Abstract
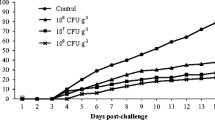
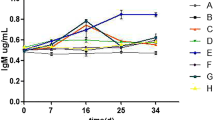
The effect of Lactococcus lactis subsp. lactis strain PTCC 1403 as a potential probiotic was investigated on the growth, hematobiochemical, immune responses, and resistance to Yersinia ruckeri infection in rainbow trout. A total of 240 fish were distributed into 12 fiberglass tanks representing four groups (× 3 replicates). Each tank was stocked with 20 fish (average initial weight: 11.81 ± 0.32 g) and fed L. lactis subsp. lactis PTCC 1403 at 0 (control, T0), 1 × 109 (T1), 2 × 109 (T2), and 3 × 109 (T3) CFU/g feed for 8 weeks. The results showed enhanced protein efficiency ratio and reduced feed conversion ratio in the fish-fed T2 diet. Further, fish-fed T2 and T3 diets showed a significantly higher survival rate than the control (p < 0.05). Trypsin, lipase, and protease activities were increased in fish-fed L. lactis subsp. lactis PTCC 1403 compared to the control (p < 0.05). Fish fed with a T2 diet showed significantly (p < 0.05) lower glucose content than other groups. The blood lysozyme activity and IgM showed significantly (p < 0.05) higher values in fish-fed T2 and T3 diets than in other groups. The antioxidative responses were increased in fish-fed T2 and T3 diets (p < 0.05). After 7 days post-Y. ruckeri challenge, the cumulative mortality rate showed the lowest value in fish fed with T1 and T2 diets, while the highest value was recorded in the control group. In conclusion, the results revealed beneficial effects of L. lactis subsp. lactis PTCC 1403 on the feed efficiency, immune response, and resistance to Y. ruckeri infection in rainbow trout.



Similar content being viewed by others
Data Availability
Data available on request due to privacy/ethical restrictions (The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions).
References
Dawood MAO, El Basuini MF, Zaineldin AI, Yilmaz S, Hasan MT, Ahmadifar E et al (2021) Antiparasitic and antibacterial functionality of essential oils: an alternative approach for sustainable aquaculture. Pathogens 10:185. https://doi.org/10.3390/pathogens10020185
Mohammadian T, Nasirpour M, Tabandeh MR, Heidary AA, Ghanei-Motlagh R, Hosseini SS (2019) Administrations of autochthonous probiotics altered juvenile rainbow trout Oncorhynchus mykiss health status, growth performance and resistance to Lactococcus garvieae, an experimental infection. Fish Shellfish Immunol 86:269–279. https://doi.org/10.1016/j.fsi.2018.11.052
Kakoolaki S, Akbary P, Zorriehzahra MJ, Salehi H, Sepahdari A, Afsharnasab M et al (2016) Camellia sinensis supplemented diet enhances the innate non-specific responses, haematological parameters and growth performance in Mugil cephalus against Photobacterium damselae. Fish Shellfish Immunol 57:379–385. https://doi.org/10.1016/j.fsi.2016.08.060
Cottrell RS, Blanchard JL, Halpern BS, Metian M, Froehlich HE (2020) Global adoption of novel aquaculture feeds could substantially reduce forage fish demand by 2030. Nat Food 1:301–308. https://doi.org/10.1038/s43016-020-0078-x
Akbary P, Aminikhoei Z (2018) Effect of water-soluble polysaccharide extract from the green alga Ulva rigida on growth performance, antioxidant enzyme activity, and immune stimulation of grey mullet Mugil cephalus. J Appl Phycol 30:1345–1353. https://doi.org/10.1007/s10811-017-1299-8
Akbary P, Jahanbakhshi A (2018) Growth yield, survival, carcass quality, haematological, biochemical parameters and innate immune responses in the grey mullet (Mugil cephalus Linneaus, 1758) fingerling induced by Immunogen® prebiotic. J Appl Anim Res 46:10–16. https://doi.org/10.1080/09712119.2016.1251927
Ringø E, Hoseinifar SH, Ghosh K, Doan HV, Beck BR, Song SK (2018) Lactic acid bacteria in finfish—an update. Front Microbiol 9:1818. https://doi.org/10.3389/fmicb.2018.01818
Dawood MA, Abo-Al-Ela HG, Hasan MT (2020) Modulation of transcriptomic profile in aquatic animals: probiotics, prebiotics and synbiotics scenarios. Fish Shellfish Immunol 97:268–282. https://doi.org/10.1016/j.fsi.2019.12.054
Dawood MAO (2021) Nutritional immunity of fish intestines: important insights for sustainable aquaculture. Rev Aquacult 13:642–663. https://doi.org/10.1111/raq.12492
Knipe H, Temperton B, Lange A, Bass D, Tyler CR (2021) Probiotics and competitive exclusion of pathogens in shrimp aquaculture. Rev Aquacult 13:324–352. https://doi.org/10.1111/raq.12477
Zaineldin AI, Hegazi S, Koshio S, Ishikawa M, Dawood MA, Dossou S et al (2020) Singular effects of Bacillus subtilis C-3102 or Saccharomyces cerevisiae type 1 on the growth, gut morphology, immunity, and stress resistance of red sea bream (Pagrus major) Ann Anim Sci 1. https://doi.org/10.2478/aoas-2020-0075
Kim D, Beck BR, Lee SM, Jeon J, Lee DW, Lee JI et al (2016) Pellet feed adsorbed with the recombinant Lactococcus lactis BFE920 expressing SiMA antigen induced strong recall vaccine effects against Streptococcus iniae infection in olive flounder (Paralichthys olivaceus). Fish Shellfish Immunol 55:374–383. https://doi.org/10.1016/j.fsi.2016.06.010
Xia Y, Lu M, Chen G, Cao J, Gao F, Wang M et al (2018) Effects of dietary Lactobacillus rhamnosus JCM1136 and Lactococcus lactis subsp. lactis JCM5805 on the growth, intestinal microbiota, morphology, immune response and disease resistance of juvenile Nile tilapia, Oreochromis niloticus. Fish Shellfish Immunol 76:368–379. https://doi.org/10.1016/j.fsi.2018.03.020
Hasan MT, Jang WJ, Tak JY, Lee BJ, Kim KW, Hur SW et al (2018) Effects of Lactococcus lactis subsp. lactis I2 with beta-glucooligosaccharides on growth, innate immunity and streptococcosis resistance in Olive flounder (Paralichthys olivaceus). Appl Microbiol Biotechnol 28:1433–1442. https://doi.org/10.4014/jmb.1805.05011
Dawood MA, Koshio S, Ishikawa M, Yokoyama S, El Basuini MF, Hossain MS et al (2016) Effects of dietary supplementation of Lactobacillus rhamnosus or/and Lactococcus lactis on the growth, gut microbiota and immune responses of red sea bream, Pagrus major. Fish Shellfish Immunol 49:275–285. https://doi.org/10.1016/j.fsi.2015.12.047
Beck BR, Kim D, Jeon J, Lee S-M, Kim HK, Kim O-J et al (2015) The effects of combined dietary probiotics Lactococcus lactis BFE920 and Lactobacillus plantarum FGL0001 on innate immunity and disease resistance in olive flounder (Paralichthys olivaceus). Fish Shellfish Immunol 42:177–183. https://doi.org/10.1016/j.fsi.2014.10.035
Kim D, Beck BR, Heo S-B, Kim J, Kim HD, Lee S-M et al (2013) Lactococcus lactis BFE920 activates the innate immune system of olive flounder (Paralichthys olivaceus), resulting in protection against Streptococcus iniae infection and enhancing feed efficiency and weight gain in large-scale field studies. Fish Shellfish Immunol 35:1585–1590. https://doi.org/10.1016/j.fsi.2013.09.008
Feng J, Chang X, Zhang Y, Yan X, Zhang J, Nie G (2019) Effects of Lactococcus lactis from Cyprinus carpio L. as probiotics on growth performance, innate immune response and disease resistance against Aeromonas hydrophila. Fish Shellfish Immunol 93:73–81. https://doi.org/10.1016/j.fsi.2019.07.028
Yao J-Y, Yuan X-M, Xu Y, Yin W-L, Lin L-Y, Pan X-Y et al (2016) Live recombinant Lactococcus lactis vaccine expressing immobilization antigen (i-Ag) for protection against Ichthyophthirius multifiliis in goldfish. Fish Shellfish Immunol 58:302–308. https://doi.org/10.1016/j.fsi.2016.09.037
Dini P, Mottaghian P, Ataie Amarloie O, Farhoodi M (2012) Comparison of the antagonistic effects of Lactococcus lactis PTCC 1403 and isolated vaginal lactobacilli against uterine and udder pathogens of dairy cows. Int J Probiotics Prebiotics 74
Ghasemzadeh J, Shekooh Saljughi Z, Akbary P, Hasani M (2018) Effects of dietary probiotic, Lactococcus lactis “subspecies PTCC 1403” on the growth parameters and survival rate of grey mullet (Mugil cephalus L.) against Lactococcus garvieae bacteria. J Anim Environ 10:367–374. http://www.aejournal.ir/article_87689.html?lang=en
Barghaman H, Yeganeh S, Keramat Amirkolaie A (2019) Effect of probiotic Lactococcus lactis (PTCC 1403) and chitin on blood and serum biochemical parameters and intestine bacteria of common carp (Cyprinus carpio). ISFJ 28:143–156. http://isfj.ir/article-1-2258-en.html
Adel M, Dawood MA, Shafiei S, Sakhaie F, Shekarabi SPH (2020) Dietary Polygonum minus extract ameliorated the growth performance, humoral immune parameters, immune-related gene expression and resistance against Yersinia ruckeri in rainbow trout (Oncorhynchus mykiss). Aquaculture 519:734738. https://doi.org/10.1016/j.aquaculture.2019.734738
Andani HRR, Tukmechi A, Meshkini S, Sheikhzadeh N (2012) Antagonistic activity of two potential probiotic bacteria from fish intestines and investigation of their effects on growth performance and immune response in rainbow trout (Oncorhynchus mykiss). J Appl Ichthyol 28:728–734. https://doi.org/10.1111/j.1439-0426.2012.01974.x
Merrifield DL, Dimitroglou A, Bradley G, Baker RTM, Davies SJ (2010) Probiotic applications for rainbow trout (Oncorhynchus mykiss Walbaum) I. Effects on growth performance, feed utilization, intestinal microbiota and related health criteria. Aquacult Nutr 16:504–510. https://doi.org/10.1111/j.1365-2095.2009.00689.x
Adel M, Yeganeh S, Dawood M, Safari R, Radhakrishnan S (2017) Effects of Pediococcus pentosaceus supplementation on growth performance, intestinal microflora and disease resistance of white shrimp, Litopenaeus vannamei. Aquacult Nutr 23:1401–1409. https://doi.org/10.1111/anu.12515
Safari R, Adel M, Lazado CC, Caipang CMA, Dadar M (2016) Host-derived probiotics Enterococcus casseliflavus improves resistance against Streptococcus iniae infection in rainbow trout (Oncorhynchus mykiss) via immunomodulation. Fish Shellfish Immunol 52:198–205. https://doi.org/10.1016/j.fsi.2016.03.020
Rodriguez-Estrada U, Satoh S, Haga Y, Fushimi H, Sweetman J (2013) Effects of inactivated Enterococcus faecalis and mannan oligosaccharide and their combination on growth, immunity, and disease protection in rainbow trout. N Am J Aquac 75:416–428. https://doi.org/10.1080/15222055.2013.799620
Eaton AD, Clesceri LS, Rice EW, Greenberg AE, Franson MAH (2005) Standard methods for the examination of water and wastewater. American public health association 1015
Heydarnejad MS, Purser GJ (2009) Agonistic acts as possible indicator of food anticipatory activity (FAA) in rainbow trout (Oncorhynchus mykiss). Iranian Journal of Veterinary Research, Shiraz University, Vol. 10, No. 2, Ser. No. 27. 137–145. https://www.sid.ir/en/journal/ViewPaper.aspx?id=141226
Adel M, Lazado CC, Safari R, Yeganeh S, Zorriehzahra MJ (2017) Aqualase®, a yeast-based in-feed probiotic, modulates intestinal microbiota, immunity and growth of rainbow trout Oncorhynchus mykiss. Aquacult Res 48:1815–1826. https://doi.org/10.1111/are.13019
Lazado CC, Caipang CMA, Kiron V (2012) Enzymes from the gut bacteria of Atlantic cod, Gadus morhua and their influence on intestinal enzyme activity. Aquacult Nutr 18:423–431. https://doi.org/10.1111/j.1365-2095.2011.00928.x
Hummel BCW (1959) A modified spectrophotometric determination of chymotrypsin, trypsin, and thrombin. Can J Biochem Physiol 37:1393–1399. https://doi.org/10.1139/o59-157
Robyt J, Whelan W (1968) The α-amylases. Starch and its derivatives. Academic Press, London. Chapman & Hall, London, pp 477–497
Furné M, Hidalgo MC, López A, García-Gallego M, Morales AE, Domezain A et al (2005) Digestive enzyme activities in Adriatic sturgeon Acipenser naccarii and rainbow trout Oncorhynchus mykiss. A comparative study. Aquaculture 250:391–398. https://doi.org/10.1016/j.aquaculture.2005.05.017
Saeidi asl MR, Adel M, Caipang CMA, Dawood MAO (2017) Immunological responses and disease resistance of rainbow trout (Oncorhynchus mykiss) juveniles following dietary administration of stinging nettle (Urtica dioica). Fish Shellfish Immunol 71:230–238. https://doi.org/10.1016/j.fsi.2017.10.016
Blaxhall PC, Daisley KW (1973) Routine haematological methods for use with fish blood. J Fish Biol 5:771–781. https://doi.org/10.1111/j.1095-8649.1973.tb04510.x
Klinger RC, Blazer VS, Echevarria CJA (1996) Effects of dietary lipid on the hematology of channel catfish, Ictalurus punctatus. Aquaculture 147:225–233. https://doi.org/10.1016/S0044-8486(96)01410-X
Mohebbi A, Nematollahi A, Dorcheh EE, Asad FG (2012) Influence of dietary garlic (Allium sativum) on the antioxidative status of rainbow trout (Oncorhynchus mykiss). Aquacult Res 43:1184–1193. https://doi.org/10.1111/j.1365-2109.2011.02922.x
Siwicki A, Anderson D (2000) Nonspecific defense mechanisms assay in fish: II. Potential killing activity of neutrophils and macrophages, lysozyme activity in serum and organs and total immunoglobulin level in serum. Olsztyn, Poland: FAO project GCP/INT/JPA, IFI p 105–112
Ellis A, Stolen J, Fletcher T, Anderson D, Robertson B, Van Muiswinkel W (1990) Lysozyme assay in techniques in fish immunology. Technique in Fish Immunology
M. Monte M, Urquhart K, Secombes CJ, Collet B, (2016) Individual monitoring of immune responses in rainbow trout after cohabitation and intraperitoneal injection challenge with Yersinia ruckeri. Fish Shellfish Immunol 55:469–478. https://doi.org/10.1016/j.fsi.2016.05.041
Ahmadifar E, Dawood MA, Moghadam MS, Shahrestanaki AH, Van Doan H, Saad AH et al (2020) The effect of Pediococcus acidilactici MA 18/5M on immune responses and mRNA levels of growth, antioxidant and immune-related genes in zebrafish (Danio rerio). Aquacult Rep 17:100374. https://doi.org/10.1016/j.aqrep.2020.100374
Dawood MA, Koshio SJA (2016) Recent advances in the role of probiotics and prebiotics in carp aquaculture: a review. Aquaculture 454:243–251. https://doi.org/10.1016/j.aquaculture.2015.12.033
Dawood MA, Koshio S, Ishikawa M, El-Sabagh M, Esteban MA, Zaineldin AI (2016) Probiotics as an environment-friendly approach to enhance red sea bream, Pagrus major growth, immune response and oxidative status. Fish Shellfish Immunol 57:170–178. https://doi.org/10.1016/j.fsi.2016.08.038
Ringø E (2020) Probiotics in shellfish aquaculture. Aquacult Fisheries 5:1–27. https://doi.org/10.1016/j.aaf.2019.12.001
Dawood MA, Magouz FI, Salem MF, Abdel-Daim HA (2019) Modulation of digestive enzyme activity, blood health, oxidative responses and growth-related gene expression in GIFT by heat-killed Lactobacillus plantarum (L-137). Aquaculture 505:127–136. https://doi.org/10.1016/j.aquaculture.2019.02.053
Melo-Bolívar JF, Ruiz Pardo RY, Hume ME, Villamil Díaz LM (2021) Multistrain probiotics use in main commercially cultured freshwater fish: a systematic review of evidence. Rev Aquacult. https://doi.org/10.1111/raq.12543
Paray BA, El-Basuini MF, Alagawany M, Albeshr MF, Farah MA, Dawood MAO (2021) Yucca schidigera usage for healthy aquatic animals: potential roles for sustainability. Animals 11:93. https://doi.org/10.3390/ani11010093
Jannathulla R, Dayal JS, Vasanthakumar D, Ambasankar K, Panigrahi A, Muralidhar M (2019) Apparent digestibility coefficients of fungal fermented plant proteins in two different penaeid shrimps—a comparative study. Aquacult Res 50:1491–1500. https://doi.org/10.1111/are.14024
Dawood MA, Koshio S (2020) Application of fermentation strategy in aquafeed for sustainable aquaculture. Rev Aquacult 12:987–1002. https://doi.org/10.1111/raq.12368
Waché Y, Auffray F, Gatesoupe F-J, Zambonino J, Gayet V, Labbé L et al (2006) Cross effects of the strain of dietary Saccharomyces cerevisiae and rearing conditions on the onset of intestinal microbiota and digestive enzymes in rainbow trout, Onchorhynchus mykiss, fry. Aquaculture 258:470–478. https://doi.org/10.1016/j.aquaculture.2006.04.002
Dawood MA, Koshio S, El-Sabagh M, Billah MM, Zaineldin AI, Zayed MM et al (2017) Changes in the growth, humoral and mucosal immune responses following β-glucan and vitamin C administration in red sea bream, Pagrus major. Aquaculture 470:214–222. https://doi.org/10.1016/j.aquaculture.2016.12.036
Dawood M, Koshio S, Ishikawa M, Yokoyama S, El Basuini M, Hossain M et al (2017) Dietary supplementation of β-glucan improves growth performance, the innate immune response and stress resistance of red sea bream, Pagrus major. Aquacult Nutr 23:148–159. https://doi.org/10.1111/anu.12376
Ghiasi M, Binaii M, Naghavi A, Rostami HK, Nori H, Amerizadeh A (2018) Inclusion of Pediococcus acidilactici as probiotic candidate in diets for beluga (Huso huso) modifies biochemical parameters and improves immune functions. Fish Physiol Biochem 44:1099–1107. https://doi.org/10.1007/s10695-018-0497-x
Dawood MA, Koshio S, Esteban MÁ (2018) Beneficial roles of feed additives as immunostimulants in aquaculture: a review. Rev Aquacult 10:950–974. https://doi.org/10.1111/raq.12209
Firouzbakhsh F, Noori F, Khalesi MK, Jani-Khalili K (2011) Effects of a probiotic, protexin, on the growth performance and hematological parameters in the Oscar (Astronotus ocellatus) fingerlings. Fish Physiol Biochem 37:833–842. https://doi.org/10.1007/s10695-011-9481-4
Cao Y, Liu H, Qin N, Ren X, Zhu B, Xia X (2020) Impact of food additives on the composition and function of gut microbiota: A review. Trends Food Sci Technol 99:295–310. https://doi.org/10.1016/j.tifs.2020.03.006
Irianto A, Austin B (2002) Use of probiotics to control furunculosis in rainbow trout, Oncorhynchus mykiss (Walbaum). J Fish Dis 25:333–342. https://doi.org/10.1046/j.1365-2761.2002.00375.x
Ahmed OM, Mahmoud AM, Abdel-Moneim A, Ashour MB (2012) Antidiabetic effects of hesperidin and naringin in type 2 diabetic rats. Diabetol Croat 41:53–67
Ansari BA, Kumar K (1988) Cypermethrin toxicity: effect on the carbohydrate metabolism of the Indian catfish, Heteropneustes fossilis. Sci Total Environ 72:161–166. https://doi.org/10.1016/0048-9697(88)90014-9
Lieke T, Meinelt T, Hoseinifar SH, Pan B, Straus DL, Steinberg CEW (2020) Sustainable aquaculture requires environmental-friendly treatment strategies for fish diseases. Rev Aquacult 12:943–965. https://doi.org/10.1111/raq.12365
Ji L, Sun G, Li J, Wang Y, Du Y, Li X et al (2017) Effect of dietary β-glucan on growth, survival and regulation of immune processes in rainbow trout (Oncorhynchus mykiss) infected by Aeromonas salmonicida. Fish Shellfish Immunol 64:56–67. https://doi.org/10.1016/j.fsi.2017.03.015
Engstad RE, Robertsen B, Frivold E (1992) Yeast glucan induces increase in lysozyme and complement-mediated haemolytic activity in Atlantic salmon blood. Fish Shellfish Immunol 2:287–297. https://doi.org/10.1016/S1050-4648(06)80033-1
Saurabh S, Sahoo P (2008) Lysozyme: an important defence molecule of fish innate immune system. Aquacult Res 39:223–239. https://doi.org/10.1111/j.1365-2109.2007.01883.x
El-Boshy ME, El-Ashram AM, AbdelHamid FM, Gadalla HA (2010) Immunomodulatory effect of dietary Saccharomyces cerevisiae, β-glucan and laminaran in mercuric chloride treated Nile tilapia (Oreochromis niloticus) and experimentally infected with Aeromonas hydrophila. Fish Shellfish Immunol 28:802–808. https://doi.org/10.1016/j.fsi.2010.01.017
Gobi N, Vaseeharan B, Chen J-C, Rekha R, Vijayakumar S, Anjugam M et al (2018) Dietary supplementation of probiotic Bacillus licheniformis Dahb1 improves growth performance, mucus and serum immune parameters, antioxidant enzyme activity as well as resistance against Aeromonas hydrophila in tilapia Oreochromis mossambicus. Fish Shellfish Immunol 74:501–508. https://doi.org/10.1016/j.fsi.2017.12.066
Guardiola FA, Porcino C, Cerezuela R, Cuesta A, Faggio C, Esteban MA (2016) Impact of date palm fruits extracts and probiotic enriched diet on antioxidant status, innate immune response and immune-related gene expression of European seabass (Dicentrarchus labrax). Fish Shellfish Immunol 52:298–308. https://doi.org/10.1016/j.fsi.2016.03.152
Iswarya A, Vaseeharan B, Anjugam M, Gobi N, Divya M, Faggio C (2018) β-1, 3 glucan binding protein based selenium nanowire enhances the immune status of Cyprinus carpio and protection against Aeromonas hydrophila infection. Fish Shellfish Immunol 83:61–75. https://doi.org/10.1016/j.fsi.2018.08.057
Salinas I, Díaz-Rosales P, Cuesta A, Meseguer J, Chabrillón M, Moriñigo MÁ et al (2006) Effect of heat-inactivated fish and non-fish derived probiotics on the innate immune parameters of a teleost fish (Sparus aurata L.). Vet Immunol Immunopathol 111:279–286. https://doi.org/10.1016/j.vetimm.2006.01.020
Alexander C, Sahu NP, Pal AK, Akhtar MS (2011) Haemato-immunological and stress responses of Labeo rohita (Hamilton) fingerlings: effect of rearing temperature and dietary gelatinized carbohydrate. J Anim Physiol Anim Nutr 95:653–663. https://doi.org/10.1111/j.1439-0396.2010.01096.x
Magnadottir B (2010) Immunological control of fish diseases. Mar Biotechnol 12:361–379. https://doi.org/10.1007/s10126-010-9279-x
Allameh SK, Ringø E, Yusoff FM, Daud HM, Ideris A (2017) Dietary supplement of Enterococcus faecalis on digestive enzyme activities, short-chain fatty acid production, immune system response and disease resistance of Javanese carp (Puntius gonionotus, Bleeker 1850). Aquacult Nutr 23:331–338. https://doi.org/10.1111/anu.12397
Sun YZ, Yang HL, Ma RL, Song K, Li JS (2012) Effect of Lactococcus lactis and Enterococcus faecium on growth performance, digestive enzymes and immune response of grouper Epinephelus coioides. Aquacult Nutr 18:281–289. https://doi.org/10.1111/j.1365-2095.2011.00894.x
Ray PD, Huang B-W, Tsuji Y (2012) Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal 24:981–900. https://doi.org/10.1016/j.cellsig.2012.01.008
Panigrahi A, Kiron V, Kobayashi T, Puangkaew J, Satoh S, Sugita H (2004) Immune responses in rainbow trout Oncorhynchus mykiss induced by a potential probiotic bacteria Lactobacillus rhamnosus JCM 1136. Vet Immunol Immunopath 102:379–388. https://doi.org/10.1016/j.vetimm.2004.08.006
Lamari F, Mahdhi A, Chakroun I, Esteban MA, Mazurais D, Amina B et al (2016) Interactions between candidate probiotics and the immune and antioxidative responses of European sea bass (Dicentrarchus labrax) larvae. J Fish Dis 39:1421–1432. https://doi.org/10.1111/jfd.12479
Acknowledgements
The authors would like to thank the Sari Agricultural Sciences and Natural Resources University (SANRU), for financial support of this research under contract number 03-1394-08.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yeganeh, S., Adel, M., Nosratimovafagh, A. et al. The Effect of Lactococcus lactis subsp. lactis PTCC 1403 on the Growth Performance, Digestive Enzymes Activity, Antioxidative Status, Immune Response, and Disease Resistance of Rainbow Trout (Oncorhynchus mykiss). Probiotics & Antimicro. Prot. 13, 1723–1733 (2021). https://doi.org/10.1007/s12602-021-09787-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-021-09787-3