Abstract
Background
Patients with Barcelona Clinic Liver Cancer Stage C (BCLC-C) hepatocellular carcinoma (HCC) can be markedly heterogeneous with varying prognosis. This study aims to establish a new subclassification system for BCLC-C HCC to better predict overall survival (OS) and to tailor therapy.
Methods
We retrospectively studied 1856 BCLC-C HCC patients between 2006 and 2017 from E-Da Hospital, Taiwan (n = 622, training cohort), Kaohsiung Medical University Hospital, Taiwan (n = 774, Taiwan validation cohort), and Stanford University Medical Center and Mayo Clinic (United States), Hanyang University Hospital (South Korea), and Ogaki Municipal Hospital (Japan) to make up the international validation cohort (n = 460).
Results
In the training cohort, significant factors associated with OS were largest tumor size ≥ 10 cm, extrahepatic spread, macrovascular invasion, and Child–Pugh class, which provided the basis, together with aged ≥ 75 years, for the substaging, through C0 to C4, of BCLC-C HCC patients. The median OS for substages C0, C1, C2, C3, and C4 were 43.8 months (95% confidence interval [CI] 32.2–53.7), 20.6 months (CI 14.1–25.9), 11.5 months (CI 8.02–14.1), 5.7 months (CI 4.02–5.98), and 3.2 months (CI 2.41–3.59), respectively, (p < 0.05). OS remained distinct among the proposed substages in the Taiwan validation cohort as well as the international validation cohort. The distinction between the substages persisted in subgroup analysis by substage combined with treatment modality. In substage C0–C3, patients receiving HCC curative therapy had a significantly better median OS than those receiving sorafenib or palliative therapy.
Conclusion
Our new substaging system provides more precise prognosis to better tailor therapy for BCLC-C HCC patients.
Similar content being viewed by others
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common cancer and the third most lethal malignancy worldwide [1, 2]. Although surveillance for HCC with alpha-fetoprotein (AFP) and ultrasound in patients at risk for HCC is recommended [3,4,5], more than 35% of patients are diagnosed with advanced-stage HCC [6]. The Barcelona Clinic Liver Cancer (BCLC) stage C includes patients who have performance status (PS) 1 or 2, extrahepatic spread (EHS), macrovascular invasion (MVI), or Child–Turcott–Pugh class (CP) A or B according to the American and European HCC practice guidelines [3,4,5]. As such, BCLC stage C patients are often quite heterogeneous, but different prognostic factors are generally considered to be of similar degree of impact in the current staging system, and the only recommended treatment for this population is sorafenib [3,4,5]. However, while sorafenib has been shown to prolong overall survival (OS) in patients with MVI and/or EHS or poor PS [7, 8], other factors such as age and other treatment modalities have also emerged as significant factors associated with survival in this population [9,10,11,12]. As a result, new scoring systems or substaging for BCLC-C HCC have been proposed [13,14,15,16,17], but many involve complex classification limiting their application in routine practice. Therefore, this study aims to develop a practical and easy-to-use substaging system for BCLC-C HCC in a training cohort in Taiwan and validate the proposed substaging system in two external validation cohorts, one from Taiwan and one from a diverse international cohort comprised of patients from the U.S. and other East Asian countries outside of Taiwan.
Materials and methods
Study design and study patients
This is a retrospective cohort study inclusive of 1856 BCLC-C HCC patients from six study centers and four countries (Taiwan, 2; USA, 2; Japan, 1, and South Korea, 1). Study patients were divided into three cohorts: The Taiwan training cohort from E-Da Hospital (n = 622, 2011–2017, Figure S1A), the Taiwan validation cohort from Kaohsiung Medical University Hospital (n = 774, 2011–2017, Figure S1B), and the international validation cohort from Stanford University Medical Center (USA), Mayo Clinic (USA), Hanyang University Seoul Hospital (South Korea), and Ogaki Municipal Hospital (Japan) (n = 460, Figure S1C). This study was approved by the Institutional Review Board at each participating institution.
Study definitions and statistical analysis
See the supplementary information.
Results
Patient baseline characteristics
Clinical features at HCC diagnosis for all study cohorts were shown in Table 1. In the total cohort (n = 1856), the median age was 65 years, the majority (81.5%) were male, about one-third had hepatitis B virus (HBV, 35.1%), one-third had hepatitis C virus (HCV, 36.6%), 7.1% had both HBV and HCV infections, the majority (64.5%) had CP class A, about half (51.0%) had MVI, and about one-third (36.6%) had EHS. The mortality rate of the total cohort was 90.8 per 100 person-years.
The Taiwan training cohort, the Taiwan validation cohort, and the international validation cohort differed significantly in all included clinical variables except for sex and median follow-up time, which ranged 6.5–8.3 months.
Training cohort and development of the new Taiwan BCLC-C substaging system (TBCSS)
Factors associated with overall survival
To select components for the subclassification of BCLC stage C patients, we examined OS in the training cohort and found significant OS differences by several patient and tumor characteristics (Table 2). The median OS was significantly longer in patients with a largest tumor size < 10 cm than in those with a tumor size ≥ 10 cm (11.5 vs 4.2 months, p < 0.0001, Figure S2A), in patients without EHS than in those with EHS (8.8 vs 4.5 months, p < 0.0001, Figure S2B), in patients without MVI than in those with MVI (8.5 vs 5.7 months, p < 0.0001, Figure S2C), and in patients with CP class A than in those with CP class B (10.3 vs 3.9 months, p < 0.0001, Figure S2D). In addition, the median OS was significantly longer in patients aged < 75 years old compared to those aged ≥ 75 years old (7.6 vs 4.5 months, p = 0.048), in patients with PS1/2 versus those with PS0 (10.1 vs 5.3 months, p < 0.0001), in patients with single tumor versus those with multiple tumors (13.2 vs 5.6 months, p < 0.0001), and in patients with AFP < 200 versus those with AFP ≥ 200 (8.3 vs 5.3 months, p < 0.0001) (Table 2).
Next, using multivariable Cox’s regression analysis (Table 2), we identified factors independently associated with OS. We found that aged ≥ 75 years old (HR 0.66; 95% CI 0.53–0.83; p < 0.0001), tumor size ≥ 10 cm (HR 0.71; 95% CI 0.59–0.85; p < 0.0001), the presence of EHS (HR 0.65; 95% CI 0.54–0.79; p < 0.0001), the presence of MVI (HR 0.74; 95% CI 0.61–0.88; p < 0.0001), CP class B (HR 0.48; 95% CI 0.40–0.58; p < 0.0001) and multiple liver tumors (HR 0.83; 95% CI 0.68–0.95; p = 0.039) were all significantly associated with worse OS, but not AFP levels.
Development of a new prognostic substaging system
Based on the significant risk factors for OS, we constructed the novel Taiwan BCLC-C substaging system (TBCSS) shown in Table 3. We classified patients with no tumor/liver risk factors and with aged < 75 years as substage C0, those with no tumor/liver risk factors but ≥ 75 years old as substage C1, and regardless of age those with any one of four tumor/liver risk factors as substage C2, those with any two of four tumor/liver risk factors as substage C3, and those with three or four tumor/liver risk factors as substage C4.
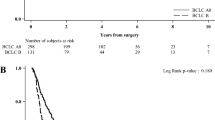
As shown in Fig. 1a, the survival curves of the substages demonstrated a significant distinction among five substages in this training cohort (p = 0.011 to < 0.0001 for comparisons between each substage from C1 to C4 against C0). The median OS ranged from 43.8 months (95% CI 32.2–53.7) for substage C0, to 20.6 months (95% CI 14.1–25.9) for C1, 11.5 months (95% CI 8.02–14.1) for C2, 5.7 months (95% CI 4.02–5.98) for C3, and 3.2 months (95% CI 2.41–3.59) for C4.
Validation of the new TBCSS
Taiwan validation cohort
The TBCSS also categorized patients in the Taiwan validation cohort with significant distinction in OS (p = 0.013 to < 0.0001 for comparison between the substages; Fig. 1b). The median OSs for the Taiwan validation cohort patients in substages C0, C1, C2, C3, and C4 were 36.8 months (95% CI 16.8–55.2), 21.1 months (95% CI 13.5–28.5), 8.6 months (95% CI 6.41–9.59), 2.9 months (95% CI 1.55–2.45), and 2.0 months (95% CI 1.40–2.60), respectively.
International validation cohort
We found similar performance of the TBCSS in our international cohort with significant distinction in OS among patients of different substages (p = 0.039 to < 0.0001; Fig. 1c). The median OSs for the international validation cohort patients in substages C0, C1, C2, C3, and C4 were 42.1 months (95% CI 30.3–51.1), 22.9 months (95% CI 15.8–30.0), 11.7 months (95% CI 8.44–13.6), 8.6 months (95% CI 5.77–10.2), and 3.4 months (95% CI 2.94–4.00), respectively.
Total cohort
Analysis of OS by substage
Combining patients from our training cohort and both our validation cohorts, we analyzed OS for each substage of the new TBCSS (p < 0.0001 for all substage comparisons; Fig. 1d). The median OS times for patients in substages C0, C1, C2, C3, and C4 were respectively 38.9 months (95% CI 28.1–47.9), 21.5 months (95% CI 16.3–25.7), 9.8 months (95% CI 7.93–10.1), 5.1 months (95% CI 4.57–5.44), and 3.3 months (95% CI 2.78–3.22) for our entire study cohort.
Sensitivity analysis with CP class A patients
As a sensitivity analysis to further isolate the effect of tumor and liver characteristics in patients with the best liver function, we analyzed only CP class A patients (n = 1197) and found that the TBCSS continued to allow great distinction in regards to OS among patients of the different substages very well (p < 0.0001 for all substage comparisons, Figure S3a–d).
Subanalysis by substage within each treatment modality
Figure S4a–d showed the median OS for substages C0, C1, C2, C3, and C4 within each treatment group. Within each treatment modality, OS was significantly different in patients of different substage (p = 0.48 to < 0.0001) except for the distinction between C1 and C0 in the sorafenib group. For example, among patients undergoing surgical resection, the median OS for substages C0, C1, C2, C3, and C4 was 65.9 months (95% CI 41.3–88.7), 44.6 months (95% CI 26.7–61.3), 23.7 months (95% CI 16.6–29.4), 9.9 months (95% CI 1.37–16.6), and 5.6 months (95% CI 1.02–8.98), respectively.
Subanalysis by treatment modality within each substage
The OS for patients undergoing different types of treatment within each substage is presented in Table 4. In substage C0, 43.6% and 31.4% of the patients received curative therapies and TACE, respectively. The median OS in patients receiving curative therapies was 49.8 months (95% CI 28.8–67.1), which was substantially longer than those receiving TACE (36.7 months, 95% CI 21.0–50.9, p = 0.092), but significantly longer than those receiving other palliative therapies (28.5 months, p < 0.0001), sorafenib (19.8 months, p = 0.04) or BSC (17.6 months, p = 0.045). The OS did not significantly differ among patients receiving TACE versus other palliative therapies, sorafenib or BSC.
In substage C1, 37.4% and 32.4% of the patients received curative therapies and TACE, respectively. The median OS was longest in patients receiving curative therapies (35.8 months, 95% CI 17.6–52.3), which did not differ significantly from those receiving TACE (25.6 months, 95% CI 13.2–36.7, p = 0.173), but significantly longer than those receiving other palliative therapies (15.1 months, 95% CI 11.2–18.7, p = 0.022), sorafenib (14.9 months, 95% CI 1.19–26.8, p = 0.046) and BSC (8.7 months 95% CI 4.31–11.7, p < 0.0001). The OS did not differ between patients receiving sorafenib and BSC.
In substage C2, most patients underwent TACE (32.2%), received sorafenib (20.6%), or had BSC (22.2%). Only 14.2% of patients underwent curative treatment but their median OS was longest among patients in substage C2 (23.8 months, 95% CI, 15.4–30.3), which was significantly longer than those undergoing TACE (12.5 months, 95% CI 9.53–14.4, p < 0.0001), other palliative therapies (7.6 months, 95% CI, 5.33–8.66, p < 0.0001), sorafenib (8.8 months, 95% CI 5.83–10.1, p < 0.0001) or BSC (4.4 months 95% CI 3.65–5.14, p < 0.0001). The OS did not differ between patients receiving other palliative therapies and sorafenib.
In substage C3, the most common intervention was BSC (34.7%), followed by sorafenib (28.1%), TACE (21.7%), and only few with curative treatment (6.9%) or other palliative therapy (6.9%). The median OS was longest in patients receiving curative therapies (9.8 months, 95% CI 1.36–16.6), which was significantly longer than in those receiving TACE (8.3 months, 95% CI 5.63–10.3, p = 0.007), other palliative therapies (3.5 months, 95% CI 2.09–3.91, p < 0.0001), sorafenib (5.4 months, 95% CI 3.54–6.45, p < 0.0001) and BSC (2.2 months 95% CI 1.58–2.41, p < 0.0001). The OS did not differ between patients receiving other palliative therapies and BSC.
In substage C4, half of the patients received BSC (47.9%) and one-quarter received sorafenib therapy (25.0%). The median OS was shortest in patients receiving BSC (2.0 months, 95% CI 1.74–2.25), which was significantly less than those receiving curative therapies (5.9 months, 95% CI 2.93–7.06, p < 0.0001), TACE (5.5 months, 95% CI 2.94–7.05, P < 0.0001), other palliative therapies (3.6 months, 95% CI 1.51–4.48, p = 0.014) and sorafenib (4.5 months, 95% CI 3.03–4.96, p < 0.0001). The OS did not differ among patients receiving curative therapies, TACE, other palliative therapies and sorafenib.
Discussion
In our large, multicenter, multinational study, we provided evidence to substage BCLC-C patients according to age, CP class and tumor characteristics (largest tumor size ≥ 10 cm, EHS, and MVI) to 5 substages C0–4 of the new TBCSS. This TBCSS derived from a Taiwan training cohort has shown great performance with clear distinction in OS rates of patients in the Taiwan validation cohort as well as the international validation cohort, despite significant differences in both patient, tumor and treatment characteristics among these three cohorts. We also showed that the performance of the TBCSS remained robust in sensitivity analysis of only CP A patients and in subgroups of patients by both substage and treatment modality. Importantly, our subgroup analysis showed that substage C0–C3 receiving curative treatment had better survival than those receiving sorafenib. Based on our findings, to maximize the survival benefit for patients with BCLC-C HCC, we proposed a novel treatment algorithm for BCLC-C patients according to the TBCSS as detailed in Fig. 2.
Proposed treatment strategy for Barcelona Clinic Liver Cancer Stage C (BCLC-C) hepatocellular carcinoma patients according to the Taiwan BCLC-C substaging system. MVI macrovascular invasion, EHS extrahepatic spread, CP class Child–Pugh class, Y/O years old, RFA radiofrequency ablation, TACE transcatheter arterial chemoembolization; systemic therapies means sorafenib
The factors used for the current TBCSS are all readily available and already included in the BCLC staging system (age, CP class, tumor size, MVI and EHS). Aging is related to a progressively decreased functional reserve of multiple organs and the reduced survival in HCC patients aged ≥ 75 years in our study have also been observed in prior studies [9,10,11]. In our total cohort, patients aged < 75 years and without any other risk factors (C0) had the best survival (median OS, 38.9 months), followed by patients aged ≥ 75 years and without one risk (C1) and those with at least one risk factor (C2–C4). Reserved liver function, as categorized by CP classification, is another important factor associated with survival among HCC patients [3,4,5]. BCLC stage C HCC patients are almost contraindicated to any HCC treatment modalities if the patients are CP class B or C [18]. In addition, we observed that tumor size is important prognostic factor associated with survival [19]. We showed that HCC patients with largest tumors ≥ 10 cm have a poorer prognosis than those with smaller tumors. It is likely due to the relationship between large tumors and the presence of factors that portent poorer prognosis, such as satellite tumors, microscopic lymphovascular involvement, and distant recurrence [20, 21].
Due to the high heterogenicity in nature and prognosis of BCLC-C population, several studies have attempted to develop substaging systems and for patients initially grouped as BCLC-C stage [13,14,15,16,17]. Prior efforts have constructed BCLC-C subclassification based on tumor size, distant metastasis, HCC type, and bile duct invasion but not other important prognostic factors, such as MVI and CP class, [16] or based on tumor burden, major portal vein invasion and distant metastasis, and CP class but without age [17]. The newly proposed TBCSS by our current study, consisting of five already collected variables for the main BCLC staging, provides a comprehensive assessment of the patient, the liver and the tumor with age, CP class, and detailed tumor factors (size, MVI, EHS). The OS rates among the substages of the TBCSS differed significantly as opposed to those seen with prior substaging systems [13, 17].
Sorafenib has generally been the only recommended therapy and the most frequently used treatment for BCLC-C patients before the introduction of the other systemic molecular targeted therapies and immunotherapy [3, 4, 22, 23], but only about 20% of our diverse cohort of BCLC patients received this treatment. While sorafenib has been shown to significantly prolong survival in unresectable HCC compared to supportive care in general, [24,25,26] we only observed significantly higher survival in sorafenib patients compared to BSC patients within BCLC-C substages C2–C4, but not among those with substage C0–1 though part of this can be due to the limited number of patients receiving sorafenib in substage C0–1 in our study (n = 11). Thus, our results indicated that sorafenib was an effective therapy for advanced HCC patients in countries in the Asia–Pacific region [26]. However, as noted above, the majority of our BCLC-C patients actually received other palliative and even curative treatments and that both curative treatment and other palliative treatment such as TACE were superior than sorafenib in regards to OS among TBCSS C0–C3 for curative treatment and C2–C3 for TACE.
Thus, our study provided support for a new treatment paradigm for the early substages of the BCLC-C group (Fig. 2). In the current study, 43.6% of BCLC-C substage C0 patients received curative therapy. The median OS could reach 49.8 (95% CI 28.8–67.1) months, which is only slightly lower than the expected survival of about 60 months among patients with very early-stage HCC (BCLC stage 0) receiving curative therapy in Taiwan as well as in West [4, 27]. It is also similar to the expected survival of about 48 months among patients with early stage HCC (BCLC stage A) receiving curative therapy. For the 31.4% of substage C0 patients in the current study who received TACE therapy, the median OS was 36.7 (95% CI 21.0–50.9) months, which was not inferior to 20–25 months among patients with intermediate stage HCC (BCLA stage B) who received TACE therapy [3,4,5, 27]. Similarly, curative therapies and TACE could provide better survival than sorafenib, other local palliative therapies and BSC among patients of BCLC substages C1–C3 in our cohort. Our results indicated that curative therapies and TACE could be recommended in a proportion of patients with previously defined advanced HCC BCLC stage C, if they meet criteria for the TBCSS substages C0-C3 and eligible for curative HCC therapies or TACE [28, 29].
However, for substage C4, neither curative therapies nor TACE provided better survival than sorafenib, indicating that sorafenib remains the best therapeutic option for the population pending future data with newly introduced systemic molecular targeted therapy, such as lenvatinib or regorafenib [30] and immunotherapy such as nivolumab or pembrolizumab [30].
The strength of our study is the large and diverse study cohort of both Eastern and Western patients of diverse liver disease etiologies and treatment modalities. Yet despite these differences in patient demographic, liver and tumor characteristics and other potential differences in regional practice patterns, our newly proposed substaging system for BCLC-C patients consistently showed divergent OS outcomes distinguishing each substage even when further stratified by treatment modality. Our study also has limitations inherent in its retrospective design such as missing data and heterogeneity in local referral and practice patterns. However, the primary outcome of our study is OS, which is an objective and universal event, and the factors included in our new TBCSS are also objective factors already included in the BCLC-C staging system. Second, whether antiviral therapy for HBV and/or HCV could improve the OS among BCLC-C HCC patients and each different substages needs further studies. Lastly, due to study time period, we were not able to study the effects of more novel systemic therapies that became available recently.
In conclusion, our study proposed a novel substaging system for BCLC-C HCC patients that can identify substage patients who may benefit from curative treatment and TACE versus sorafenib. We advocate for curative treatment or TACE for TBCSS substage C0–C1 and also in patients substage C2–C3 prior to systemic therapy, but we confirmed the current recommendation for systemic therapy or supportive care for substage C4 patients. We also recommend further validation of the TBCSS in larger scale prospective studies that also investigate the effectiveness of new systemic therapies for BCLC-C patients.
Abbreviations
- HCC:
-
Hepatocellular carcinoma
- BCLC:
-
Barcelona Clinic Liver Cancer
- PS:
-
Performance status
- MVI:
-
Macrovascular invasion
- EHS:
-
Extrahepatic spread
- CP class:
-
Child–Pugh class
- OS:
-
Overall survival
- HBV:
-
Hepatitis B virus
- HCV:
-
Hepatitis C virus
- AFP:
-
Alpha-fetoprotein
- RFA:
-
Radiofrequency ablation
- TACE:
-
Transcatheter arterial chemoembolization
- HAIC:
-
Hepatic artery infusion chemotherapy
- BSC:
-
Best supportive care
- HR:
-
Hazard ratio
- CI:
-
Confidence interval
- HKLC:
-
Hong Kong Liver Cancer staging
- TBCSS:
-
Taiwan Barcelona Clinic Liver Cancer Stage C substaging system
References
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin 2015;65(2):87–108
Bertuccio P, Turati F, Carioli G, Rodriguez T, La Vecchia C, Malvezzi M, et al. Global trends and predictions in hepatocellular carcinoma mortality. J Hepatol 2017;67(2):302–309
Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018;67(1):358–380
European Association for the Study of the Liver. Electronic address EEE, European Association for the Study of the L. EASL Clinical Practice Guidelines: management of hepatocellular carcinoma. J Hepatol 2018;69(1):182–236
Omata M, Cheng AL, Kokudo N, Kudo M, Lee JM, Jia J, et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int 2017;11(4):317–370
Mittal S, Kanwal F, Ying J, Chung R, Sada YH, Temple S, et al. Effectiveness of surveillance for hepatocellular carcinoma in clinical practice: a United States cohort. J Hepatol 2016;65(6):1148–1154
Cheng AL, Guan Z, Chen Z, Tsao CJ, Qin S, Kim JS, et al. Efficacy and safety of sorafenib in patients with advanced hepatocellular carcinoma according to baseline status: subset analyses of the phase III Sorafenib Asia-Pacific trial. Eur J Cancer 2012;48(10):1452–1465
Bruix J, Cheng AL, Meinhardt G, Nakajima K, De Sanctis Y, Llovet J. Prognostic factors and predictors of sorafenib benefit in patients with hepatocellular carcinoma: analysis of two phase III studies. J Hepatol 2017;67(5):999–1008
De Toni EN, Schlesinger-Raab A, Fuchs M, Schepp W, Ehmer U, Geisler F, et al. Age independent survival benefit for patients with hepatocellular carcinoma (HCC) without metastases at diagnosis: a population-based study. Gut 2020;69(1):168–176
Guo H, Wu T, Lu Q, Dong J, Ren YF, Nan KJ, et al. Hepatocellular carcinoma in elderly: clinical characteristics, treatments and outcomes compared with younger adults. PLoS One 2017;12(9):e0184160
Nishikawa H, Kimura T, Kita R, Osaki Y. Treatment for hepatocellular carcinoma in elderly patients: a literature review. J Cancer 2013;4(8):635–643
Yeh ML, Huang CF, Huang CI, Hsieh MY, Hou NJ, Lin IH, et al. The prognostic factors between different viral etiologies among advanced hepatocellular carcinoma patients receiving sorafenib treatment. Kaohsiung J Med Sci 2019;35(10):624–632
Yau T, Tang VY, Yao TJ, Fan ST, Lo CM, Poon RT. Development of Hong Kong Liver Cancer staging system with treatment stratification for patients with hepatocellular carcinoma. Gastroenterology 2014;146(7):1691e3–1700e3
Adhoute X, Penaranda G, Raoul JL, Blanc JF, Edeline J, Conroy G, et al. Prognosis of advanced hepatocellular carcinoma: a new stratification of Barcelona Clinic Liver Cancer stage C: results from a French multicenter study. Eur J Gastroenterol Hepatol 2016;28(4):433–440
Sinn DH, Cho JY, Gwak GY, Paik YH, Choi MS, Lee JH, et al. Different survival of Barcelona clinic liver cancer stage C hepatocellular carcinoma patients by the extent of portal vein invasion and the type of extrahepatic spread. PLoS One 2015;10(4):e0124434
Jun CH, Yoon JH, Cho E, Shin SS, Cho SB, Kim HJ, et al. Barcelona clinic liver cancer-stage C hepatocellular carcinoma: a novel approach to subclassification and treatment. Medicine (Baltimore) 2017;96(17):e6745
Lee DW, Yim HJ, Seo YS, Na SK, Kim SY, Suh SJ, et al. Prognostic assessment using a new substaging system for Barcelona clinic liver cancer stage C hepatocellular carcinoma: a nationwide study. Liver Int 2019;39(6):1109–1119
Giannini EG, Bucci L, Garuti F, Brunacci M, Lenzi B, Valente M, et al. Patients with advanced hepatocellular carcinoma need a personalized management: a lesson from clinical practice. Hepatology 2018;67(5):1784–1796
Dai CY, Lin CY, Tsai PC, Lin PY, Yeh ML, Huang CF, et al. Impact of tumor size on the prognosis of hepatocellular carcinoma in patients who underwent liver resection. J Chin Med Assoc 2018;81(2):155–163
Kokudo T, Hasegawa K, Matsuyama Y, Takayama T, Izumi N, Kadoya M, et al. Survival benefit of liver resection for hepatocellular carcinoma associated with portal vein invasion. J Hepatol 2016;65(5):938–943
Lin CW, Chen YS, Lin CC, Lee PH, Lo GH, Hsu CC, et al. Significant predictors of overall survival in patients with hepatocellular carcinoma after surgical resection. PLoS One 2018;13(9):e0202650
Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet 2018;391(10127):1301–1314
Villanueva A. Hepatocellular carcinoma. N Engl J Med 2019;380(15):1450–1462
Hsiao P, Hsieh KC, Chen YS, Hsu CC, Lo GH, Li YC, et al. Sorafenib with concurrent multiple-line therapies improves overall survival in advanced stage hepatocellular carcinoma. Medicine (Baltimore) 2019;98(25):e16074
Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008;359(4):378–390
Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 2009;10(1):25–34
Surveillance g, Diagnosis g, Staging g, Surgery g, Local ablation g, group TTH, et al. Management consensus guideline for hepatocellular carcinoma: 2016 updated by the Taiwan Liver Cancer Association and the Gastroenterological Society of Taiwan. J Formos Med Assoc 2018;117(5):381–403
Ho MC, Hasegawa K, Chen XP, Nagano H, Lee YJ, Chau GY, et al. Surgery for intermediate and advanced hepatocellular carcinoma: a consensus report from the 5th Asia-Pacific primary liver cancer expert meeting (APPLE 2014). Liver Cancer 2016;5(4):245–256
Chen ZH, Zhang XP, Lu YG, Li LQ, Chen MS, Wen TF, et al. Actual long-term survival in HCC patients with portal vein tumor thrombus after liver resection: a nationwide study. Hepatol Int 2020;14(5):754–764
Llovet JM, Lencioni R. mRECIST for HCC: performance and novel refinements. J Hepatol 2020;72(2):288–306
Funding
This study was supported by Grants from MOST (105-2314-B-650-004-MY3 and 108-2314-B-214-006-MY2), E-Da Hospital-National Taiwan University Hospital Joint Research Program (108-EDN11 and 109-EDN03), the E-Da Hospital (EDAHP109044, EDAHP109045, EDAHP109053, EDPJ105056, EDPJ106094, EDPJ107076, and EDPJ1080696) to Chih-Wen Lin, and was supported in part by Kaohsiung Medical University Grants KMU-DK109002, Research Center Grant, Cohort Research Center KMU-TC108B07 and Center of Cancer Research KMU-TCA04-3.
Author information
Authors and Affiliations
Contributions
Lin CW, Chen YS, Lo GH, Wu TC, Yen JH, and Yen ML recruited the patients, collected the data, performed data analysis, and participated in the review and revision of the manuscript together with Dai CY, Huang JF, Chung WL, Roberts L, Jun DW, Toyoda H, and Yasuda S. Lin CW designed the study and drafted the manuscript together with Nguyen MH and Yu ML. All of the authors provided important suggestions pertaining to the manuscript, reviewed and approved the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
Mindie H. Nguyen: Advisory Board and/or consultation: Exact Sciences, Bayer, Eisai, Laboratory of Advanced Medicine. Wan-Long Chuang: Consultation: AbbVie, Bristol-Myers Squibb Merck, PharmaEssentia. Ming-Lung Yu: Research grant from Abbott, BMS, Gilead and Merck; onsultant of Abbvie, Abbott, Ascletis, BMS, Gilead, Merck and Roche; Speaker of Abbvie, Abbott, BMS, Gilead, Merck, and IPSEN. Hidenori Toyoda: Speaker of AbbVie and Gilead. Other authors have no conflicts to declare.
Ethical approval
This study was approved by the Institutional Review Board at each participating institution.
Informed consent
Informed consent was not required in this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lin, CW., Chen, YS., Lo, GH. et al. Resubclassification and clinical management for Barcelona Clinic Liver Cancer Stage C hepatocellular carcinoma. Hepatol Int 15, 946–956 (2021). https://doi.org/10.1007/s12072-021-10169-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12072-021-10169-8