller MV et al (1981) Empirical atomic sensitivity factors for quantitative analysis by electron spectroscopy for chemical analysis. Surf Interface Anal 3:211–225. https://doi.org/10.1002/sia.740030506
Magalhães JL, Moreira LM, Rodrigues-Filho UP et al (2002) Surface chemistry of the iron tetraazamacrocycle on the aminopropyl-modified surface of oxidized n-Si(100) by AFM and XPS. Surf Interface Anal 33:293–298. https://doi.org/10.1002/sia.1186
Scofield JH (1976) Hartree-Slater subshell photoionization cross-sections at 1254 and 1487 eV. J Electron SpectrosRelat Phenomena 8:129–137. https://doi.org/10.1016/0368-2048(76)80015-1
Zhao D, Huo Q, Feng J et al (1998) Nonionic triblock and star diblock copolymer and oligomericsufactant syntheses of highly ordered, hydrothermally stable, mesoporous silica structures. J Am ChemSoc 120:6024–6036. https://doi.org/10.1021/ja974025i
Rodríguez-Gómez A, Platero F, Caballero A, Colón G (2018) Improving the direct synthesis of hydrogen peroxide from hydrogen and oxygen over Au-Pd/SBA-15 catalysts by selective functionalization. MolCatal 445:142–151. https://doi.org/10.1016/j.mcat.2017.10.034
Thommes M, Kaneko K, Neimark AV et al (2015) Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure ApplChem 87:1051–1069. https://doi.org/10.1515/pac-2014-1117
Choma J, Jaroniec M (2007) Applicability of classical methods of pore size analysis for MCM-41 and SBA-15 silicas. Appl Surf Sci 253:5587–5590. https://doi.org/10.1016/j.apsusc.2006.12.059
Luz GE, Lima SH, Melo ACR et al (2010) Direct synthesis and characterization of LaSBA-15 mesoporous molecular sieves. J Mater Sci 45:1117–1122. https://doi.org/10.1007/s10853-009-4054-y
Meynen V, Cool P, Vansant EF (2009) Verified syntheses of mesoporous materials. Microporous Mesoporous Mater 125:170–223. https://doi.org/10.1016/j.micromeso.2009.03.046
Ma Z, Dai S (2011) Development of novel supported gold catalysts: a materials perspective. Nano Res 4:3–32. https://doi.org/10.1007/s12274-010-0025-5
Gutiérrez LF, Hamoudi S, Belkacemi K (2011) Synthesis of gold catalysts supported on mesoporous silica materials: recent developments. Catalysts 1(1):97–154
Kučerová G, Strunk J, Muhler M, Behm RJ (2017) Effect of Titania surface modification of mesoporous silica SBA-15 supported Au catalysts: activity and stability in the CO oxidation reaction. J Catal 356:214–228. https://doi.org/10.1016/j.jcat.2017.09.017
Katiyar A, Ji L, Smirniotis P, Pinto NG (2005) Protein adsorption on the mesoporous molecular sieve silicate SBA-15: effects of pH and pore size. J Chromatogr A 1069:119–126. https://doi.org/10.1016/j.chroma.2004.10.077
Benamor T, Vidal L, Lebeau B, Marichal C (2012) Influence of synthesis parameters on the physico-chemical characteristics of SBA-15 type ordered mesoporous silica. Microporous Mesoporous Mater 153:100–114. https://doi.org/10.1016/j.micromeso.2011.12.016
Tiruvalam RC, Pritchard JC, Dimitratos N et al (2011) Aberration corrected analytical electron microscopy studies of sol-immobilized Au + Pd, Au{Pd} and Pd{Au} catalysts used for benzyl alcohol oxidation and hydrogen peroxide production. Faraday Discuss 152:63. https://doi.org/10.1039/c1fd00020a
Odio OF, Lartundo-Rojas L, Santiago-Jacinto P et al (2014) Sorption of gold by naked and thiol-capped magnetite nanoparticles: an XPS approach. J PhysChem C 118:2776–2791. https://doi.org/10.1021/jp409653t
Guo S, Zhang S, Fang Q et al (2018) Gold-palladium nanoalloys supported by graphene oxide and lamellar TiO2 for direct synthesis of hydrogen peroxide. ACS Appl Mater Interfaces 10:40599–40607. https://doi.org/10.1021/acsami.8b17342
SiavashMoakhar R, Jalali M, Kushwaha A et al (2018) AuPd bimetallic nanoparticle decorated TiO2 rutile nanorod arrays for enhanced photoelectrochemical water splitting. J ApplElectrochem 48:995–1007. https://doi.org/10.1007/s10800-018-1231-1
Bharti B, Kumar S, Lee H-N, Kumar R (2016) Formation of oxygen vacancies and Ti3+ state in TiO2 thin film and enhanced optical properties by air plasma treatment. Sci Rep 6:32355. https://doi.org/10.1038/srep32355
Sanjinés R, Tang H, Berger H et al (1994) Electronic structure of anatase TiO2 oxide. J ApplPhys 75:2945–2951. https://doi.org/10.1063/1.356190
Chenakin SP, Melaet G, Szukiewicz R, Kruse N (2014) XPS study of the surface chemical state of a Pd/(SiO2+TiO2) catalyst after methane oxidation and SO2 treatment. J Catal 312:1–11. https://doi.org/10.1016/j.jcat.2014.01.008
Paparazzo E, Fanfoni M, Severini E, Priori S (1992) Evidence of Si–OH species at the surface of aged silica. J VacSciTechnol A 10:2892–2896. https://doi.org/10.1116/1.577726
Post P, Wurlitzer L, Maus-Friedrichs W, Weber A (2018) Characterization and applications of nanoparticles modified in-flight with silica or silica-organic coatings. Nanomaterials 8:530. https://doi.org/10.3390/nano8070530
Wang Z-L, Yan J-M, Ping Y et al (2013) An efficient CoAuPd/C catalyst for hydrogen generation from formic acid at room temperature. AngewChemieInt Ed 52:4406–4409. https://doi.org/10.1002/anie.201301009
Qian K, Huang W (2011) Au–Pd alloying-promoted thermal decomposition of PdO supported on SiO2 and its effect on the catalytic performance in CO oxidation. Catal Today 164:320–324. https://doi.org/10.1016/j.cattod.2010.10.018
Venezia A (2003) Activity of SiO2 supported gold-palladium catalysts in CO oxidation. ApplCatal A Gen 251:359–368. https://doi.org/10.1016/S0926-860X(03)00343-0
Malkhasian AYS, Narasimharao K (2017) Structural and photocatalytic properties of Pd-deposited semiconductors with different morphology. RSC Adv 7:55633–55645. https://doi.org/10.1039/C7RA11080D
Wang S, Zhao Q, Wei H et al (2013) Aggregation-free gold nanoparticles in ordered mesoporous carbons: toward highly active and stable heterogeneous catalysts. J Am ChemSoc 135:11849–11860. https://doi.org/10.1021/ja403822d
Savara A, Chan-Thaw CE, Sutton JE et al (2017) Molecular origin of the selectivity differences between palladium and gold-palladium in benzyl alcohol oxidation: different oxygen adsorption properties. ChemCatChem 9:253–257. https://doi.org/10.1002/cctc.201601295
Enache DI, Knight DW, Hutchings GJ (2005) Solvent-free oxidation of primary alcohols to aldehydes using supported gold catalysts. Catal Lett 103:43–52. https://doi.org/10.1007/s10562-005-6501-y
Jiang Y, Chen M, Yang Y et al (2018) Facile synthesis of AuPd nanoparticles anchored on TiO2nanosheets for efficient dehydrogenation of formic acid. Nanotechnology. https://doi.org/10.1088/1361-6528/aac79e
Cao E, Sankar M, Nowicka E et al (2013) Selective suppression of disproportionation reaction in solvent-less benzyl alcohol oxidation catalysed by supported Au–Pd nanoparticles. Catal Today 203:146–152. https://doi.org/10.1016/j.cattod.2012.05.023
Cao E, Sankar M, Firth S et al (2011) Reaction and Raman spectroscopic studies of alcohol oxidation on gold-palladium catalysts in microstructured reactors. ChemEng J 167:734–743. https://doi.org/10.1016/j.cej.2010.08.082
Wang D, Villa A, Porta F et al (2008) Bimetallic gold/palladium catalysts: correlation between nanostructure and synergistic effects. J PhysChem C 112:8617–8622. https://doi.org/10.1021/jp800805e
Abad A, Almela C, Corma A, García H (2006) Efficient chemoselective alcohol oxidation using oxygen as oxidant. Superior performance of gold over palladium catalysts. Tetrahedron 62:6666–6672. https://doi.org/10.1016/j.tet.2006.01.118
Acknowledgements
The authors acknowledge financial support from FAPEPI and CNPq and the technical support of Center for Strategic Technology of the Northeast (CETENE-PE).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
da Silva, J.M., Sousa, R.C., Costa, J.C.S. et al. Base-Free Benzyl Alcohol Aerobic Oxidation Catalyzed by AuPdNPs Supported on SBA-15 and TiO2/SBA-15 Mesoporous Materials. Catal Lett 152, 585–599 (2022). https://doi.org/10.1007/s10562-021-03624-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-021-03624-6