Abstract
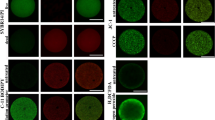
Estimation of realized fecundity (Freal, number of viable oocytes produced) is an essential, yet seldom-achieved element in the understanding of marine animal production and population dynamics. We used the Neutral Red (NR) vital stain to determine oocyte viability in spawns and gonad strippings of four species of commercially-important bivalves: Cerastoderma edule (L), Crassostrea gigas Thunberg, Mytilus edulis L, and Tapes philippinarum (Adams and Reeve). The utility of Trypan Blue as a complementary mortal stain was also assessed, and found to be unnecessary. Normal, live oocytes stained with NR, and were either spherical (mature oocytes) or pedunculated (immature oocytes); atresic/abnormal oocytes also stained with NR, allowing their ready recognition due to their abnormal shapes and cytoplasmic retraction. Dead oocytes did not stain with NR. Across the species studied, a considerable and highly variable proportion of spawned or stripped oocytes was either dead or non-viable; quantitative counts were performed for Cerastoderma edule, the only species for which > 5 spawns occurred. The high level and variability (34–85%) of dead or non-viable oocytes is consistent with a reproductive Red Queen dilemma, in which greater oocyte numbers do not translate to commensurately greater real fecundities, and also with a Sweepstakes Reproductive Success strategy, in which a large range of Freal confronts the considerable variability of intertidal environmental conditions. Neutral Red vital staining is a promising tool for the elucidation and optimization of crucial yet previously intractable aspects of bivalve hatchery production, genetic improvement, restocking, stock management, and conservation.




Similar content being viewed by others
Data availability
All files from this study are available from the authors upon reasonable request.
References
Adiyodi K, Adiyodi R (1983) Reproductive biology of invertebrates. Vol. I: Oogenesis, oviposition, and oosorption. Wiley, New York
Aguirre-Martínez GV, Buratti S, Fabbri E, DelValls AT, Martín-Díaz ML (2013) Using lysosomal membrane stability of haemocytes in Ruditapes philippinarum as a biomarker of cellular stress to assess contamination by caffeine, ibuprofen, carbamazepine and novobiocin. J Environ Sci 25:1408–1418
Angel-Dapa MA, Rodríguez-Jaramillo C, Cáceres-Martínez CJ, Saucedo PE (2010) Changes in lipid content of oocytes of the penshell Atrina maura as a criterion of gamete development and quality: a study of histochemistry and digital image analysis. J Shellfish Res 29:407–413
Aoyama S (1989) The Mutu Bay scallop fisheries: scallop culture, stock enhancement, and resource management. In: Caddy JF (ed) Marine invertebrate fisheries: their assessment and management. Wiley, New York, pp 525–539
Arnold WS (2008) Application of larval release for restocking and stock enhancement of coastal marine bivalve populations. Rev Fish Sci 16:65–71
Babich B, Borenfreund E (1993) Applications of the neutral red cytotoxicity assay to risk assessment of aquatic contaminants: an overview. In: Environmental toxicology and risk assessment, W. Landis, J. Hughes and M. Lewis. ASTM International, West Conshohocken, PA, pp 215–219
Barros J, Velasco L, Winkler F (2018) Heritability, genetic correlations and genotype by environment interactions in productive traits of the Caribbean scallop, Argopecten nucleus(Mollusca: Bivalvia). Aquaculture 488:39–48
Beninger PG (2017) Caveat observator: the many faces of pre-spawning atresia in marine bivalve reproductive cycles. Mar Biol:164–163. https://doi.org/10.1007/s00227-017-3194-x
Beninger PG, Boldina I (2018) Quantitative considerations in mudflat ecology. In: Mudflat ecology. P.G. Beninger. Springer, Cham, Switzerland, pp 389–419
Beninger PG, Boldina I, Katsanevakis S (2012) Strengthening statistical usage in marine ecology. J Exp Mar Biol Ecol 426:97–108
Borenfreund E, Puerner JA (1984) A simple quantitative procedure using monolayer cultures for cytotoxicity assays (HTD/NR-90). J Tissue Cult Meth 9:7–9
Borenfreund E, Puerner JA (1985) Toxicity determined in vitro by morphological alterations and neutral red absorption. Toxicol Lett 24:119–124
Borenfreund E, Babich H, Martin-Alguacil N (1988) Comparisons of two in vitro cytotoxicity assays—the neutral red (NR) and tetrazolium MTT tests. Toxicol in vitro 2:1–6
Boudry P (2009) Genetic variation and selective breeding in hatchery-propagated molluscan shellfish. In: New technologies in aquaculture. G. Burnell and G. Allan. CRC Press-Woodhead Publishing Ltd, Boca Raton FL, pp 87–108
Buttner JK, Weston S (2010) Softshell clam culture: hatchery phase, broodstock care through seed production. Northeastern Regional Aquaculture Center Publication No. 202-2010, 12 pp
Caers M, Coutteau P, Cure K, Morales V, Gajardo G, Sorgeloos P (1999) The Chilean scallop Argopecten purpuratus (Lamarck, 1819): II. Manipulation of the fatty acid composition and lipid content of the eggs via lipid supplementation of the broodstock diet. Comp Biochem Physiol B: Biochem Mol Biol 123:97–103
Caers M, Utting S, Coutteau P et al (2002) Impact of the supplementation of a docosahexaenoic acid–rich emulsion on the reproductive output of oyster broodstock, Crassostrea gigas. Mar Biol 140:1157–1166
Cannuel R, Beninger PG (2005) Is oyster broodstock feeding always necessary? A study using oocyte quality predictors and validators in Crassostrea gigas. Aquat Liv Res 18:35–43
Chérel D, Beninger PG (2017) Oocyte atresia characteristics and effect on reproductive effort of Manila clam Tapes philippinarum (Adams and Reeve, 1850). J Shellfish Res 36:549–557
Chérel D, Beninger PG (2019) Oocyte atresia and its effect on reproductive effort of the common cockle Cerastoderma edule (Linneaus, 1758). J Shellfish Res 38:603–609. https://doi.org/10.2983/035.038.0300
Chérel D, Beninger PG, Le Pennec G (2020) Two enigmas may solve each other: the oocyte coat and atresia in the common cockle, Cerastoderma edule (Linnaeus, 1758). Mar Biol 167:104. https://doi.org/10.1007/s00227-020-03718-6
Chiba K, Kawakami K, Tohyama K (1998) Simultaneous evaluation of cell viability by neutral red, MTT and crystal violet staining assays of the same cells. Toxicol in vitro 12:251–258
Corporeau C, Vanderplancke G, Boulais M, Suquet M, Quéré C, Boudry P, Huvet A, Madec S (2012) Proteomic identification of quality factors for oocytes in the Pacific oyster Crassostrea gigas. J Proteom 75:5554–5563
Crippen RW, Perrier J (1974) The use of neutral red and Evans blue for live-dead determinations of marine plankton (with comments on the use of rotenone for inhibition of grazing). Stain Technol 49:97–104
Da Luz DS, Da Silva DG, Souza MM et al (2016) Efficiency of Neutral Red, Evans Blue and MTT to assess viability of the freshwater microalgae Desmodesmus communis and Ediastrum boryanum. Phycol Res 64:56–60
Dressel DM, Heinle DR, Grote MC (1972) Vital staining to sort dead and live copepods 1, 2, 3. Chesapeake Sci 13:156–159
Ehrlich P (1894) Ueber Neutralrot. Allg med Zentral-Zeit 20:
Elliott DT, Tang KW (2009) Simple staining method for differentiating live and dead marine zooplankton in field samples. Limnol Oceanogr: Methods 7:585–594
Gallager SM, Mann R (1986) Growth and survival of larvae of Mercenaria mercenaria (L.) and Crassostrea virginica (Gmelin) relative to broodstock conditioning and lipid content of eggs. Aquaculture 56:105–121
Gallager SM, Mann R, Sasaki GC (1986) Lipid as an index of growth and viability in three species of bivalve larvae. Aquaculture 56:81–103
García-Corona JL, Rodríguez-Jaramillo C, Saucedo PE, López-Carvallo JA, Arcos-Ortega GF, Mazón-Suástegui JM (2018) Internal energy management associated with seasonal gonad development and oocyte quality in the Horsemussel Modiolus capax (Bivalvia; Mytilidae). J Shellfish Res 37:475–484
Gomez ED, Mingoa-Licuanan SS (2006) Achievements and lessons learned in restocking giant clams in the Philippines. Fish Res 80:46–52. https://doi.org/10.1016/j.fishres.2006.03.017
Gómez-Robles E, RodrIguez-Jaramillo C, Saucedo PE (2005) Digital image analysis of lipid and protein histochemical markers for easuring oocyte development and quality in pearl oyster Pinctada mazatlanica (Hanley, 1856). Journal of Shellfish Research 24:1197–1202. https://doi.org/10.2983/0730-8000(2005)24[1197:DIAOLA]2.0.CO;2
González-Wangüemert M, Basso L, Balau A, Costa J, Renault L, Serrão EA, Duarte CM, Hendriks IE (2018) Gene pool and connectivity patterns of Pinna nobilis in the Balearic Islands (Spain, Western Mediterranean Sea): implications for its conservation through restocking. Aquat Conserv: Mar Freshw Ecosyst 29:175–188. https://doi.org/10.1002/aqc.2976
Hammond M, Goodwin J, Dvorak H (1980) Quantitative measurements of neutral red uptake and excretion by mammalian cells. J Reticuloendothel Soc 27:337
Hedgecock D, Pudovkin AI (2011) Sweepstakes Reproductive Success in highly fecund marine fish and shellfish: a review and commentary. Bull Mar Sci 87:971–1002. https://doi.org/10.5343/bms.2010.1051
His E, Beiras R, Seaman M (1999) The assessment of marine pollution-bioassays with bivalve embryos and larvae. Adv Mar Biol 37:1–178
Horvath TG, Lamberti GA (1999) Mortality of zebra mussel, Dreissena polymorpha, veligers during downstream transport. Freshw biol 42:69–76
Hu W, Culloty S, Darmody G, Lynch S, Davenport J, Ramirez-Garcia S, Dawson K, Lynch I, Doyle H, Sheehan D (2015) Neutral red retention time assay in determination of toxicity of nanoparticles. Mar Environ Res 111:158–161. https://doi.org/10.1016/j.marenvres.2015.05.007
Jacques PJ (1969) Endocytosis. Lysosomes in biology and pathology 2:395–420
Jennings S, Kaiser MJ, Reynolds JD (2001) Marine fisheries ecology. Blackwell Scientific Publication
Joaquim S, Gaspar MB, Matias D, Ben-Hamadou R, Arnold WS (2008) Rebuilding viable spawner patches of the overfished Spisula solida (Mollusca: Bivalvia): a preliminary contribution to fishery sustainability. ICES J Mar Sci 65:60–64
Koslow JA (1992) Fecundity and the stock–recruitment relationship. Can J of Fish Aquat Sci 49:210–217
Kreeger DA, Gatenby CM, Bergstrom PW (2018) Restoration potential of several native species of Bivalve Molluscs for water quality improvement in Mid-Atlantic Watersheds. Journal Shellfish Res 37:1121. https://doi.org/10.2983/035.037.0524
Kwok AKH, Lai TYY, Li WWY, Yew DTW, Wong VWY (2004) Trypan blue- and indocyanine green-assisted epiretinal membrane surgery: clinical and histopathological studies. Eye 18:882–888. https://doi.org/10.1038/sj.eye.6701359
Liu X, Rodeheaver DP, White JC, Wright AM, Walker LM, Zhang F, Shannon S (2018) A comparison of in vitro cytotoxicity assays in medical device regulatory studies. Regul Toxicol Pharmacol 97:24–32. https://doi.org/10.1016/j.yrtph.2018.06.003
Lowe D, Moore M, Evans B (1992) Contaminant impact on interactions of molecular probes with lysosomes in living hepatocytes from dab Limanda limanda. Mar Ecol Prog Ser 91:135–140. https://doi.org/10.3354/meps091135
Lowe D, Fossato V, Depledge M (1995a) Contaminant-induced lysosomal membrane damage in blood cells of mussels Mytilus galloprovincialis from the Venice Lagoon: an in vitro study. Mar Ecol Prog Ser 129:189–196. https://doi.org/10.3354/meps129189
Lowe DM, Soverchia C, Moore MN (1995b) Lysosomal membrane responses in the blood and digestive cells of mussels experimentally exposed to fluoranthene. Aquat Toxicol 33:105–112. https://doi.org/10.1016/0166-445X(95)00015-V
Massapina C, Joaquim S, Matias D, Devauchelle N (1999) Oocyte and embryo quality in Crassostrea gigas (Portuguese strain) during a spawning period in Algarve, South Portugal. Aquat Living Resour 12:327–333
Maunder MN, Thorson JT (2019) Modeling temporal variation in recruitment in fisheries stock assessment: a review of theory and practice. Fish Res 217:71–86
Mejuto J (1984) Primeros datos sobre la dinámica de la población de Cerastoderma edule (L.) de la Ría do Pasaxe (NW de Galicia): estrategias de explotación. Actas IV Simp Iber Estud Bentos Mar 1:83–102
Militz TA, Southgate PC (2021) Chapter 3: Culture of giant clams. In: Shumway, S (ed) Molluscan shellfish aquaculture: a practical guide. 5M Books Ltd, Essex, pp 61-85
Moens T, Beninger PG (2018) Meiofauna: an inconspicuous but important player in mudflat ecology. In: Beninger PG (ed) Mudflat Ecology. Springer International Publishing, Cham, pp 91–147
Nemes Z, Dietz R, Lüth JB, Gomba S, Hackenthal E, Gross F (1979) The pharmacological relevance of vital staining with neutral red. Experientia 35:1475–1476. https://doi.org/10.1007/BF01962793
Nevejan N, Courtens V, Hauva M, Gajardo G, Sorgeloos P (2003) Effect of lipid emulsions on production and fatty acid composition of eggs of the scallop Argopecten purpuratus. Mar Biol 143:327–338
Patetsini E, Dimitriadis VK, Kaloyianni M (2013) Biomarkers in marine mussels, Mytilus galloprovincialis, exposed to environmentally relevant levels of the pesticides, chlorpyrifos and penoxsulam. Aquat Toxicol 126:338–345. https://doi.org/10.1016/j.aquatox.2012.09.009
Pérez Camacho A, Román G (1984) Crecimiento, reproducción, mortalidad y producción del berberecho Cerastoderma edule (L.), en la Ría de Arousa. Cuad Area Cienc Mariñ Sem Est Gal 499–507
Plough LV (2018) Fine-scale temporal analysis of genotype-dependent mortality at settlement in the Pacific oyster Crassostrea gigas. J Exp Mar Biol Ecol 501:90–98
Plough L, Shin G, Hedgecock D (2016) Genetic inviability is a major driver of type III survivorship in experimental families of a highly fecund marine bivalve. Mol Ecol 25:895–910
Rajab GZE-A, Demer JL (2019) Long-term results of surgical excision of conjunctival retention cyst using trypan blue with methylcellulose. Am J Ophthalmol Case Rep 14:28–31. https://doi.org/10.1016/j.ajoc.2019.01.010
Repetto G, del Peso A, Zurita JL (2008) Neutral red uptake assay for the estimation of cell viability/cytotoxicity. Nat Protoc 3:1125–1131. https://doi.org/10.1038/nprot.2008.75
Shantharam AK, Padilla DK, Peterson BJ, Doall M, Lobue C, Webb A (2019) Macrofaunal community structure following the restocking of Northern Quahog (Mercenaria mercenaria) to Great South Bay, Long Island, NY. J Shelf Res 38:259–270. https://doi.org/10.2983/035.038.0206
Smolarz K, Hallmann A, Zabrzańska S, Pietrasik A (2017) Elevated gonadal atresia as biomarker of endocrine disruptors: field and experimental studies using Mytilus trossulus (L.) and 17-alpha ethinylestradiol (EE2). Mar Poll Bull 120:58–67. https://doi.org/10.1016/j.marpolbul.2017.04.007
Strober W (2015) Trypan Blue exclusion test of cell viability. Curr Protoc Immunol 111:A3.B.1-A3.B.3. doi:https://doi.org/10.1002/0471142735.ima03bs111
Svendsen C, Spurgeon DJ, Hankard PK, Weeks JM (2004) A review of lysosomal membrane stability measured by neutral red retention: is it a workable earthworm biomarker? Ecotoxicol Environ Safety 57:20–29. https://doi.org/10.1016/j.ecoenv.2003.08.009
Talbot P, Chacon RS (1981) A triple-stain technique for evaluating normal acrosome reactions of human sperm. J Exp Zool 215:201–208. https://doi.org/10.1002/jez.1402150210
Tang KW, Freund CS, Schweitzer CL (2006) Occurrence of copepod carcasses in the lower Chesapeake Bay and their decomposition by ambient microbes. Estuar Coast Shelf Sci 68:499–508
Triglia D, Braa SS, Yonan C, Naughton GK (1991) In vitro toxicity of various classes of test agents using the neutral red assay on a human three-dimensional physiologic skin model. In Vitro Cell Devel Biol-Animal 27:239–244
Uttig SD, Spencer BE (1991) The hatchery culture of bivalve mollusc larvae and juveniles. Laboratory Leaflet, Ministry of Agriculture, Fisheries and Food Fisheries Research, Lowestoft 68:31 pp
Utting SD, Millican P (1997) Techniques for the hatchery conditioning of bivalve broodstocks and the subsequent effect on egg quality and larval viability. Aquaculture 155:45–54
Valdez-Ramirez ME, Donval A, Le Pennec M (2002) Ultrastructural and histochemical criteria for determining normality in mature oocytes of the Pacific oyster Crassostrea gigas. J Shellfish Res 21:707–714
Wales R (1959) The differential staining of human and dog spermatozoa. Aust J Exp Biol Med 37:433–439. https://doi.org/10.1038/icb.1959.44
Weeks JM, Svendsen C (1996) Neutral red retention by lysosomes from earthworm (Lumbricus rubellus) coelomocytes: A simple biomarker of exposure to soil copper. Environ Toxicol Chem 15:1801–1805. https://doi.org/10.1002/etc.5620151022
Wijsman JWM, Troost K, Fang J, Roncarati A (2019) Global production of marine bivalves. Trends and challenges. In: Smaal AC, Ferreira JG, Grant J et al (eds) Goods and services of marine bivalves. Springer International Publishing, Cham, pp 7–26
Winckler J (1974) Vitalfärbung von Lysosomen und anderen Zellorganellen der Ratte mit Neutralrot. Prog Histochem Cyto 6:III–89. https://doi.org/10.1016/S0079-6336(74)80001-X
Wourms J (1987) Oogenesis. In: Reproduction of marine invertebrates, Vol 9: General aspects, seeking unity in diversity. Blackwell Scientifc Publications, Palo Alto, pp 117–124
Zetsche EM, Meysman FJ (2012) Dead or alive? Viability assessment of micro-and mesoplankton. J Plankton Res 34:493–509
Acknowledgements
We thank Mathilde Clairambault for her patience and perseverance in the laboratory spawning trials. We are indebted to David Berteau and employees of Chellet-Berteau Production for their warm welcome and permission to sample on-site.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed by the authors.
Conflict of interest
The authors declare no competing interests.
Additional information
Handling Editor: Gavin Burnell
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Beninger, P.G., Chérel, D. & Kessler, L. Examining bivalve fecundity: oocyte viability revealed by Neutral Red vital staining. Aquacult Int 29, 1219–1231 (2021). https://doi.org/10.1007/s10499-021-00686-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-021-00686-6