Abstract
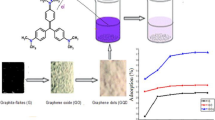
Ethylene diaminetetraacetic acid (EDTA)-functionalized graphene was synthesized from Nigerian coal using a chemical exfoliation method and the graphene was applied for the removal of Congo red dye from aqueous solutions. The synthesized coal graphene and the raw coal were characterized using Fourier transform infrared (FTIR) spectroscopy, X-ray diffraction (XRD) spectroscopy, scanning electron microscopy (SEM)–energy-dispersive X-ray (EDX) spectroscopy, measurement of pHpzc (pH of point of zero charge), and Boehm titrations. The SEM data revealed surface roughness which is enhanced in the prepared graphene while the EDX revealed an increase in carbon content, the main constituent of graphene, from about 26% in the raw coal to about 80% in the prepared graphene. Various adsorption variables, such as pH, contact time, concentration of Congo red, and temperature, were varied for the removal of the dye using raw coal and the synthesized coal graphene. The Liu isotherm gave the best fit of the equilibrium data than the Langmuir, Freundlich, and Dubinin-Radushkevich models. The maximum adsorption capacities of the raw coal and synthesized coal graphene at 25°C are 109.1 mg/g and 129.0 mg/g, respectively. The Avrami fractional-order kinetic model was the best model for description of the kinetic data. The model had the lowest values of standard deviation than the pseudo-first-order and pseudo-second-order models. The adsorption process of the two materials occurred via two stages as proved by intraparticle diffusion model. The adsorption process of the Congo red removal was spontaneous, feasible, and endothermic. The study conclusively revealed the graphene nanomaterial to be a viable adsorbent for textile wastewater treatment.








Similar content being viewed by others
Data availability
The data used in this study are available from the corresponding author on request.
References
Abbasizadeh S, Keshtkar AR, Mousavian MA (2014) Sorption of heavy metal ions from aqueous solution by a novel cast PVA/TiO2 nanohybrid adsorbent functionalized with amine groups. J Ind Eng Chem 20(4):1656–1664
Adebayo MA (2019) Adsorption of congo red from aqueous solutions using clay–corn cob–FeCl3 composite. FUTA J Res Sci 15:61–74
Adebayo MA, Areo FI (2021) Removal of phenol and 4-nitrophenol from wastewater using a composite prepared from clay and Cocos nucifera shell: kinetic, equilibrium and thermodynamic studies. Resour Environ Sustain 3:100020. https://doi.org/10.1016/j.resenv.2021.100020
Adebayo MA, Prola LDT, Lima EC, Puchana-Rosero MJ, Cataluña R, Saucier C, Umpierres CS, Vaghetti JCP, da Silva LG, Ruggiero R (2014) Adsorption of Procion blue MX-R dye from aqueous solutions by lignin chemically modified with aluminium and manganese. J Hazard Mater 268:43–50. https://doi.org/10.1016/j.jhazmat.2014.01.005
Adebayo MA, Adebomi JI, Abe TO, Areo FI (2020) Removal of aqueous Congo red and malachite green using ackee apple seed–bentonite composite. Colloids Interf Sci Commun 38:100311. https://doi.org/10.1016/j.colcom.2020.100311
Adesemuyi MF, Adebayo MA, Akinola AO, Olasehinde FE, Adewole KA, Lajide L (2020) Preparation and characterisation of biochars from elephant grass and their utilisation for aqueous nitrate removal: effect of pyrolysis temperature. J Environ Chem Eng 8:104507. https://doi.org/10.1016/j.jece.2020.104507
Alhashimi HA, Aktas CB (2017) Life cycle environmental and economic performance of biochar compared with activated carbon: a meta-analysis. Resour Conserv Recycl 118:13–26
Ali I (2012) New generation adsorbents for water treatment. Chem Rev 112:5073–5091
Boehm HP (2002) Surface oxides on carbon and their analysis: a critical assessment. Carbon 40:145–149
Bozorgi M, Abbasizadeh S, Samani F, Mousavi SE (2018) Performance of synthesized cast and electrospun PVA/chitosan/ZnO-NH2 nano-adsorbents in single and simultaneous adsorption of cadmium and nickel ions from wastewater. Environ Sci Pollut Res 25(18):17457–17472. https://doi.org/10.1007/s11356-018-1936-z
Chong KY, Chia CH, Zakaria S, Sajab MS (2014) Vaterite calcium carbonate for the adsorption of Congo red from aqueous solutions. J Environ Chem Eng 2:2156–2161
Cui L, Wang Y, Gao L, Hu L, Yan L, Wei Q, Du B (2015) EDTA functionalized magnetic graphene oxide for removal of Pb(II), Hg(II) and Cu(II) in water treatment: adsorption mechanism and separation property. Chem Eng J 281:1–10. https://doi.org/10.1016/j.cej.2015.06.043
Danish M, Hashim R, Rafatullah M, Sulaiman O, Ahmad A, Govind G (2011) Adsorption of Pb(II) ions from aqueous solutions by date bead carbon activated with ZnCl2. Clean-Soil, Air, Water 39:392–399
de Assis LK, Damasceno BS, Carvalho MN, Oliveira EHC, Ghislandi MG (2020) Adsorption capacity comparison between graphene oxide and graphene nanoplatelets for the removal of colored textile dyes from wastewater. Environ Technol 41:2360–2371. https://doi.org/10.1080/09593330.2019.1567603
dos Santos DC, Adebayo MA, Pereira SFP, Prola LDT, Cataluña R, Lima EC, Gally CR, Saucier C, Machado FM (2014) Application of carbon composite adsorbents prepared from coffee wastes and red mud for the removal of textile dyes from aqueous solutions: kinetic, equilibrium, and thermodynamic studies. Korean J Chem Eng 31:1470–1479. https://doi.org/10.1007/s11814-014-0086-3
Du Q, Sun J, Li Y, Yang X, Wang X, Wang Z, Xia L (2014) Highly enhanced adsorption of congo red onto graphene oxide/chitosan fibers by wet-chemical etching off silica nanoparticles. Chem Eng J 245:99–106. https://doi.org/10.1016/j.cej.2014.02.006
Fahdil A, Al-Niaimi D, Muhi FH (2019) Kinetic and thermodynamic study on the removal of Congo red from the aqueous solution using graphene oxide/magnesium oxide nanocomposite. J Biochem Tech 4:1–10
Galashev AE, Polukhin VA (2014) Removal of copper from graphene by bombardment with argon clusters: computer experiment. Phys Met Metallogr 115:697–704
Ghann WE, Kang H, Uddin J, Chowdhury FA, Khondaker SI, Moniruzzaman M, Kabir MH, Rahman MM (2019) Synthesis and characterization of reduced graphene oxide and their application in dye-sensitized solar cells. ChemEng 3:7. https://doi.org/10.3390/chemengineering3010007
Gul K, Khan H, Muhammad N, Ara B, Zia TUH (2020) Removal of toxic malachite green dye from aqueous environment using reduced magnetic graphene oxide as an efficient and reusable adsorbent. Sep Purif Technol:1–14. https://doi.org/10.1080/01496395.2020.1839498
Guo T, Bulin C (2021) Facile preparation of MgO/graphene oxide nanocomposite for efficient removal of aqueous Congo red: adsorption performance and interaction mechanism. Res Chem Intermed 47:945–971. https://doi.org/10.1007/s11164-020-04310-9
Hairom NHH, Mohammad AW, Kadhum AAH (2014) Nanofiltration of hazardous Congo red dye: performance and flux decline analysis. J Water Process Eng 4:99–106
Kumari S, Mankotia D, Chauhan G (2016) Crosslinked cellulose dialdehyde for Congo red removal from its aqueous solutions. J Environ Chem Eng 4:1126–1136
Li L, Li X, Duan H, Wang X, Luo C (2014) Removal of Congo red by magnetic mesoporous titanium dioxide–graphene oxide core–shell microspheres for water purification. Dalton Trans 43:8431–8438. https://doi.org/10.1039/c3dt53474j
Lima EC, Cestari AR, Adebayo MA (2016) Comments on the paper: a critical review of the applicability of Avrami fractional kinetic equation in adsorption-based water treatment studies. Desal Water Treat 57:19566–19571. https://doi.org/10.1080/19443994.2015
Liu Y, Xu H, Yang SF, Tay JH (2003) A general model for biosorption of Cd2+, Cu2+ and Zn2+ by aerobic granules. J Biotechnol 102:233–239
Mahmoudi E, Azizkhani S, Mohammad A, Ng LY, Benamor A, Ang WL, Ba-Abbad M (2020) Simultaneous removal of Congo red and cadmium (II) from aqueous solutions using graphene oxide–silica composite as a multifunctional adsorbent. J Environ Sci 98:151–160. https://doi.org/10.1016/j.jes.2020.05.013
Nupearachchi CN, Mahatantila K, Vithanage M (2017) Application of graphene for decontamination of water; implications for sorptive removal. Groundw Sustain Dev 5:206–215. https://doi.org/10.1016/j.gsd.2017.06.006
Omidi S, Kakanejadifard A (2018) Eco-friendly synthesis of graphene–chitosan composite hydrogel as efficient adsorbent for Congo red. RSC Adv 8:12179–12189. https://doi.org/10.1039/c8ra00510a
Pang LSK, Wilson MA (1993) Nanotubes from coal. Energy Fuel 7:436–437
Pang LSK, Vassallo AM, Wilson MA (1991) Fullerenes from coal. Nature 352:480
Pham VT, Tran TV, Nguyen TD, Tham NTH, Quang PTT, Uyen DTT, Le NTH, Vo DN, Thanh NT, Bach LG (2019) Adsorption behavior of Congo red dye from aqueous solutions onto exfoliated graphite as an adsorbent: kinetic and isotherm studies. Mater Today: Proc 18:4449–4457
Querol X, Fernández-Turiel JL, Lopez-Soler A (1995) Trace elements in coal and their behaviour during combustion in a large power station. Fuel 74:331–343
Rafi M, Samiey B, Cheng C (2018) Study of adsorption mechanism of Congo red on graphene oxide/PAMAM nanocomposite. Mater 11:496. https://doi.org/10.3390/ma11040496
Rao A, Raj AM, Manoj B (2017) Extraction and characterization of preformed mixed phase graphene sheets from graphitized sub-bituminous coal. Asian J Chem 29:2425–2428. https://doi.org/10.14233/ajchem.2017.20722
Rehman A, Zulfiqar S, Shakir I, Aly Aboud MF, Shahid M, Warsi MF (2020) Nanocrystalline hematite a-Fe2O3 synthesis with different precursors and their composites with graphene oxide. Ceram Int 46:8227–8237. https://doi.org/10.1016/j.ceramint.2019.12.050
Ren H, Cunha E, Sun Q, Li Z, Kinloch IA, Young RJ, Fan Z (2019) Surface functionality analysis by Boehm titration of graphene nanoplatelets functionalized via a solvent-free cycloaddition reaction. Nanoscale Adv 1:1432–1441. https://doi.org/10.1039/c8na00280k
Repo E, Koivula R, Harjula R, Sillanpää M (2013) Effect of EDTA and some other interfering species on the adsorption of Co(II) by EDTA-modified chitosan. Desalination 321:93–102
Ribas MC, Franco M, Adebayo MA, Parkes GM, Lima EC, Féris LA (2020) Adsorption of Procion Red MX-5B dye from aqueous solution using a homemade peach activated carbon compared with commercial activated carbon. Appl Water Sci 10:154. https://doi.org/10.1007/s13201-020-01237-9
Robinson T, McMullan G, Marchant R, Nigam P (2001) Remediation of dyes in textile effluent: a critical review on current treatment technologies with a proposed alternative. Bioresour Technol 77:247–225
Rodrigues AE, Silva CM (2016) What’s wrong with Lagergreen pseudo first order model for adsorption kinetics? Chem Eng J 306:1138–1142. https://doi.org/10.1016/j.cej.2016.08.055
Rovani S, Fernandes A, Prola LDT, Lima EC, Santos WO, Adebayo MA (2014) Removal of Cibacron Brilliant Yellow 3G-P Dye from aqueous solutions by Brazilian peats as biosorbents. Chem Eng Commun 201:1431–1458. https://doi.org/10.1080/00986445.2013.81695
Shafiee M, Abedi MA, Abbasizadeh S, Sheshdeh RK, Mousavi SE, Shohani S (2020) Effect of zeolite hydroxyl active site distribution on adsorption of Pb(II) and Ni(II) pollutants from water system by polymeric nanofibers. Sep Purif Technol 55:1994–2011. https://doi.org/10.1080/01496395.2019.1624572
Shah NS, Khan JA, Sayed M, Khan ZH, Iqbal J, Arshad S, Junaid M, Khan HM (2020) Sep. Synergistic effects of H2O2 and S2O82− in the gamma radiation induced degradation of congo-red dye: kinetics and toxicities evaluation. Purif Technol 233:115966. https://doi.org/10.1016/j.seppur.2019.115966
Shin SR, Li Y, Jang HL, Khoshakhlagh P, Akbari M, Nasajpour A, Zhang YS, Tamayol A, Khademhosseini A (2016) Graphene-based materials for tissue engineering. Adv Drug Deliv Rev 105:255–274
Shinn JH (1996) Visualization of complex hydrocarbon reaction systems. Prepr of Pap – Am Chem Soc Div Fuel Chem 41:510–515
Sohn HI, Gordin ML, Xu T, Chen S, Lv D, Song J, Manivannan A, Wang D (2014) Porous spherical carbon/sulphur nanocomposite by aerosol synthesis: the effect of pore structure and morphology on their electrochemical performance as Lithium/sulphur battery cathodes. ACS Appl Mater Interfaces 6:596–606
Stephen LU (2000) Ultraviolet/visible light adsorption spectrometry in clinical chemistry. John Wiley and Sons Ltd, Chichester, pp 1699–1171
Suhas PJM, Carrott MML, Carrott R, Singh R, Singh LP, Chaudhary M (2017) An innovative approach to develop microporous activated carbons in oxidising atmosphere. J Clean Prod 156:549–555
Suraj G, Iyer C, Rugmini S, Lalithambika M (1997) The effect of micronization on kaolinites and their sorption behavior. Appl Clay Sci 12:111–130
Tabrez AK, Kumar D (2004) Removal of some basic dyes from artificial wastewater by adsorption on Akash Kinari Coal. J Sci Ind Res 63:355–364
Thue PS, Adebayo MA, Lima EC, Sieliechi JM, Machado FM, Dotto GL, Vaghetti JCP, Dias SLP (2016) Preparation, characterization and application of microwave-assisted activated carbons from wood chips for removal of phenol from aqueous solution. J Mol Liq 223:1067–1080. https://doi.org/10.1016/j.molliq.2016.09.032
Vassilev SV (1994) Trace elements in solid waste products from coal burning at some Bulgarian thermoelectric power stations. Fuel 73:367–374
Ward CR, Spears DA, Booth CA, Staton I (1999) Mineral matter and trace elements in coals of the Gunnedah Basin New South Wales. Australia Int J Coal Geol 40:281–308
Weber J, Wolfe N (1987) Kinetic studies of the reduction of aromatic azo dyes in anaerobic waste water. Environ Toxicol Chem 6:911–919
Wei F, Ren Q, Liang Z, Chen D (2019) Synthesis of graphene oxide/metal-organic frameworks composite materials for removal of Congo red from wastewater. ChemistrySelect 4:5755–5762. https://doi.org/10.1002/slct.201900363
Xu J, Xu D, Zhu B, Cheng B, Jiang C (2018) Adsorptive removal of an anionic dye Congo red by flower-like hierarchical magnesium oxide (MgO)-graphene oxide composite microspheres. Appl Surf Sci 435:1136–1142. https://doi.org/10.1016/j.apsusc.2017.11.232
Yang Q, Choi H, Dionysiou DD (2007) Nanocrystalline cobalt oxide immobilized on titanium dioxide nanoparticles for the heterogeneous activation of peroxymonosulfate. Appl Catal B Environ 74:170–178
Yao Y, Miao S, Liub S, Ma LP, Sun H, Wang S (2012) Synthesis, characterization, and adsorption properties of magnetic Fe3O4@graphene nanocomposite. Chem Eng J 184:326–332. https://doi.org/10.1016/j.cej.2011.12.017
Yao J, Wen D, Shen J, Wang J (2016) Zero discharge process for dyeing wastewater treatment. J Water Process Eng 11:98–103
Zhang Y, Yan L, Xu W, Guo X, Cui L, Gao L, Wei Q, Du B (2014) Adsorption of Pb(II) and Hg(II) from aqueous solution using magnetic CoFe2O4-reduced graphene oxide. J Mol Liq 191:177–182
Author information
Authors and Affiliations
Contributions
Temilolu J. Popoola: investigation, data acquisition, conceptualization, and preparation of the draft manuscript.
Afamefuna E. Okoronkwo: conceptualization, methodology, and supervision.
Olugbenga O. Oluwasina: methodology, editing, and supervision.
Matthew A. Adebayo: data analysis, supervision, editing, and review of the manuscript.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Tito Roberto Cadaval Jr
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(PDF 395 kb)
Rights and permissions
About this article
Cite this article
Popoola, T.J., Okoronkwo, A.E., Oluwasina, O.O. et al. Preparation, characterization, and application of a homemade graphene for the removal of Congo red from aqueous solutions. Environ Sci Pollut Res 28, 52174–52187 (2021). https://doi.org/10.1007/s11356-021-14434-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-14434-z