Abstract
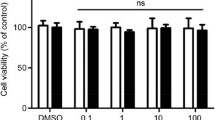
Macrophage proliferation is known to correlate with macrophage accumulation in atherosclerotic plaque, and therefore its inhibition and secondary reduction of plaque inflammation may have therapeutic beneficial effects on atherosclerosis. Recently, we reported that a peptide corresponding to positions 41–51 of royalisin (which consists of 51 amino acid residues), a potent antibacterial protein contained in royal jelly (RJ), can specifically bind to oxidized LDL (Ox-LDL), a major components of atherosclerotic lesions. Here, we investigated the interaction of RJ proteins including royalisin with LDL and Ox-LDL. Measurement of LDL oxidation by the production of thiobarbituric acid reactive substances and conjugated dienes, and by electrophoretic mobility on polyacrylamide gel electrophoresis showed that RJ proteins including royalisin and the degradation products of major RJ protein (MRJP) 1 and MRJP3 can induce oxidation of LDL and Ox-LDL. Surface plasmon resonance experiments showed that these RJ proteins can exhibit much higher binding affinity to LDL than Ox-LDL (the equilibrium dissociation constant, KD = 8.35 and 49.65 μg proteins/mL for LDL and Ox-LDL, respectively). Experiments using cultured mouse J774A.1 macrophage cells proved that these RJ proteins can inhibit macrophage proliferation markedly and concentration-dependently, regardless of the absence or presence of LDL and Ox-LDL, but hardly affect lipid accumulation in macrophages. These results suggest that RJ proteins including royalisin and degradation products of MRJP1/MRJP3 may have therapeutic beneficial effects on atherosclerosis owing to the reduction of plaque inflammation. Further studies of these RJ proteins may lead to the discovery of novel anti-atherosclerotic drugs.







Similar content being viewed by others
References
Ross R (1999) Mechanism of disease: atherosclerosis—an inflammatory disease. N Engl Med 340:115–126. https://doi.org/10.1056/NEJM199901143400207
Glass CK, Witztum JL (2001) Atherosclerosis: the road ahead. Cell 104:503–516. https://doi.org/10.1016/S0092-8674(01)00238-0
Sternberg D, Parthasarathy S, Carew TE, Khoo JC, Witztum JL (1989) Beyond cholesterol: modification of low-density lipoprotein that increases its atherogenicity. N Engl J Med 320:915–924. https://doi.org/10.1056/NEJM198904063201407
Rosenfeld ME (1991) Oxidized LDL affects multiple atherogenic cellular responses. Circulation 83:2137–2140. https://doi.org/10.1161/01.cir.83.6.2137
Witztum JL (1993) Role of oxidised low-density lipoprotein in atherogenesis. Br Heart J 69:S12–S18. https://doi.org/10.1136/hrt.69.1_suppl.s12
Nishi K, Itabe H, Uno M, Kitazato KT, Horiguchi H, Shinno K, Nagahiro S (2002) Oxidized LDL in carotid plaques and plasma associates with plaque instability. Arterioscler Thromb Vasc Biol 22:1649–1654. https://doi.org/10.1161/01.atv.0000033829.14012.18
Sigala F, Kotsinas A, Savari P, Fillis K, Markantonis S, Iliodromitis EK, Gorgoulis VG, Andreadou I (2010) Oxidized LDL in human carotid plaques is related to symptomatic carotid disease and lesion instability. J Vasc Surg 52:704–713. https://doi.org/10.1016/j.jvs.2010.03.047
Ramos-Arellano LE, Muñoz-Valle JF, De la Cruz-Mosso U, Salgado-Bernabé AB, Castro-Alarcón N, Isela Parra-Rojas I (2014) Circulating CD36 and oxLDL levels are associated with cardiovascular risk factors in young subjects. BMC Cardiovasc Disord 14:54. https://doi.org/10.1186/1471-2261-14-54
Tang J, Lobatto ME, Hassing L, van der Staay S, van Rijs SM, Calcagno C, Braza MS, Baxter S, Fay F, Sanchez-Gaytan BL, Duivenvoorden R, Sager HB, Astudillo YM, Leong W, Ramachandran S, Storm G, Pérez-Medina C, Reiner T, Cormode DP, Strijkers GJ, Stroes ESG, Swirski FK, Nahrendorf M, Fisher EA, Fayad ZA, Mulder WJM (2015) Inhibiting macrophage proliferation suppresses atherosclerotic plaque inflammation. Sci Adv 1:e1400223. https://doi.org/10.1126/sciadv.1400223
Yamada S, Senokuchi T, Matsumura T, Morita Y, Ishii N, Fukuda K, Murakami-Nishida S, Nishida S, Kawasaki S, Motoshima H, Furukawa N, Komohara Y, Fujiwara Y, Koga T, Yamagata K, Takeya M, Araki E (2018) Inhibition of local macrophage growth ameliorates focal inflammation and suppresses atherosclerosis. Arterioscler Thromb Vasc Biol 38:994–1006. https://doi.org/10.1161/ATVBAHA.117.310320
Nagai T, Inoue R (2004) Preparation and functional properties of water extract and alkaline extract of royal jelly. Food Chem 84:181–186. https://doi.org/10.1016/S0308-8146(03)00198-5
Melliou E, Chinou I (2005) Chemistry and bioactivity of royal jelly from Greece. J Agri Food Chem 53:8987–8992. https://doi.org/10.1021/jf051550p
Ramadan MF, AI-Ghamdi A (2012) Bioactive compounds and health-promoting properties of royal jelly: a review. J Funct Foods 4:39–52. https://doi.org/10.1016/j.jff.2011.12.007
Fujiwara S, Imai J, Fujiwara M, Yaeshima T, Kawashima T, Kobayashi KA (1990) A potent antibacterial protein in royal jelly. Purification and determination of the primary structure of royalisin. J Biol Chem 265:11333–11337. https://doi.org/10.1016/S0021-9258(19)38596-5
Shen L, Liu D, Li M, Jin F, Din M, Parnell LD, Lai CQ (2012) Mechanism of action of recombinant acc-royalisin from royal jelly of Asian honeybee against gram-positive bacteria. PLoS ONE 7:e47194. https://doi.org/10.1371/journal.pone.0047194
Bílikova K, Huang SC, Lin IP, Šimúth J, Peng CC (2015) Structure and antimicrobial activity relationship of royalisin, an antimicrobial peptide from royal jelly of apis mellifera. Peptides 68:190–196. https://doi.org/10.1016/j.peptides.2015.03.001
Sato A, Unuma H, Yamazaki Y, Ebina K (2018) A fluorescently labeled undecapeptide derived from a protein in royal jelly of the honeybee-royalisin-for specific detection of oxidized low-density lipoprotein. J Pept Sci 24:e3072. https://doi.org/10.1002/psc.3072
Sato A, Aoki J, Ebina K (2012) Synthetic biotinylated peptide compound, BP21, specifically recognizes lysophosphatidylcholine micelles. Chem Biol Drug Des 80:417–425. https://doi.org/10.1111/j.1747-0285.2012.01413.x
Sato A, Kumagai T, Aoki J, Ebina K (2012) Synthetic biotinylated peptide compounds derived from asp-hemolysin: novel potent inhibitors of platelet-activating factor. Eur J Pharmacol 685:205–212. https://doi.org/10.1016/j.ejphar.2012.04.025
Sato A, Yamazaki M, Watanabe H, Sakurai E, Ebina K (2020) Human estrogen sulfotransferase and its related fluorescently labeled decapeptides specifically interact with oxidized low-density lipoprotein. J Pept Sci 26:e3274. https://doi.org/10.1002/psc.3274
Bíliková K, Wu G, Šimúth J (2001) Isolation of a peptide fraction from honeebee royal jelly as a potential antifoulbrood factor. Apidologie 32:275–283. https://doi.org/10.1051/apido:2001129
Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Klenk DC (1985) Measurement of protein using bicinchoninic acid. Anal Biochem 150:76–85. https://doi.org/10.1016/0003-2697(85)90442-7
Havel RJ, Eder HA, Bragdon JH (1955) The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest 34:1345–1353. https://doi.org/10.1172/JCI103182
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193:265–275
Corongiu FP, Banni S (1994) Detection of conjugated dienes by second derivative ultraviolet spectrophotometry. Methods Enzymol 233:303–310. https://doi.org/10.1016/S0076-6879(94)33033-6
Staprans I, Rapp JH, Pan X-M, Kim KY, Feingold KR (1994) Oxidized lipids in the diet are a source of oxidized lipids in chylomicrons of human serum. Arterioscler Thromb 14:1900–1905. https://doi.org/10.1161/01.ATV.14.12.1900
Sato A, Yamanaka H, Oe K, Yamazaki Y, Ebina K (2014) Novel fluorescently labeled peptide compounds for detection of oxidized low-density lipoprotein at high specificity. Chem Biol Drug Des 84:443–449. https://doi.org/10.1111/cbdd.12333
Sato A, Yamanaka H, Oe K, Yokoyama I, Yamazaki Y, Ebina K (2015) Highly stable, fluorescence-labeled heptapeptides substituted with a d-amino acid for the specific detection of oxidized low-density lipoprotein in plasma. Chem Biol Drug Des 85:348–355. https://doi.org/10.1111/cbdd.12399
Ahmad S, Campos MG, Fratini F, Altaye SZ, Li J (2020) New insights into biological and pharmaceutical properties of royal jelly. Int J Mol Sci 21:382. https://doi.org/10.3390/ijms21020382
Tamura S, Amano S, Kono T, Kondoh J, Yamaguchi K, Kobayashi S, Ayabe T, Moriyama T (2009) Molecular characteristics and physiological functions of major royal jelly protein 1 oligomer. Proteomics 9:5534–5543. https://doi.org/10.1002/pmic.200900541
Nozaki R, Tamura S, Ito A, Moriyama T, Yamaguchi K, Kono T (2012) A rapid method to isolate soluble royal jelly proteins. Food Chem 134:2332–2337. https://doi.org/10.1016/j.foodchem.2012.03.106
Mitra S, Goyal T, Mehta JL (2011) Oxidized LDL LOX-1 and atherosclerosis. Cardiovasc Drugs Ther 25:419–429. https://doi.org/10.1007/s10557-011-6341-5
Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial, or not-profit sectors. The authors thank Ms. Hiromi Yoshida (Common Instrument Center, Institute of Development, Aging and Cancer, Tohoku University) for surface plasmon resonance measurement. We would like to thank Editage (Tokyo, Japan) for editing and reviewing this manuscript for English language.
Author information
Authors and Affiliations
Contributions
AS and KE conceived and designed the experiments. AS and HU carried out the experiments and analyzed the data. AS wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest associated with this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sato, A., Unuma, H. & Ebina, K. Royal Jelly Proteins Inhibit Macrophage Proliferation: Interactions with Native- and Oxidized-Low Density Lipoprotein. Protein J 40, 699–708 (2021). https://doi.org/10.1007/s10930-021-09998-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10930-021-09998-1