Abstract
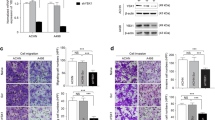
Metastasis is inevitable in about 30% of patients with primary renal cell carcinoma after nephrectomy treatment. APOBEC1 complementation factor (A1CF), an RNA binding protein, participates in tumor progressions such as growth, apoptosis, differentiation, and invasion. Here, we explored biological functions of A1CF and provided a new insight into renal cell carcinoma metastasis. Wound healing assay was conducted to detect migration in A1CF overexpression and knockdown stable cell lines. Quantitative PCR and western blot assays were utilized to test transcriptional and translation levels of A1CF and SMAD3 in A1CF overexpression and knockdown renal carcinoma cells. Nuclear and cytoplasmic protein separation assays were conducted to evaluate the subcellular distribution of A1CF and SMAD3. Immunoprecipitation assay was conducted to detect the interaction between A1CF and SMAD3. Our study demonstrated A1CF overexpression facilitated cell migration in renal carcinoma cells. A1CF deficiency downregulated expression of SMAD3, Snail1, and N-cadherin. In addition, A1CF promoted nucleus translocation of SMAD3 and interacted with SMAD3. SMAD3 knockdown attenuated cell migration induced by A1CF overexpression. Our study suggested A1CF facilitated cell migration by promoting nucleus translocation of SMAD3 in renal cell carcinoma cells.




Similar content being viewed by others
References
Batlle R, Andrés E, Gonzalez L, Llonch E, Igea A, Gutierrez-Prat N, Berenguer-Llergo A, Nebreda AR (2019) Regulation of tumor angiogenesis and mesenchymal-endothelial transition by p38α through TGF-β and JNK signaling. Nat Commun 10:3071
Chen H, Liu J, Wang H, Cheng Q, Zhou C, Chen X, Ye F (2019a) Inhibition of RNA-binding protein Musashi-1 suppresses malignant properties and reverses paclitaxel resistance in ovarian carcinoma. J Cancer 10:1580–1592
Chen X, Xiong D, Yang H, Ye L, Mei S, Wu J, Chen S, Shang X, Wang K, Huang L (2019b) Long noncoding RNA OPA-interacting protein 5 antisense transcript 1 upregulated SMAD3 expression to contribute to metastasis of cervical cancer by sponging miR-143-3p. J Cell Physiol 234:5264–5275
Han P, Wang XH, Han YF, Chen GM (2019) MicroRNA-140’s inhibition on the cell migration and invasion of non-small cell lung cancer by down-regulating Smad3 expression. Eur Rev Med Pharmacol Sci 23:9471–9479
Hao J, Hu Y, Li Y, Zhou Q, Lv X (2017) Involvement of JNK signaling in IL4-induced M2 macrophage polarization. Exp Cell Res 357:155–162
Hsieh JJ, Purdue MP, Signoretti S, Swanton C, Albiges L, Schmidinger M, Heng DY, Larkin J, Ficarra V (2017) Renal cell carcinoma. Nat Rev Dis Primers 3:17009
Jonasch E, Gao J, Rathmell WK (2014) Renal cell carcinoma. BMJ 349:g4797
Kaszak I, Witkowska-Piłaszewicz O, Niewiadomska Z, Dworecka-Kaszak B, Ngosa Toka F, Jurka P (2020) Role of cadherins in cancer-a review. Int J Mol Sci 21:7624
Lellek H, Kirsten R, Diehl I, Apostel F, Buck F, Greeve J (2000) Purification and molecular cloning of a novel essential component of the apolipoprotein B mRNA editing enzyme-complex. J Biol Chem 275:19848–19856
Li W, Li X, Gao LN, You CG (2020) Integrated analysis of the functions and prognostic values of RNA binding proteins in lung squamous cell carcinoma. Front Genet 11:185
Lindemann RK, Nordheim A, Dittmer J (2003) Interfering with TGFbeta-induced Smad3 nuclear accumulation differentially affects TGFbeta-dependent gene expression. Mol Cancer 2:20
Liou YF, Hsieh YS, Hung TW, Chen PN, Chang YZ, Kao SH, Lin SW, Chang HR (2019) Thymoquinone inhibits metastasis of renal cell carcinoma cell 786-O-SI3 associating with downregulation of MMP-2 and u-PA and suppression of PI3K/Src signaling. Int J Med Sci 16:686–695
Liu Q, Chen CY, Chen GL (2020) High APOBEC1 complementation factor expression positively modulates the proliferation, invasion, and migration of endometrial cancer cells through regulating P53/P21 signaling pathway. Cancer Biother Radiopharm. https://doi.org/10.1089/cbr.2020.3957
Liu R, Pei Q, Shou T, Zhang W, Hu J, Li W (2019) Apoptotic effect of green synthesized gold nanoparticles from Curcuma wenyujin extract against human renal cell carcinoma A498 cells. Int J Nanomedicine 14:4091–4103
Mandal G, Biswas S, Roy Chowdhury S, Chatterjee A, Purohit S, Khamaru P, Chakraborty S, Mandal PK, Gupta A, de la Mare JA, Edkins AL, Bhattacharyya A (2018) Heterodimer formation by Oct4 and Smad3 differentially regulates epithelial-to-mesenchymal transition-associated factors in breast cancer progression. Biochim Biophys Acta Mol basis Dis 1864:2053–2066
Ni D, Liu J, Hu Y, Liu Y, Gu Y, Zhou Q, Xie Y (2019) A1CF-Axin2 signal axis regulates apoptosis and migration in Wilms tumor-derived cells through Wnt/beta-catenin pathway. In Vitro Cell Dev Biol Anim 55:252–259
Ni D, Yi Q, Liu J, Hu Y, Lv T, Tan G, Liu Y, Xu L, Xia H, Zhou Q, Xie Y (2020) A1CF-promoted colony formation and proliferation of RCC depends on DKK1-MEK/ERK signal axis. Gene 730:144299
Ou YC, Li JR, Wang JD, Chang CY, Wu CC, Chen WY, Kuan YH, Liao SL, Lu HC, Chen CJ (2019) Fibronectin promotes cell growth and migration in human renal cell carcinoma cells. Int J Mol Sci 20:2792
Oudard S, Vano Y (2015) The role of rechallenge with targeted therapies in metastatic renal-cell carcinoma. Curr Opin Urol 25:402–410
Owens B (2016) Kidney cancer. Nature 537:S97
Park JH, Kim M, Yim B, Park CY (2021) Nitric oxide attenuated transforming growth factor-β induced myofibroblast differentiation of human keratocytes. Sci Rep 11:8183
Petersen M, Pardali E, van der Horst G, Cheung H, van den Hoogen C, van der Pluijm G, Ten Dijke P (2010) Smad2 and Smad3 have opposing roles in breast cancer bone metastasis by differentially affecting tumor angiogenesis. Oncogene 29:1351–1361
Poomsawat S, Punyasingh J, Vejchapipat P (2015) Aberrant expression of p-Smad3 in oral carcinogenesis. Clin Oral Investig 19:613–618
Qian Z, Zhang Q, Hu Y, Zhang T, Li J, Liu Z, Zheng H, Gao Y, Jia W, Hu A, Li B, Hao J (2018) Investigating the mechanism by which SMAD3 induces PAX6 transcription to promote the development of non-small cell lung cancer. Respir Res 19:262
Sekimoto G, Matsuzaki K, Yoshida K, Mori S, Murata M, Seki T, Matsui H, Fujisawa J, Okazaki K (2007) Reversible Smad-dependent signaling between tumor suppression and oncogenesis. Cancer Res 67:5090–5096
Shi Y, Wang X, Xu Z, He Y, Guo C, He L, Huan C, Cai C, Huang J, Zhang J, Li Y, Zeng C, Zhang X, Wang L, Ke Y, Cheng H (2020) PDLIM5 inhibits STUB1-mediated degradation of SMAD3 and promotes the migration and invasion of lung cancer cells. J Biol Chem 295:13798–13811
Snyder EM, McCarty C, Mehalow A, Svenson KL, Murray SA, Korstanje R, Braun RE (2017) APOBEC1 complementation factor (A1CF) is dispensable for C-to-U RNA editing in vivo. RNA 23:457–465
Song Y, Shao L, Xue Y, Ruan X, Liu X, Yang C, Zheng J, Shen S, Chen J, Li Z, Liu Y (2019) Inhibition of the aberrant A1CF-FAM224A-miR-590-3p-ZNF143 positive feedback loop attenuated malignant biological behaviors of glioma cells. J Exp Clin Cancer Res 38:248
Stegmüller J, Huynh MA, Yuan Z, Konishi Y, Bonni A (2008) TGFbeta-Smad2 signaling regulates the Cdh1-APC/SnoN pathway of axonal morphogenesis. J Neurosci 28:1961–1969
Tang L, Zhao P, Kong D (2019) Muscleblind-like 1 destabilizes Snail mRNA and suppresses the metastasis of colorectal cancer cells via the Snail/E-cadherin axis. Int J Oncol 54:955–965
Tang PM, Zhou S, Meng XM, Wang QM, Li CJ, Lian GY, Huang XR, Tang YJ, Guan XY, Yan BP, To KF, Lan HY (2017) Smad3 promotes cancer progression by inhibiting E4BP4-mediated NK cell development. Nat Commun 8:14677
Warburton D, Shi W, Xu B (2013) TGF-β-Smad3 signaling in emphysema and pulmonary fibrosis: an epigenetic aberration of normal development? Am J Physiol Lung Cell Mol Physiol 304:L83–L85
Wolff I, May M, Hoschke B, Zigeuner R, Cindolo L, Hutterer G, Schips L, De Cobelli O, Rocco B, De Nunzio C, Tubaro A, Coman I, Feciche B, Truss M, Dalpiaz O, Figenshau RS, Madison K, Sánchez-Chapado M, Santiago Martin MD, Salzano L, Lotrecchiano G, Shariat SF, Hohenfellner M, Waidelich R, Stief C, Miller K, Pahernik S, Brookman-May S (2016) Do we need new high-risk criteria for surgically treated renal cancer patients to improve the outcome of future clinical trials in the adjuvant setting? Results of a comprehensive analysis based on the multicenter CORONA database. Eur J Surg Oncol 42:744–750
Xie W, Aisner S, Baredes S, Sreepada G, Shah R, Reiss M (2013) Alterations of Smad expression and activation in defining 2 subtypes of human head and neck squamous cell carcinoma. Head Neck 35:76–85
Xie Y, Lv X, Ni D, Liu J, Hu Y, Liu Y, Liu Y, Liu R, Zhao H, Lu Z, Zhou Q (2019) HPD degradation regulated by the TTC36-STK33-PELI1 signaling axis induces tyrosinemia and neurological damage. Nat Commun 10:4266
Yang S, Zhang H, Yang H, Zhang J, Wang J, Luo T, Jiang Y, Hua H (2021) SEPHS1 promotes SMAD2/3/4 expression and hepatocellular carcinoma cells invasion. Exp Hematol Oncol 10:17
Zhang H, Yang K, Ren T, Huang Y, Tang X, Guo W (2018) miR-16-5p inhibits chordoma cell proliferation, invasion and metastasis by targeting Smad3. Cell Death Dis 9:680
Zhang X, Min KW, Liggett J, Baek SJ (2013) Disruption of the transforming growth factor-β pathway by tolfenamic acid via the ERK MAP kinase pathway. Carcinogenesis 34:2900–2907
Zhao B, Liu L, Mao J, Zhang Z, Wang Q, Li Q (2018) PIM1 mediates epithelial-mesenchymal transition by targeting Smads and c-Myc in the nucleus and potentiates clear-cell renal-cell carcinoma oncogenesis. Cell Death Dis 9:307
Zhou L, Hao J, Yuan Y, Peng R, Wang H, Ni D, Gu Y, Huang L, Mao Z, Lyu Z, Du Y, Liu Z, Li Y, Ju P, Long Y, Liu J, Zhou Q (2016a) EIYMNVPV motif is essential for A1CF nucleus localization and A1CF (-8aa) promotes proliferation of MDA-MB-231 cells via up-regulation of IL-6. Int J Mol Sci 17:811
Zhou Q, Han LR, Zhou YX, Li Y (2016b) MiR-195 suppresses cervical cancer migration and invasion through targeting Smad3. Int J Gynecol Cancer 26:817–824
Zhou Y, Mao H, Li S, Cao S, Li Z, Zhuang S, Fan J, Dong X, Borkan SC, Wang Y, Yu X (2010) HSP72 inhibits Smad3 activation and nuclear translocation in renal epithelial-to-mesenchymal transition. J Am Soc Nephrol 21:598–609
Acknowledgements
The authors are thankful for all the fellows in the M.O.E. Key Laboratory of Laboratory Medical Diagnostics, the College of Laboratory Medicine, Chongqing Medical University.
Funding
The work was funded by the National Science Foundation for Young Scientists of China (Grant No. 31701218) and the National Natural Science Foundation of China (Grant No. 82030065 and 81873932).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Editor: Tetsuji Okamoto.
Supplementary Information
Supplementary Figure 1
A1CF upregulated SMAD3 expression in HEK293T cells. Protein levels and relative grayscale values of SMAD3 in A1CF overexpression (A) and knockdown (B, C) HEK293T cells. **P <0.01; ***P <0.001 (PNG 429 kb)
Supplementary Figure 2
STR analyses of HEK293T cell line. Peak charts of locus in HEK293T cell line. (PNG 521 kb)
Supplementary Figure 3
STR analyses of 786-O cell line. Peak charts of locus in 786-O cell line. (PNG 1247 kb)
Supplementary Figure 4
STR analyses of A498 cell line. Peak charts of locus in A498 cell line. (PNG 1847 kb)
Supplementary Figure 5
PCR analysis of mycoplasma infection. Agarose gel image of mycoplasma PCR detection. (PNG 348 kb)
Supplementary Table 1
The genotyping results of STR locus in HEK293T cell. STR profiles of HEK293T cell line were exactly matched with HEK293T (CVCL_QW54) in ExPASy database (8 core loci plus Amelogenin). (XLSX 10 kb)
Supplementary Table 2
The genotyping results of STR locus in 786-O cell. STR profiles of 786-O cell line were matched with 786-O (CVCL_1051) in ExPASy database. (XLSX 10 kb)
Supplementary Table 3
The genotyping results of STR locus in A498 cell. STR profiles of A498 cell line were matched with A498 (CVCL_1056) in ExPASy database. (XLSX 10 kb)
Rights and permissions
About this article
Cite this article
Xia, H., Liu, Y., Xu, L. et al. APOBEC1 complementation factor facilitates cell migration by promoting nucleus translocation of SMAD3 in renal cell carcinoma cells. In Vitro Cell.Dev.Biol.-Animal 57, 501–509 (2021). https://doi.org/10.1007/s11626-021-00589-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11626-021-00589-z