Abstract
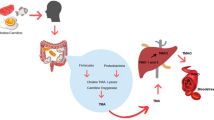
Trimethylamine (TMA) is a gut microbiota-derived metabolite which comes from diets rich of choline, betaine or l-carnitine and could be further converted to Trimethylamine-N-oxide (TMAO) in the liver. As the function of gut microbiota and its metabolites being explored so far, studies suggest that TMAO may be a potential risk factor of cardiovascular diseases independent of other traditional risk factors. However, the precise role of TMAO is controversial as some converse results were discovered. In recent studies, it is hypothesized that TMA may also participate in the progression of cardiovascular diseases and some cytotoxic effect of TMA has been discovered. Thus, exploring the relationship between TMA, TMAO and CVD may bring a novel insight into the diagnosis and therapy of cardiovascular diseases. In this review, we discussed the factors which influence the TMA/TMAO’s process of metabolism in the human body. We have also summarized the pathogenic effect of TMA/TMAO in cardiovascular diseases, as well as the limitation of some controversial discoveries.


Similar content being viewed by others
Abbreviations
- TMA:
-
Trimethylamine
- TMAO:
-
Trimethylamine-N-oxide
- AS:
-
Atherosclerosis
- DMB:
-
3, 3-Dimethyl-1butanol
- NLRP3:
-
Nucleotide-binding oligomerization domain-like receptor family pyrin domain containing 3
- γBB:
-
γ-Butyrobetaine
- PERK:
-
Protein kinase R-like endoplasmic reticulum kinase
- SR-A:
-
Scavenger receptor-A
- MAPK:
-
Mitogen-activated protein kinase
- JNK:
-
C-JUN NH2-terminal protein kinase
- NF-κB:
-
Nuclear factor kappa B
- CD36:
-
Cluster of differentiation 36
- HSP:
-
Heat shock protein
- GRP:
-
Glucose-related protein
- TXNIP:
-
Thioredoxin-interactive protein
- ROS:
-
Reactive oxygen species
- HMGB1:
-
High mobility group box 1
- TNF-α:
-
Tumor necrosis factor α
- IL-6:
-
Interleukin 6
- VCAM-1:
-
Vascular cell adhesion molecule 1
- PKC:
-
Protein kinase C
- SIRT3:
-
Sirtuin 3
- SOD2:
-
Superoxide dismutase 2
- ERS:
-
Endoplasmic reticulum stress
References
Koh, A., De Vadder, F., Kovatcheva-Datchary, P., & Bäckhed, F. (2016). From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell, 165, 1332–1345. https://doi.org/10.1016/j.cell.2016.05.041
Bu, F., Zhang, S., Duan, Z., Ding, Y., Chen, T., Wang, R., Feng, Z., Shi, G., Zhou, J., & Chen, Y. (2020). A critical review on the relationship of herbal medicine, Akkermansia muciniphila, and human health. Biomedicine & Pharmacotherapy, 128, 110352. https://doi.org/10.1016/j.biopha.2020.110352
Tedelind, S., Westberg, F., Kjerrulf, M., & Vidal, A. (2007). Anti-inflammatory properties of the short-chain fatty acids acetate and propionate: a study with relevance to inflammatory bowel disease. World Journal of Gastroenterology, 13, 2826–2832. https://doi.org/10.3748/wjg.v13.i20.2826
Heianza, Y., Ma, W., Manson, J. E., Rexrode, K. M., & Qi, L. (2017). Gut microbiota metabolites and risk of major adverse cardiovascular disease events and death: A systematic review and meta-analysis of prospective studies. Journal of the American Heart Association. https://doi.org/10.1161/jaha.116.004947
Moludi, J., Maleki, V., Jafari-Vayghyan, H., Vaghef-Mehrabany, E., & Alizadeh, M. (2020). Metabolic endotoxemia and cardiovascular disease: A systematic review about potential roles of prebiotics and probiotics. Clinical and Experimental Pharmacology and Physiology, 47, 927–939. https://doi.org/10.1111/1440-1681.13250
Koeth, R. A., Wang, Z., Levison, B. S., Buffa, J. A., Org, E., Sheehy, B. T., Britt, E. B., Fu, X., Wu, Y., Li, L., Smith, J. D., DiDonato, J. A., Chen, J., Li, H., Wu, G. D., Lewis, J. D., Warrier, M., Brown, J. M., Krauss, R. M., … Hazen, S. L. (2013). Intestinal microbiota metabolism of l-carnitine, a nutrient in red meat, promotes atherosclerosis. Natural Medicines, 19, 576–585. https://doi.org/10.1038/nm.3145
Ufnal, M., & Nowiński, A. (2019). Is increased plasma TMAO a compensatory response to hydrostatic and osmotic stress in cardiovascular diseases? Medical Hypotheses, 130, 109271. https://doi.org/10.1016/j.mehy.2019.109271
Wang, Z., Klipfell, E., Bennett, B. J., Koeth, R., Levison, B. S., Dugar, B., Feldstein, A. E., Britt, E. B., Fu, X., Chung, Y. M., Wu, Y., Schauer, P., Smith, J. D., Allayee, H., Tang, W. H., DiDonato, J. A., Lusis, A. J., & Hazen, S. L. (2011). Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature, 472, 57–63. https://doi.org/10.1038/nature09922
Papandreou, C., Moré, M., & Bellamine, A. (2020). Trimethylamine N-oxide in relation to cardiometabolic health-cause or effect? Nutrients. https://doi.org/10.3390/nu12051330
Roberts, A. B., Gu, X., Buffa, J. A., Hurd, A. G., Wang, Z., Zhu, W., Gupta, N., Skye, S. M., Cody, D. B., Levison, B. S., Barrington, W. T., Russell, M. W., Reed, J. M., Duzan, A., Lang, J. M., Fu, X., Li, L., Myers, A. J., Rachakonda, S., … Hazen, S. L. (2018). Development of a gut microbe-targeted nonlethal therapeutic to inhibit thrombosis potential. Natural Medicines, 24, 1407–1417. https://doi.org/10.1038/s41591-018-0128-1
Tang, W. H. W., Backhed, F., Landmesser, U., & Hazen, S. L. (2019). Intestinal Microbiota in Cardiovascular Health and Disease: JACC State-of-the-Art Review. Journal of the American College of Cardiology, 73, 2089–2105. https://doi.org/10.1016/j.jacc.2019.03.024
Zhu, Y., Jameson, E., Crosatti, M., Schäfer, H., Rajakumar, K., Bugg, T. D., & Chen, Y. (2014). Carnitine metabolism to trimethylamine by an unusual Rieske-type oxygenase from human microbiota. Proceedings of the National Academy of Sciences of the United States of America, 111, 4268–4273. https://doi.org/10.1073/pnas.1316569111
Jie, Z., Xia, H., Zhong, S. L., Feng, Q., Li, S., Liang, S., Zhong, H., Liu, Z., Gao, Y., Zhao, H., Zhang, D., Su, Z., Fang, Z., Lan, Z., Li, J., Xiao, L., Li, J., Li, R., Li, X., … Kristiansen, K. (2017). The gut microbiome in atherosclerotic cardiovascular disease. Nature Communications, 8, 845. https://doi.org/10.1038/s41467-017-00900-1
Koeth, R. A., Levison, B. S., Culley, M. K., Buffa, J. A., Wang, Z., Gregory, J. C., Org, E., Wu, Y., Li, L., Smith, J. D., Tang, W. H. W., DiDonato, J. A., Lusis, A. J., & Hazen, S. L. (2014). γ-Butyrobetaine is a proatherogenic intermediate in gut microbial metabolism of l-carnitine to TMAO. Cell Metabolism, 20, 799–812. https://doi.org/10.1016/j.cmet.2014.10.006
Yin, J., Liao, S. X., He, Y., Wang, S., Xia, G. H., Liu, F. T., Zhu, J. J., You, C., Chen, Q., Zhou, L., Pan, S. Y., & Zhou, H. W. (2015). Dysbiosis of gut microbiota with reduced trimethylamine-N-oxide level in patients with large-artery atherosclerotic stroke or transient ischemic attack. Journal of the American Heart Association. https://doi.org/10.1161/JAHA.115.002699
Xu, K. Y., Xia, G. H., Lu, J. Q., Chen, M. X., Zhen, X., Wang, S., You, C., Nie, J., Zhou, H. W., & Yin, J. (2017). Impaired renal function and dysbiosis of gut microbiota contribute to increased trimethylamine-N-oxide in chronic kidney disease patients. Science and Reports, 7, 1445. https://doi.org/10.1038/s41598-017-01387-y
Romano, K. A., Vivas, E. I., Amador-Noguez, D., & Rey, F. E. (2015). Intestinal microbiota composition modulates choline bioavailability from diet and accumulation of the proatherogenic metabolite trimethylamine-N-oxide. MBio. https://doi.org/10.1128/mBio.02481-14
Jaworska, K., Huc, T., Samborowska, E., Dobrowolski, L., Bielinska, K., Gawlak, M., & Ufnal, M. (2017). Hypertension in rats is associated with an increased permeability of the colon to TMA, a gut bacteria metabolite. PLoS ONE, 12, e0189310. https://doi.org/10.1371/journal.pone.0189310
Ufnal, M., Zadlo, A., & Ostaszewski, R. (2015). TMAO: A small molecule of great expectations. Nutrition, 31, 1317–1323. https://doi.org/10.1016/j.nut.2015.05.006
Koeth, R. A., Lam-Galvez, B. R., Kirsop, J., Wang, Z., Levison, B. S., Gu, X., Copeland, M. F., Bartlett, D., Cody, D. B., Dai, H. J., Culley, M. K., Li, X. S., Fu, X., Wu, Y., Li, L., DiDonato, J. A., Tang, W. H. W., Garcia-Garcia, J. C., & Hazen, S. L. (2019). l-Carnitine in omnivorous diets induces an atherogenic gut microbial pathway in humans. Journal of Clinical Investigation, 129, 373–387. https://doi.org/10.1172/jci94601
Lopez-Garcia, E., Rodriguez-Artalejo, F., Li, T. Y., Fung, T. T., Li, S., Willett, W. C., Rimm, E. B., & Hu, F. B. (2014). The Mediterranean-style dietary pattern and mortality among men and women with cardiovascular disease. American Journal of Clinical Nutrition, 99, 172–180. https://doi.org/10.3945/ajcn.113.068106
Griffin, L. E., Djuric, Z., Angiletta, C. J., Mitchell, C. M., Baugh, M. E., Davy, K. P., & Neilson, A. P. (2019). A Mediterranean diet does not alter plasma trimethylamine N-oxide concentrations in healthy adults at risk for colon cancer. Food & Function, 10, 2138–2147. https://doi.org/10.1039/c9fo00333a
Zhu, W., Buffa, J. A., Wang, Z., Warrier, M., Schugar, R., Shih, D. M., Gupta, N., Gregory, J. C., Org, E., Fu, X., Li, L., DiDonato, J. A., Lusis, A. J., Brown, J. M., & Hazen, S. L. (2018). Flavin monooxygenase 3, the host hepatic enzyme in the metaorganismal trimethylamine N-oxide-generating pathway, modulates platelet responsiveness and thrombosis risk. Journal of Thrombosis and Haemostasis, 16, 1857–1872. https://doi.org/10.1111/jth.14234
Miao, J., Ling, A. V., Manthena, P. V., Gearing, M. E., Graham, M. J., Crooke, R. M., Croce, K. J., Esquejo, R. M., Clish, C. B., Vicent, D., & Biddinger, S. B. (2015). Flavin-containing monooxygenase 3 as a potential player in diabetes-associated atherosclerosis. Nature Communications, 6, 6498. https://doi.org/10.1038/ncomms7498
Bennett, B. J., de Aguiar Vallim, T. Q., Wang, Z., Shih, D. M., Meng, Y., Gregory, J., Allayee, H., Lee, R., Graham, M., Crooke, R., Edwards, P. A., Hazen, S. L., & Lusis, A. J. (2013). Trimethylamine-N-oxide, a metabolite associated with atherosclerosis, exhibits complex genetic and dietary regulation. Cell Metabolism, 17, 49–60. https://doi.org/10.1016/j.cmet.2012.12.011
Welch, W. J., & Brown, C. R. (1996). Influence of molecular and chemical chaperones on protein folding. Cell Stress & Chaperones, 1, 109–115. https://doi.org/10.1379/1466-1268(1996)001
Ma, J., & Li, H. (2018). The Role of gut microbiota in atherosclerosis and hypertension. Frontiers in Pharmacology, 9, 1082. https://doi.org/10.3389/fphar.2018.01082
Ge, X., Zheng, L., Zhuang, R., Yu, P., Xu, Z., Liu, G., Xi, X., Zhou, X., & Fan, H. (2020). The gut microbial metabolite trimethylamine n-oxide and hypertension risk: a systematic review and dose-response meta-analysis. Advances in Nutrition, 11, 66–76. https://doi.org/10.1093/advances/nmz064
Zhang, W. Q., Wang, Y. J., Zhang, A., Ding, Y. J., Zhang, X. N., Jia, Q. J., Zhu, Y. P., Li, Y. Y., Lv, S. C., & Zhang, J. P. (2021). TMA/TMAO in hypertension: novel horizons and potential therapies. Journal of Cardiovascular Translational Research. https://doi.org/10.1007/s12265-021-10115-x
Ferrario, C. M. (2006). Role of angiotensin II in cardiovascular disease therapeutic implications of more than a century of research. Journal of the Renin Angiotensin Aldosterone System, 7, 3–14. https://doi.org/10.3317/jraas.2006.003
Karbach, S. H., Schonfelder, T., Brandao, I., Wilms, E., Hormann, N., Jackel, S., Schuler, R., Finger, S., Knorr, M., Lagrange, J., Brandt, M., Waisman, A., Kossmann, S., Schafer, K., Munzel, T., Reinhardt, C., & Wenzel, P. (2016). Gut microbiota promote angiotensin ii-induced arterial hypertension and vascular dysfunction. Journal of the American Heart Association. https://doi.org/10.1161/JAHA.116.003698
Ufnal, M., Jazwiec, R., Dadlez, M., Drapala, A., Sikora, M., & Skrzypecki, J. (2014). Trimethylamine-N-oxide: a carnitine-derived metabolite that prolongs the hypertensive effect of angiotensin II in rats. Canadian Journal of Cardiology, 30, 1700–1705. https://doi.org/10.1016/j.cjca.2014.09.010
Madhur, M. S., Lob, H. E., McCann, L. A., Iwakura, Y., Blinder, Y., Guzik, T. J., & Harrison, D. G. (2010). Interleukin 17 promotes angiotensin II-induced hypertension and vascular dysfunction. Hypertension, 55, 500–507. https://doi.org/10.1161/hypertensionaha.109.145094
Chen, M. L., Zhu, X. H., Ran, L., Lang, H. D., Yi, L., & Mi, M. T. (2017). Trimethylamine-N-oxide induces vascular inflammation by activating the NLRP3 inflammasome through the SIRT3-SOD2-mtROS signaling pathway. Journal of the American Heart Association. https://doi.org/10.1161/jaha.117.006347
Shaw, M. H., Kamada, N., Kim, Y. G., & Nunez, G. (2012). Microbiota-induced IL-1beta, but not IL-6, is critical for the development of steady-state TH17 cells in the intestine. Journal of Experimental Medicine, 209, 251–258. https://doi.org/10.1084/jem.20111703
Boini, K. M., Hussain, T., Li, P. L., & Koka, S. (2017). Trimethylamine-N-oxide instigates NLRP3 inflammasome activation and endothelial dysfunction. Cellular Physiology and Biochemistry, 44, 152–162. https://doi.org/10.1159/000484623
Dannenberg, L., Zikeli, D., Benkhoff, M., Ahlbrecht, S., Kelm, M., Levkau, B., & Polzin, A. (2020). Targeting the human microbiome and its metabolite TMAO in cardiovascular prevention and therapy. Pharmacology & Therapeutics. https://doi.org/10.1016/j.pharmthera.2020.107584
Chen, H., Li, J., Li, N., Liu, H., & Tang, J. (2019). Increased circulating trimethylamine N-oxide plays a contributory role in the development of endothelial dysfunction and hypertension in the RUPP rat model of preeclampsia. Hypertension in Pregnancy., 38, 96–104. https://doi.org/10.1080/10641955.2019.1584630
Hsu, C. N., Chang-Chien, G. P., Lin, S., Hou, C. Y., & Tain, Y. L. (2019). Targeting on gut microbial metabolite trimethylamine-N-oxide and short-chain fatty acid to prevent maternal high-fructose-diet-induced developmental programming of hypertension in adult male offspring. Molecular Nutrition & Food Research, 63, e1900073. https://doi.org/10.1002/mnfr.201900073
Yancey, P. H. (2005). Organic osmolytes as compatible, metabolic and counteracting cytoprotectants in high osmolarity and other stresses. Journal of Experimental Biology, 208, 2819–2830. https://doi.org/10.1242/jeb.01730
Huc, T., Drapala, A., Gawrys, M., Konop, M., Bielinska, K., Zaorska, E., Samborowska, E., Wyczalkowska-Tomasik, A., Paczek, L., Dadlez, M., & Ufnal, M. (2018). Chronic, low-dose TMAO treatment reduces diastolic dysfunction and heart fibrosis in hypertensive rats. American Journal of Physiology:Heart and Circulatory Physiology, 315, H1805–H1820. https://doi.org/10.1152/ajpheart.00536.2018
Wang, Z., Roberts, A. B., Buffa, J. A., Levison, B. S., Zhu, W., Org, E., Gu, X., Huang, Y., Zamanian-Daryoush, M., Culley, M. K., DiDonato, A. J., Fu, X., Hazen, J. E., Krajcik, D., DiDonato, J. A., Lusis, A. J., & Hazen, S. L. (2015). Non-lethal inhibition of gut microbial trimethylamine production for the treatment of atherosclerosis. Cell, 163, 1585–1595. https://doi.org/10.1016/j.cell.2015.11.055
Li, X., Geng, J., Zhao, J., Ni, Q., Zhao, C., Zheng, Y., Chen, X., & Wang, L. (2019). Trimethylamine N-oxide exacerbates cardiac fibrosis via activating the NLRP3 inflammasome. Frontiers in Physiology., 10, 866. https://doi.org/10.3389/fphys.2019.00866
Sun, X., Jiao, X., Ma, Y., Liu, Y., Zhang, L., He, Y., & Chen, Y. (2016). Trimethylamine N-oxide induces inflammation and endothelial dysfunction in human umbilical vein endothelial cells via activating ROS-TXNIP-NLRP3 inflammasome. Biochemical and Biophysical Research Communications, 481, 63–70. https://doi.org/10.1016/j.bbrc.2016.11.017
Sho, T., & Xu, J. (2019). Role and mechanism of ROS scavengers in alleviating NLRP3-mediated inflammation. Biotechnology and Applied Biochemistry, 66, 4–13. https://doi.org/10.1002/bab.1700
Seldin, M. M., Meng, Y., Qi, H., Zhu, W., Wang, Z., Hazen, S. L., Lusis, A. J., & Shih, D. M. (2016). Trimethylamine N-oxide promotes vascular inflammation through signaling of mitogen-activated protein kinase and nuclear factor-κB. Journal of the American Heart Association. https://doi.org/10.1161/jaha.115.002767
Singh, G. B., Zhang, Y., Boini, K. M., & Koka, S. (2019). High mobility group box 1 mediates TMAO-induced endothelial dysfunction. International Journal of Molecular Sciences. https://doi.org/10.3390/ijms20143570
Ma, G., Pan, B., Chen, Y., Guo, C., Zhao, M., Zheng, L., & Chen, B. (2017). Trimethylamine N-oxide in atherogenesis: impairing endothelial self-repair capacity and enhancing monocyte adhesion. Bioscience Reports. https://doi.org/10.1042/bsr20160244
Ke, Y., Li, D., Zhao, M., Liu, C., Liu, J., Zeng, A., Shi, X., Cheng, S., Pan, B., Zheng, L., & Hong, H. (2018). Gut flora-dependent metabolite Trimethylamine-N-oxide accelerates endothelial cell senescence and vascular aging through oxidative stress. Free Radical Biology & Medicine, 116, 88–100. https://doi.org/10.1016/j.freeradbiomed.2018.01.007
Geng, J., Yang, C., Wang, B., Zhang, X., Hu, T., Gu, Y., & Li, J. (2018). Trimethylamine N-oxide promotes atherosclerosis via CD36-dependent MAPK/JNK pathway. Biomedicine & Pharmacotherapy, 97, 941–947. https://doi.org/10.1016/j.biopha.2017.11.016
Mohammadi, A., Vahabzadeh, Z., Jamalzadeh, S., & Khalili, T. (2018). Trimethylamine-N-oxide, as a risk factor for atherosclerosis, induces stress in J774A.1 murine macrophages. Advances in Medical Sciences, 63, 57–63. https://doi.org/10.1016/j.advms.2017.06.006
Mohammadi, A., Gholamhoseyniannajar, A., Yaghoobi, M. M., Jahani, Y., & Vahabzadeh, Z. (2015). Expression levels of heat shock protein 60 and glucose-regulated protein 78 in response to trimethylamine-N-oxide treatment in murine macrophage J774A.1 cell line. Cellular and Molecular Biology (Noisy-Le-Grand), 61, 94–100
Yang, S., Li, X., Yang, F., Zhao, R., Pan, X., Liang, J., Tian, L., Li, X., Liu, L., Xing, Y., & Wu, M. (2019). Gut microbiota-dependent marker TMAO in promoting cardiovascular disease: inflammation mechanism, clinical prognostic, and potential as a therapeutic target. Frontiers in Pharmacology, 10, 1360. https://doi.org/10.3389/fphar.2019.01360
Ross, R., & Glomset, J. A. (1973). Atherosclerosis and the arterial smooth muscle cell: Proliferation of smooth muscle is a key event in the genesis of the lesions of atherosclerosis. Science, 180, 1332–1339. https://doi.org/10.1126/science.180.4093.1332
Charach, G., Rabinovich, P. D., Konikoff, F. M., Grosskopf, I., Weintraub, M. S., & Gilat, T. (1998). Decreased fecal bile acid output in patients with coronary atherosclerosis. Journal of Medicine, 29, 125–136
Ross, R. (1999). Atherosclerosis–an inflammatory disease. New England Journal of Medicine, 340, 115–126. https://doi.org/10.1056/nejm199901143400207
Liu, X., Xie, Z., Sun, M., Wang, X., Li, J., Cui, J., Zhang, F., Yin, L., Huang, D., Hou, J., Tian, J., & Yu, B. (2018). Plasma trimethylamine N-oxide is associated with vulnerable plaque characteristics in CAD patients as assessed by optical coherence tomography. International Journal of Cardiology, 265, 18–23. https://doi.org/10.1016/j.ijcard.2018.04.126
Zhu, W., Gregory, J. C., Org, E., Buffa, J. A., Gupta, N., Wang, Z., Li, L., Fu, X., Wu, Y., Mehrabian, M., Sartor, R. B., McIntyre, T. M., Silverstein, R. L., Tang, W. H. W., DiDonato, J. A., Brown, J. M., Lusis, A. J., & Hazen, S. L. (2016). Gut microbial metabolite TMAO enhances platelet hyperreactivity and thrombosis risk. Cell, 165, 111–124. https://doi.org/10.1016/j.cell.2016.02.011
Haissman, J. M., Haugaard, A. K., Ostrowski, S. R., Berge, R. K., Hov, J. R., Trøseid, M., & Nielsen, S. D. (2017). Microbiota-dependent metabolite and cardiovascular disease marker trimethylamine-N-oxide (TMAO) is associated with monocyte activation but not platelet function in untreated HIV infection. BMC Infectious Diseases, 17, 445. https://doi.org/10.1186/s12879-017-2547-x
Nam, H. S. (2019). Gut microbiota and ischemic stroke: The role of trimethylamine N-oxide. Journal of Stroke, 21, 151–159. https://doi.org/10.5853/jos.2019.00472
Farhangi, M. A., Vajdi, M., & Asghari-Jafarabadi, M. (2020). Gut microbiota-associated metabolite trimethylamine N-Oxide and the risk of stroke: a systematic review and dose-response meta-analysis. Nutrition Journal, 19, 76. https://doi.org/10.1186/s12937-020-00592-2
Senthong, V., Wang, Z., Fan, Y., Wu, Y., Hazen, S. L., & Tang, W. H. (2016). Trimethylamine N-oxide and mortality risk in patients with peripheral artery disease. Journal of the American Heart Association. https://doi.org/10.1161/jaha.116.004237
Randrianarisoa, E., Lehn-Stefan, A., Wang, X., Hoene, M., Peter, A., Heinzmann, S. S., Zhao, X., Königsrainer, I., Königsrainer, A., Balletshofer, B., Machann, J., Schick, F., Fritsche, A., Häring, H. U., Xu, G., Lehmann, R., & Stefan, N. (2016). Relationship of serum trimethylamine N-oxide (TMAO) levels with early atherosclerosis in humans. Science and Reports, 6, 26745. https://doi.org/10.1038/srep26745
Tang, W. H., Wang, Z., Fan, Y., Levison, B., Hazen, J. E., Donahue, L. M., Wu, Y., & Hazen, S. L. (2014). Prognostic value of elevated levels of intestinal microbe-generated metabolite trimethylamine-N-oxide in patients with heart failure: refining the gut hypothesis. Journal of the American College of Cardiology, 64, 1908–1914. https://doi.org/10.1016/j.jacc.2014.02.617
Dambrova, M., Latkovskis, G., Kuka, J., Strele, I., Konrade, I., Grinberga, S., Hartmane, D., Pugovics, O., Erglis, A., & Liepinsh, E. (2016). Diabetes is associated with higher trimethylamine N-oxide plasma levels. Experimental and Clinical Endocrinology and Diabetes, 124, 251–256. https://doi.org/10.1055/s-0035-1569330
Shan, Z., Sun, T., Huang, H., Chen, S., Chen, L., Luo, C., Yang, W., Yang, X., Yao, P., Cheng, J., Hu, F. B., & Liu, L. (2017). Association between microbiota-dependent metabolite trimethylamine-N-oxide and type 2 diabetes. American Journal of Clinical Nutrition, 106, 888–894. https://doi.org/10.3945/ajcn.117.157107
Henning, R. J. (2018). Type-2 diabetes mellitus and cardiovascular disease. Future Cardiology, 14, 491–509. https://doi.org/10.2217/fca-2018-0045
Chen, S., Henderson, A., Petriello, M. C., Romano, K. A., Gearing, M., Miao, J., Schell, M., Sandoval-Espinola, W. J., Tao, J., Sha, B., Graham, M., Crooke, R., Kleinridders, A., Balskus, E. P., Rey, F. E., Morris, A. J., & Biddinger, S. B. (2019). Trimethylamine N-Oxide Binds and Activates PERK to Promote Metabolic Dysfunction. Cell Metabolism, 30, 1141-1151.e1145. https://doi.org/10.1016/j.cmet.2019.08.021
Lupachyk, S., Watcho, P., Stavniichuk, R., Shevalye, H., & Obrosova, I. G. (2013). Endoplasmic reticulum stress plays a key role in the pathogenesis of diabetic peripheral neuropathy. Diabetes, 62, 944–952. https://doi.org/10.2337/db12-0716
Senft, D., & Ronai, Z. A. (2015). UPR, autophagy, and mitochondria crosstalk underlies the ER stress response. Trends in Biochemical Sciences, 40, 141–148. https://doi.org/10.1016/j.tibs.2015.01.002
Papandreou, C., Bulló, M., Zheng, Y., Ruiz-Canela, M., Yu, E., Guasch-Ferré, M., Toledo, E., Clish, C., Corella, D., Estruch, R., Ros, E., Fitó, M., Arós, F., Fiol, M., Lapetra, J., Serra-Majem, L., Gómez-Gracia, E., Liang, L., Fragkiadakis, G. A., & Salas-Salvadó, J. (2018). Plasma trimethylamine-N-oxide and related metabolites are associated with type 2 diabetes risk in the Prevención con Dieta Mediterránea (PREDIMED) trial. American Journal of Clinical Nutrition, 108, 163–173. https://doi.org/10.1093/ajcn/nqy058
Dumas, M. E., Rothwell, A. R., Hoyles, L., Aranias, T., Chilloux, J., Calderari, S., Noll, E. M., Péan, N., Boulangé, C. L., Blancher, C., Barton, R. H., Gu, Q., Fearnside, J. F., Deshayes, C., Hue, C., Scott, J., Nicholson, J. K., & Gauguier, D. (2017). Microbial-host co-metabolites are prodromal markers predicting phenotypic heterogeneity in behavior, obesity, and impaired glucose tolerance. Cell Reports, 20, 136–148. https://doi.org/10.1016/j.celrep.2017.06.039
Tang, W. H., Wang, Z., Shrestha, K., Borowski, A. G., Wu, Y., Troughton, R. W., Klein, A. L., & Hazen, S. L. (2015). Intestinal microbiota-dependent phosphatidylcholine metabolites, diastolic dysfunction, and adverse clinical outcomes in chronic systolic heart failure. Journal of Cardiac Failure, 21, 91–96. https://doi.org/10.1016/j.cardfail.2014.11.006
Trøseid, M., Ueland, T., Hov, J. R., Svardal, A., Gregersen, I., Dahl, C. P., Aakhus, S., Gude, E., Bjørndal, B., Halvorsen, B., Karlsen, T. H., Aukrust, P., Gullestad, L., Berge, R. K., & Yndestad, A. (2015). Microbiota-dependent metabolite trimethylamine-N-oxide is associated with disease severity and survival of patients with chronic heart failure. Journal of Internal Medicine, 277, 717–726. https://doi.org/10.1111/joim.12328
Hayashi, T., Yamashita, T., Watanabe, H., Kami, K., Yoshida, N., Tabata, T., Emoto, T., Sasaki, N., Mizoguchi, T., Irino, Y., Toh, R., Shinohara, M., Okada, Y., Ogawa, W., Yamada, T., & Hirata, K. I. (2018). Gut Microbiome and plasma microbiome-related metabolites in patients with decompensated and compensated heart failure. Circulation Journal, 83, 182–192. https://doi.org/10.1253/circj.CJ-18-0468
Organ, C. L., Otsuka, H., Bhushan, S., Wang, Z., Bradley, J., Trivedi, R., Polhemus, D. J., Tang, W. H., Wu, Y., Hazen, S. L., & Lefer, D. J. (2016). choline diet and its gut microbe-derived metabolite, trimethylamine N-Oxide, exacerbate pressure overload-induced heart failure. Circulation. Heart Failure, 9, e002314. https://doi.org/10.1161/circheartfailure.115.002314
Meng, G., Zhou, X., Wang, M., Zhou, L., Wang, Z., Wang, M., Deng, J., Wang, Y., Zhou, Z., Zhang, Y., Lai, Y., Zhang, Q., Yang, X., Yu, L., & Jiang, H. (2019). Gut microbe-derived metabolite trimethylamine N-oxide activates the cardiac autonomic nervous system and facilitates ischemia-induced ventricular arrhythmia via two different pathways. eBioMedicine, 44, 656–664. https://doi.org/10.1016/j.ebiom.2019.03.066
Yu, L., Meng, G., Huang, B., Zhou, X., Stavrakis, S., Wang, M., Li, X., Zhou, L., Wang, Y., Wang, M., Wang, Z., Deng, J., Po, S. S., & Jiang, H. (2018). A potential relationship between gut microbes and atrial fibrillation: Trimethylamine N-oxide, a gut microbe-derived metabolite, facilitates the progression of atrial fibrillation. International Journal of Cardiology, 255, 92–98. https://doi.org/10.1016/j.ijcard.2017.11.071
Jia, Q., Li, H., Zhou, H., Zhang, X., Zhang, A., Xie, Y., Li, Y., Lv, S., & Zhang, J. (2019). Role and effective therapeutic target of gut microbiota in heart failure. Cardiovascular Therapeutic, 2019, 5164298. https://doi.org/10.1155/2019/5164298
Li, Z., Wu, Z., Yan, J., Liu, H., Liu, Q., Deng, Y., Ou, C., & Chen, M. (2019). Gut microbe-derived metabolite trimethylamine N-oxide induces cardiac hypertrophy and fibrosis. Laboratory Investigation, 99, 346–357. https://doi.org/10.1038/s41374-018-0091-y
Chen, K., Zheng, X., Feng, M., Li, D., & Zhang, H. (2017). Gut Microbiota-dependent metabolite trimethylamine N-oxide contributes to cardiac dysfunction in western diet-induced obese mice. Frontiers in Physiology, 8, 139. https://doi.org/10.3389/fphys.2017.00139
Savi, M., Bocchi, L., Bresciani, L., Falco, A., Quaini, F., Mena, P., Brighenti, F., Crozier, A., Stilli, D., & Del Rio, D. (2018). Trimethylamine-N-oxide (TMAO)-induced impairment of cardiomyocyte function and the protective role of urolithin B-glucuronide. Molecules. https://doi.org/10.3390/molecules23030549
Makrecka-Kuka, M., Volska, K., Antone, U., Vilskersts, R., Grinberga, S., Bandere, D., Liepinsh, E., & Dambrova, M. (2017). Trimethylamine N-oxide impairs pyruvate and fatty acid oxidation in cardiac mitochondria. Toxicology Letters, 267, 32–38. https://doi.org/10.1016/j.toxlet.2016.12.017
Gawrys-Kopczynska, M., Konop, M., Maksymiuk, K., Kraszewska, K., Derzsi, L., Sozanski, K., Holyst, R., Pilz, M., Samborowska, E., Dobrowolski, L., Jaworska, K., Mogilnicka, I., & Ufnal, M. (2020). TMAO, a seafood-derived molecule, produces diuresis and reduces mortality in heart failure rats. eLife. https://doi.org/10.7554/eLife.57028
Querio, G., Antoniotti, S., Levi, R., & Gallo, M. P. (2019). Trimethylamine N-oxide does not impact viability, ros production, and mitochondrial membrane potential of adult rat cardiomyocytes. International Journal of Molecular Sciences. https://doi.org/10.3390/ijms20123045
Albert, C. L., & Tang, W. H. W. (2018). Metabolic biomarkers in heart failure. Heart Failure Clinics, 14, 109–118. https://doi.org/10.1016/j.hfc.2017.08.011
Schiattarella, G.G., Sannino, A., Toscano, E., Giugliano, G., Gargiulo, G., Franzone, A., Trimarco, B., Esposito, G. and Perrino, C. (2017). Gut microbe-generated metabolite trimethylamine-N-oxide as cardiovascular risk biomarker: a systematic review and dose-response meta-analysis. European Heart Journal 38:2948–2956. https://doi.org/10.1093/eurheartj/ehx342
Li, X. S., Obeid, S., Klingenberg, R., Gencer, B., Mach, F., Raber, L., Windecker, S., Rodondi, N., Nanchen, D., Muller, O., Miranda, M. X., Matter, C. M., Wu, Y., Li, L., Wang, Z., Alamri, H. S., Gogonea, V., Chung, Y. M., Tang, W. H., … Luscher, T. F. (2017). Gut microbiota-dependent trimethylamine N-oxide in acute coronary syndromes: a prognostic marker for incident cardiovascular events beyond traditional risk factors. European Heart Journal, 38, 814–824. https://doi.org/10.1093/eurheartj/ehw582
Suzuki, T., Heaney, L. M., Jones, D. J., & Ng, L. L. (2017). Trimethylamine N-oxide and risk stratification after acute myocardial infarction. Clinical Chemistry, 63, 420–428. https://doi.org/10.1373/clinchem.2016.264853
Jaworska, K., Bielinska, K., Gawrys-Kopczynska, M., & Ufnal, M. (2019). TMA (trimethylamine), but not its oxide TMAO (trimethylamine-oxide), exerts haemodynamic effects: implications for interpretation of cardiovascular actions of gut microbiome. Cardiovascular Research, 115, 1948–1949. https://doi.org/10.1093/cvr/cvz231
Jaworska, K., Hering, D., Mosieniak, G., Bielak-Zmijewska, A., Pilz, M., Konwerski, M., Gasecka, A., Kaplon-Cieslicka, A., Filipiak, K., Sikora, E., Holyst, R., & Ufnal, M. (2019). TMA, a forgotten uremic toxin, but not TMAO is involved in cardiovascular pathology. Toxins (Basel). https://doi.org/10.3390/toxins11090490
Suska, A., Ibanez, A. B., Lundstrom, I., & Berghard, A. (2009). G protein-coupled receptor mediated trimethylamine sensing. Biosensors & Bioelectronics, 25, 715–720. https://doi.org/10.1016/j.bios.2009.08.012
Chhibber-Goel, J., Gaur, A., Singhal, V., Parakh, N., Bhargava, B., & Sharma, A. (2016). The complex metabolism of trimethylamine in humans: endogenous and exogenous sources. Expert Reviews in Molecular Medicine, 18, e8. https://doi.org/10.1017/erm.2016.6
Jaworska, K., Konop, M., Hutsch, T., Perlejewski, K., Radkowski, M., Grochowska, M., Bielak-Zmijewska, A., Mosieniak, G., Sikora, E., & Ufnal, M. (2020). Trimethylamine But not trimethylamine oxide increases with age in rat plasma and affects smooth muscle cells viability. Journals of Gerontology Series A, Biological Sciences and Medical Sciences, 75, 1276–1283. https://doi.org/10.1093/gerona/glz181
Restini, C. B. A., Fink, G. D., & Watts, S. W. (2021). Vascular reactivity stimulated by TMA and TMAO: Are perivascular adipose tissue and endothelium involved? Pharmacological Research, 163, 105273. https://doi.org/10.1016/j.phrs.2020.105273
Gao, X., Liu, X., Xu, J., Xue, C., Xue, Y., & Wang, Y. (2014). Dietary trimethylamine N-oxide exacerbates impaired glucose tolerance in mice fed a high fat diet. Journal of Bioscience and Bioengineering, 118, 476–481. https://doi.org/10.1016/j.jbiosc.2014.03.001
Acknowledgements
This study was supported by the National Natural Science Foundation of China (Grant Nos. 82070299, 81870221, 81670249 and 31271226 to Dr. Wei Jiang)
Funding
National Natural Science Foundation of China (Grant Nos. 82070299, 81870221, 81670249 and 31271226 to Dr. Wei Jiang).
Author information
Authors and Affiliations
Contributions
SH was responsible for the collection and arrangement of articles associated with TMA/TMAO and cardiovascular diseases, manuscript writing and revision. HJ was responsible for figures drawing and part of the manuscript writing. CZ was responsible for the organization and revision of the manuscript. WJ made important intellectual contribution in research design, technical guidance and manuscript revision.
Corresponding author
Ethics declarations
Conflicts of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Handling Editor as “Dipak K Dube”.
Rights and permissions
About this article
Cite this article
He, S., Jiang, H., Zhuo, C. et al. Trimethylamine/Trimethylamine-N-Oxide as a Key Between Diet and Cardiovascular Diseases. Cardiovasc Toxicol 21, 593–604 (2021). https://doi.org/10.1007/s12012-021-09656-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12012-021-09656-z