Abstract
Incorporating species’ ability to adaptively respond to climate change is critical for robustly predicting persistence. One such example could be the adaptive role of algal symbionts in setting coral thermal tolerance under global warming and ocean acidification. Using a global ecological and evolutionary model of competing branching and mounding coral morphotypes, we show symbiont shuffling (towards taxa with increased heat tolerance) was more effective than symbiont evolution in delaying coral-cover declines, but stronger warming rates (high emissions scenarios) outpace the ability of these adaptive processes and limit coral persistence. Acidification has a small impact on reef degradation rates relative to warming. Global patterns in coral reef vulnerability to climate are sensitive to the interaction of warming rate and adaptive capacity and cannot be predicted by either factor alone. Overall, our results show how models of spatially resolved adaptive mechanisms can inform conservation decisions.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Code availability
All Matlab code can be found at https://github.com/VeloSteve/Coral-Model-V12 under the following: https://doi.org/10.5281/zenodo.2639126.
References
IPCC Special Report on the Ocean and Cryosphere in a Changing Climate (eds Pörtner, H.-O. et al.) (IPCC, 2019).
Urban, M. C. Accelerating extinction risk from climate change. Science 348, 571–573 (2015).
McCauley, D. J. & Pinsky, M. L. Marine defaunation: animal loss in the global ocean. Science 347, 1255641 (2015).
Hoffmann, A. A. & Sgrò, C. M. Climate change and evolutionary adaptation. Nature 470, 479–485 (2011).
Somero, G. N. The physiology of climate change: how potentials for acclimatization and genetic adaptation will determine ‘winners’ and ‘losers’. J. Exp. Biol. 213, 912–920 (2010).
Kearney, M. & Porter, W. Mechanistic niche modelling: combining physiological and spatial data to predict species’ ranges. Ecol. Lett. 12, 334–350 (2009).
Foden, W. B. et al. Identifying the world’s most climate change vulnerable species: a systematic trait-based assessment of all birds, amphibians and corals. PLoS ONE 8, e65427 (2013).
West, J. M. & Salm, R. V. Resistance and resilience to coral bleaching: implications for coral reef conservation and management. Conserv. Biol. 17, 956–967 (2003).
Baskett, M. L., Nisbet, R. M., Kappel, C. V., Mumby, P. J. & Gaines, S. D. Conservation management approaches to protecting the capacity for corals to respond to climate change: a theoretical comparison. Glob. Change Biol. 16, 1229–1246 (2010).
Beyer, H. L. et al. Risk-sensitive planning for conserving coral reefs under rapid climate change. Conserv. Lett. 11, e12587 (2018).
Walsworth, T. E. et al. Management for network diversity speeds evolutionary adaptation to climate change. Nat. Clim. Change 9, 632–636 (2019).
Hughes, T. P. et al. Spatial and temporal patterns of mass bleaching of corals in the Anthropocene. Science 359, 80–83 (2018).
Donner, S. D., Skirving, W. J., Little, C. M., Oppenheimer, M. & Hoegh-Guldberg, O. Global assessment of coral bleaching and required rates of adaptation under climate change. Glob. Change Biol. 11, 2251–2265 (2005).
Frieler, K. et al. Limiting global warming to 2 °C is unlikely to save most coral reefs. Nat. Clim. Change 3, 165–170 (2012).
Van Hooidonk, R., Maynard, J. A., Manzello, D. & Planes, S. Opposite latitudinal gradients in projected ocean acidification and bleaching impacts on coral reefs. Glob. Change Biol. 20, 103–112 (2014).
Logan, C. A., Dunne, J. P., Eakin, C. M. & Donner, S. D. Incorporating adaptive responses into future projections of coral bleaching. Glob. Change Biol. 20, 125–139 (2014).
Bay, R. A., Rose, N. H., Logan, C. A. & Palumbi, S. R. Genomic models predict successful coral adaptation if future ocean warming rates are reduced. Sci. Adv. 3, e1701413 (2017).
Matz, M. V., Treml, E. A. & Haller, B. C. Estimating the potential for coral adaptation to global warming across the Indo-West Pacific. Glob. Change Biol. 26, 3473–3481 (2020).
Baskett, M. L., Gaines, S. D. & Nisbet, R. M. Symbiont diversity may help coral reefs survive moderate climate change. Ecol. Appl. 19, 3–17 (2009).
Matz, M. V., Treml, E. A., Aglyamova, G. V. & Bay, L. K. Potential and limits for rapid genetic adaptation to warming in a Great Barrier Reef coral. PLoS Genet. 14, e1007220 (2018).
Muscatine, L., Falkowski, P. G., Porter, J. W. & Dubinsky, Z. Fate of photosynthetic fixed carbon in light- and shade-adapted colonies of the symbiotic coral Stylophora pistillata. Proc. R. Soc. Lond. B. 222, 181–202 (1984).
Csaszar, N. B., Ralph, P. J., Frankham, R., Berkelmans, R. & van Oppen, M. J. Estimating the potential for adaptation of corals to climate warming. PLoS ONE 5, e9751 (2010).
Howells, E. J. et al. Coral thermal tolerance shaped by local adaptation of photosymbionts. Nat. Clim. Change 2, 116–120 (2012).
Buerger, P. et al. Heat-evolved microalgal symbionts increase coral bleaching tolerance. Sci. Adv. 6, eaba2498 (2020).
Baker, A. C. Flexibility and specificity in coral–algal symbiosis: diversity, ecology, and biogeography of Symbiodinium. Annu. Rev. Ecol. Evol. Syst. 34, 661–689 (2003).
Berkelmans, R. & van van Oppen, M. J. H. The role of zooxanthellae in the thermal tolerance of corals: a ‘nugget of hope’ for coral reefs in an era of climate change. Proc. R. Soc. Lond. B 273, 2305–2312 (2006).
National Academies of Sciences, Engineering, and Medicine A research Review of Interventions to Increase the Persistence and Resilience of Coral Reefs (National Academies Press, 2019).
National Academies of Sciences, Engineering, and Medicine A Decision Framework for Interventions to Increase the Persistence and Resilience of Coral Reefs (National Academies Press, 2019).
Darling, E. S., Alvarez-Filip, L., Oliver, T. A., McClanahan, T. R. & Côté, I. M. Evaluating life-history strategies of reef corals from species traits. Ecol. Lett. 15, 1378–1386 (2012).
Chan, N. C. S. & Connolly, S. R. Sensitivity of coral calcification to ocean acidification: a meta-analysis. Glob. Change Biol. 19, 282–290 (2013).
Hughes, T. P. et al. Global warming transforms coral reef assemblages. Nature 556, 492–496 (2018).
Darling, E. S. et al. Relationships between structural complexity, coral traits, and reef fish assemblages. Coral Reefs 36, 561–575 (2017).
Howells, E. J. et al. Corals in the hottest reefs in the world exhibit symbiont fidelity not flexibility. Mol. Ecol. 29, 899–911 (2020).
Madin, J. S., Hughes, T. P. & Connolly, S. R. Calcification, storm damage and population resilience of tabular corals under climate change. PLoS ONE 7, e46637 (2012).
Hoegh-Guldberg, O. et al. in Global Warming of 1.5°C (eds Masson-Delmotte, V. et al.) Ch. 3 (IPCC, 2018).
Darling, E. S. et al. Social–environmental drivers inform strategic management of coral reefs in the Anthropocene. Nat. Ecol. Evol. 3, 1341–1350 (2019).
Wilkinson, C. R. Global and local threats to coral reef functioning and existence: review and predictions. Mar. Freshw. Res. 50, 867–878 (1999).
Palumbi, S. R., Barshis, D. J., Traylor-Knowles, N. & Bay, R. A. Mechanisms of reef coral resistance to future climate change. Science 344, 895–898 (2014).
Kleypas, J. A. et al. Larval connectivity across temperature gradients and its potential effect on heat tolerance in coral populations. Glob. Change Biol. 22, 3539–3549 (2016).
Heron, S. F. et al. Validation of reef-scale thermal stress satellite products for coral bleaching monitoring. Remote Sens. 8, 59 (2016).
Safaie, A. et al. High frequency temperature variability reduces the risk of coral bleaching. Nat. Commun. 9, 1671 (2018).
Forster, P. M. et al. Evaluating adjusted forcing and model spread for historical and future scenarios in the CMIP5 generation of climate models. J. Geophys. Res. Atmos. 118, 1139–1150 (2013).
Ziegler, M., Eguíluz, V. & Duarte, C. et al. Rare symbionts may contribute to the resilience of coral–algal assemblages. ISME J 12, 161–172 (2018).
Sampayo, E. M., Ridgway, T., Bongaerts, P. & Hoegh-Guldberg, O. Bleaching susceptibility and mortality of corals are determined by fine-scale differences in symbiont type. Proc. Natl Acad. Sci. USA 105, 10444–10449 (2008).
Thornhill, D. J., Xiang, Y. U., Fitt, W. K. & Santos, S. R. Reef endemism, host specificity and temporal stability in populations of symbiotic dinoflagellates from two ecologically dominant Caribbean corals. PLoS ONE 4, e6262 (2009).
Stat, M., Loh, W. K. W., LaJeunesse, T. C., Hoegh-Guldberg, O. & Carter, D. A. Stability of coral–endosymbiont associations during and after a thermal stress event in the southern Great Barrier Reef. Coral Reefs 28, 709–713 (2009).
Chakravarti, L. J., Beltran, V. H. & van Oppen, M. J. Rapid thermal adaptation in photosymbionts of reef-building corals. Glob. Change Biol. 23, 4675–4688 (2017).
Schulte, P. M., Healy, T. M. & Fangue, N. A. Thermal performance curves, phenotypic plasticity, and the time scales of temperature exposure. Integr. Comp. Biol. 51, 691–702 (2011).
Loya, Y. et al. Coral bleaching: the winners and the losers. Ecol. Lett. 4, 122–131 (2001).
Langmead, O. & Sheppard, C. Coral reef community dynamics and disturbance: a simulation model. Ecol. Modell. 175, 271–290 (2004).
Chancerelle, Y. Methods to estimate actual surface areas of scleractinian coral at the colony- and community-scale. Oceanol. Acta 23, 211–219 (2000).
Falkowski, P. G., Dubinsky, Z., Muscatine, L. & Porter, J. W. Light and the bioenergetics of a symbiotic coral. Bioscience 34, 705–709 (1984).
Huston, M. Variation in coral growth rates with depth at Discovery Bay, Jamaica. Coral Reefs 4, 19–25 (1985).
Hoogenboom, M., Beraud, E. & Ferrier-Pagès, C. Relationship between symbiont density and photosynthetic carbon acquisition in the temperate coral Cladocora caespitosa. Coral Reefs 29, 21–29 (2010).
Cunning, R. & Baker, A. C. Not just who, but how many: the importance of partner abundance in reef coral symbioses. Front. Microbiol. 5, 400 (2014).
McClanahan, T., Muthiga, N. & Mangi, S. Coral and algal changes after the 1998 coral bleaching: interaction with reef management and herbivores on Kenyan reefs. Coral Reefs 19, 380–391 (2001).
Fitt, W. K., McFarland, F. K., Warner, M. E. & Chilcoat, G. C. Seasonal patterns of tissue biomass and densities of symbiotic dinoflagellates in reef corals and relation to coral bleaching. Limnol. Oceanogr. 45, 677–685 (2000).
Eppley, R. W. Temperature and phytoplankton growth in the sea. Fish. Bull. 70, 1063–1085 (1972).
Jon, N. Biodiversity and ecosystem functioning: a complex adaptive systems approach. Limnol. Oceanogr. 49, 1269–1277 (2004).
Mousseau, T. A. & Roff, D. A. Natural selection and the heritability of fitness components. Heredity 59, 181–197 (1987).
Lynch, M. The rate of polygenic mutation. Genet. Res. 51, 137–148 (1988).
Donner, S. D., Rickbeil, G. J. & Heron, S. F. A new, high-resolution global mass coral bleaching database. PLoS ONE 12, e0175490 (2017).
Cunning, R., Gillette, P., Capo, T., Galvez, K. & Baker, A. C. Growth tradeoffs associated with thermotolerant symbionts in the coral Pocillopora damicornis are lost in warmer oceans. Coral Reefs 34, 155–160 (2015).
Silverstein, R. N., Cunning, R. & Baker, A. C. Change in algal symbiont communities after bleaching, not prior heat exposure, increases heat tolerance of reef corals. Glob. Change Biol. 21, 236–249 (2015).
Dunne, J. P. et al. GFDL’s ESM2 global coupled climate–carbon Earth system models part I: physical formulation and baseline simulation characteristics. J. Clim. 25, 6646–6665 (2012).
Dunne, J. P. et al. GFDL’s ESM2 global coupled climate–carbon Earth system models Part II: carbon system formulation and baseline simulation characteristics. J. Clim. 26, 2247–2267 (2012).
Lough, J. M. & Barnes, D. J. Environmental controls on growth of the massive coral Porites. J. Exp. Mar. Biol. Ecol. 245, 225–243 (2000).
UNEP-WCMC, WorldFish Centre, WRI & TNC. Global Distribution of Warm-water Coral Reefs, Compiled From Multiple Sources Including the Millennium Coral Reef Mapping Project. Version 4.1 (UN Environment World Conservation Monitoring Centre. Data, 2021); https://doi.org/10.34892/t2wk-5t34
van Hooidonk, R., Maynard, J. A. & Planes, S. Temporary refugia for coral reefs in a warming world. Nat. Clim. Change 3, 508–511 (2013).
Fitt, W., Brown, B., Warner, M. & Dunne, R. Coral bleaching: interpretation of thermal tolerance limits and thermal thresholds in tropical corals. Coral Reefs 20, 51–65 (2001).
González-Espinosa, P. C. & Donner, S. D. Predicting cold-water bleaching in corals: role of temperature, and potential integration of light exposure. Mar. Ecol. Prog. Ser. 642, 133–146 (2020).
Acknowledgements
This work was supported by a NOAA Coral Reef Conservation grant to J.P.D. and S.D.D., a Coral Reef Alliance Coral Adaptation Challenge grant to C.A.L. and S.D.D., and an ROA supplement to NSF DEB #1655475 to C.A.L. and M.L.B. We thank C. M. Eakin for helpful initial discussions in the development of the global model. The contents in this manuscript are solely the opinions of the authors and do not constitute a statement of policy, decision or position on behalf of NOAA or the US Government.
Author information
Authors and Affiliations
Contributions
C.A.L., J.P.D. and S.D.D. conceived and designed the global model; C.A.L. and J.S.R. developed and tested the computer code; C.A.L., J.P.D., J.S.R. and S.D.D. analysed the results; C.A.L. and J.S.R. wrote the paper. C.A.L., J.P.D., J.S.R., S.D.D. and M.L.B. critically revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Climate Change thanks M. Matz and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Coral and symbiont ecological and evolutionary global model diagram.
The left-hand boxes (a) describe the symbiont fitness curve and genetic dynamics. The right-hand boxes (b) describe the coral and symbiont population dynamics.
Extended Data Fig. 2 Relative coral extent across all reef cells in a 400-year model run with no anthropogenic warming and no adaptive capacity.
In all model runs, branching corals (blue) are initialized at 80% and mounding corals (red) at 20% of a fixed pre-warming carrying capacity (K) in 1861 averaged across all reef cells. Initializing coral morphotypes to the inverse of these proportions (80% mounding: 20% branching) results in a similar outcome (~ 90% branching and 1% mounding corals) by 1950. Shaded colors represent the 50% interquartile range around the mean for all reef cells.
Extended Data Fig. 3 Percentage of ‘healthy’ reef cells globally in four RCP emissions scenarios from 1980 to 2100.
Model trajectories are shown with no evolution (black), shuffling with a +1 °C advantage (red), evolution (blue), and combined shuffling and evolution (purple). A reef is considered ‘healthy’ if it is not in a bleached or mortality state (see Methods). SST (grey) is the mean and 25th-75th percentile increase in annual maximum temperatures across all reef grid cells. Bar plots indicate number of bleaching events per year in each model run.
Extended Data Fig. 4 In each model year, reef cells are defined as being in a ‘healthy’, ‘bleached’, or ‘mortality’ state.
Arrows represent transitions between states. 1) ‘Bleaching’ occurs when symbiont populations drop <30% of the minimum population size in the previous year or when bleaching occurs ≥2 times in the previous decade. 2) ‘Mortality’ is defined if a reef bleaches but does not recover within five years, or 3) if coral populations drop to <2x the seed value. 4-5) Recovery occurs if coral and symbiont populations increase to >4x their respective seed value or coral populations grow above 10% of carrying capacity.
Extended Data Fig. 5 Sensitivity analysis of percent ‘healthy’ coral reef cells when the model is calibrated to estimated bleaching frequencies of 3 or 5% between 1985-2010.
In the main text, model output is calibrated to a 5% bleaching frequency during this time. The effect of changing the target to 3 % is shown for RCP4.5 and RCP8.5 scenarios. Projected trajectories are shown with and without symbiont evolution (E=1 vs. E=0), and with or without shuffling (+1.0 °C advantage) in the tolerant population. The effect of increasing pCO2 on coral growth rates is also included (OA=1) with evolution and shuffling.
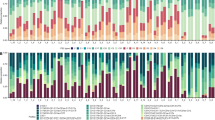
Extended Data Fig. 6 Global mean fraction of corals hosting heat-tolerant symbionts in branching (heat-sensitive) corals and mounding (heat-tolerant) corals.
The mean value is calculated for all reef cells (n=1,925) for all RCPs in shuffling (+1.0 °C advantage) simulations. For most reefs, fidelity to heat-tolerant symbiont occurs following a rapid transition between 2010-2040 through 2100.
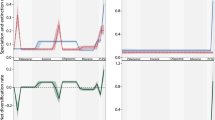
Extended Data Fig. 7 Fine-scale symbiont shuffling dynamics in four example reef cells.
Temperature is monthly SST with the optimal temperature (gi) for each symbiont type overlaid in yellow (top). Symbiont density (bottom) is in terms of cells per cm2 of coral area for a heat-sensitive (solid lines) and heat-tolerant (dashed lines) symbiont population in each coral morphotype. Realistic seasonal fluctuations in symbiont density (a,b) and reversion can occur (c, d), but reversion is uncommon under future warming; (d) represents a model run with no anthropogenic warming in which reversion occurs several times during a 200-year period. Bleaching events are shown in black circles.
Extended Data Fig. 8 Global change in mean symbiont genotype (gi or optimal temperature in °C) and average increase in annual maximum sea surface temperatures (SST) in model runs with symbiont evolution for all RCPs.
Median (solid lines) and interquartile range (shaded) is shown across all reef cells (n=1,925) for mounding (heat-tolerant) and branching (heat-sensitive) corals. Across all RCP scenarios and all reefs, the increase in symbiont optimal thermal tolerance ranged between 0.3°C and 1.8°C.
Extended Data Fig. 9 Global maps of warming rate and SST variability.
Values represent change in each temperature metric between the historical period (1861-1900) and 2080 (a-d, g-h) as well as future variability between 2050-2080 (e-f, i-j) for RCP 4.5 and RCP 8.5 climate scenarios. In panels (a) to (f), inputs are filtered to include only maximum monthly mean SST. Panels (g) through (j) include all months.
Supplementary information
Supplementary Information
Supplementary Tables 1–4.
Rights and permissions
About this article
Cite this article
Logan, C.A., Dunne, J.P., Ryan, J.S. et al. Quantifying global potential for coral evolutionary response to climate change. Nat. Clim. Chang. 11, 537–542 (2021). https://doi.org/10.1038/s41558-021-01037-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41558-021-01037-2
This article is cited by
-
Systematic review of the uncertainty of coral reef futures under climate change
Nature Communications (2024)
-
Lineage-specific symbionts mediate differential coral responses to thermal stress
Microbiome (2023)
-
Emergent increase in coral thermal tolerance reduces mass bleaching under climate change
Nature Communications (2023)
-
Coral–algal endosymbiosis characterized using RNAi and single-cell RNA-seq
Nature Microbiology (2023)
-
From polyps to pixels: understanding coral reef resilience to local and global change across scales
Landscape Ecology (2023)