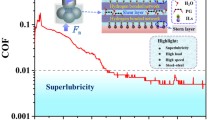
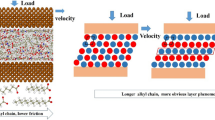
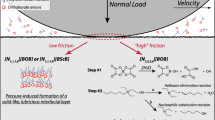
Abstract
Background
Ionic liquids (ILs) have recently attracted considerable attention in tribology owing to their unique physico-chemical properties and promising lubrication performance when used in a wide range of material pairs.
Objective
The aim of this review article is to summarize recent advances in our knowledge related to the lubrication mechanisms of neat ILs, with a particular focus on nanoscale studies dealing with the behavior of ILs in the boundary lubrication regime.
Methods
We first discuss the current state-of-the-art concerning the normal pressure-dependent lubrication mechanism of ILs and then focus on the dynamic behavior of ILs upon nanoconfinment. Finally, we summarize recent research efforts aiming to control the tribological response of ILs by changing the surface charge density, evaluate the effects of impurities on the lubricity of ILs, and shed light on the IL tribochemistry at small length scales.
Results
While the field of IL-mediated lubrication has made significant progress, several open questions still remain, including the effects of temperature, impurities, and surface roughness on the friction response and dynamic behaviors of nanoconfined ILs. Additionally, a mechanistic understanding of the tribochemical reactivity of ILs is still lacking.
Conclusions
To harness the full potential of ILs for tribological applications, significant work is still required to establish links between the IL structure, lubrication mechanism(s), and performance. These advancements will be instrumental for the predictive design, development, and implementation of ILs with enhanced tribological properties in next-generation lubricants for a variety of applications across several sectors, including manufacturing and transportation.






Similar content being viewed by others
References
Stark R, Lindow K (2017) Sustainability dynamics. In: Stark R, Seliger G, Bonvoisin J (eds) Sustainable manufacturing, sustainable production, life cycle engineering and management. Springer, Cham. https://doi.org/10.1007/978-3-319-48514-0_2
Holmberg K, Andersson P, Erdemir A (2012) Global energy consumption due to friction in passenger cars. Tribol Int 47:221–234. https://doi.org/10.1016/j.triboint.2011.11.022
IEA (2016) (2016) CO2 emissions from fuel combustion 2016. OECD Publishing, Paris. https://doi.org/10.1787/co2_fuel-2016-en
Carpick RW, Jackson A, Lee P et al (2017) Tribology opportunities for enhancing America’s energy efficiency. A report to the Advanced Research Projects Agency-Energy (APPA-E) at the U.S. Department of Energy
Ye C, Liu W, Chen Y et al (2001) Room-temperature ionic liquids: a novel versatile lubricant. Chem Commun 2244–2245. https://doi.org/10.1039/B106935G
Palacio M, Bhushan B (2008) Ultrathin wear-resistant ionic liquid films for novel MEMS/NEMS applications. Adv Mater 20:1194–1198. https://doi.org/10.1002/adma.200702006
Somers A, Howlett P, MacFarlane D, Forsyth M (2013) A review of ionic liquid lubricants. Lubricants 1:3–21
Zhou Y, Qu J (2017) Ionic liquids as lubricant additives: a review. ACS Appl Mater Interfaces 9:3209–3222. https://doi.org/10.1021/acsami.6b12489
Rogers RD, Seddon KR (2003) Ionic liquids - solvents of the future? Science 302:792–793. https://doi.org/10.1126/science.1090313
Shakeel A, Mahmood H, Farooq U et al (2019) Rheology of pure ionic liquids and their complex fluids: a review. ACS Sustain Chem Eng 7:13586–13626. https://doi.org/10.1021/acssuschemeng.9b02232
Rodríguez H, Brennecke JF (2006) Temperature and composition dependence of the density and viscosity of binary mixtures of water + ionic liquid. J Chem Eng Data 51:2145–2155. https://doi.org/10.1021/je0602824
Harris LKR, Kanakubo M, Woolf A, Data JCE (2005) Temperature and pressure dependence of the viscosity of the ionic liquid 1-butyl-3-methylimidazolium tetrafluoroborate : viscosity and density relationships in ionic liquids. J Chem Eng Data 50:800181
Torrecilla JS, Rafione T, García J, Rodrígue F (2008) Effect of relative humidity of air on density, apparent molar volume, viscosity, surface tension, and water content of 1-ethyl-3-methylimidazolium ethylsulfate ionic liquid. J Chem Eng Data 53:923–928. https://doi.org/10.1021/je700523b
Lhermerout R, Diederichs C, Perkin S (2018) Are ionic liquids good boundary lubricants? A molecular perspective Lubricants 6:9. https://doi.org/10.3390/lubricants6010009
Espinosa-Marzal RM, Han M, Arcifa A et al (2018) Ionic liquids at interfaces and their tribological behavior. In: Wandelt K. (ed) Reference module in chemistry, molecular sciences and chemical engineering. Elsevier, Oxford, UK, pp 172–194
Perkin S (2012) Ionic liquids in confined geometries. Phys Chem Chem Phys 14:5052–5062. https://doi.org/10.1039/c2cp23814d
Niedermeyer H, Hallett JP, Villar-Garcia IJ et al (2012) Mixtures of ionic liquids. Chem Soc Rev 41:7780–7802. https://doi.org/10.1039/c2cs35177c
Qu J, Bansal DG, Yu B et al (2012) Antiwear performance and mechanism of an oil-miscible ionic liquid as a lubricant additive. ACS Appl Mater Interfaces 4:997–1002. https://doi.org/10.1021/am201646k
Guo W, Zhou Y, Sang X et al (2017) Atom probe tomography unveils formation mechanisms of wear-protective tribofilms by ZDDP, ionic liquid, and their combination. ACS Appl Mater Interfaces 9:23152–23163. https://doi.org/10.1021/acsami.7b04719
Qu J, Barnhill WC, Luo H et al (2015) Synergistic effects between phosphonium-alkylphosphate ionic liquids and zinc dialkyldithiophosphate (ZDDP) as lubricant additives. Adv Mater 27:4767–4774. https://doi.org/10.1002/adma.201502037
Zhou Y, Dyck J, Graham TW et al (2014) Ionic liquids composed of phosphonium cations and organophosphate, carboxylate, and sulfonate anions as lubricant antiwear additives. Langmuir 30:13301–13311. https://doi.org/10.1021/la5032366
Barnhill WC, Qu J, Luo H et al (2014) Phosphonium-organophosphate ionic liquids as lubricant additives: effects of cation structure on physicochemical and tribological characteristics. ACS Appl Mater Interfaces 6:22585–22593. https://doi.org/10.1021/am506702u
Cooper PK, Wear CJ, Li H, Atkin R (2017) Ionic liquid lubrication of stainless steel: friction is inversely correlated with interfacial liquid nanostructure. ACS Sustain Chem Eng 5:11737–11743. https://doi.org/10.1021/acssuschemeng.7b03262
Hayes R, Warr GG, Atkin R (2015) Structure and nanostructure in ionic liquids. Chem Rev 115:6357–6426. https://doi.org/10.1021/cr500411q
Zhou H, Rouha M, Feng G et al (2012) Nanoscale perturbations of room temperature ionic liquid structure at charged and uncharged interfaces. ACS Nano 6:9818–9827. https://doi.org/10.1021/nn303355b
Uysal A, Zhou H, Feng G et al (2014) Structural origins of potential dependent hysteresis at the electrified graphene/ionic liquid interface. J Phys Chem C 118:569–574. https://doi.org/10.1021/jp4111025
Fedorov MV, Kornyshev AA (2008) Towards understanding the structure and capacitance of electrical double layer in ionic liquids. Electrochim Acta 53:6835–6840. https://doi.org/10.1016/j.electacta.2008.02.065
Horn RG, Israelachvili JN (1981) Direct measurement of structural forces between two surfaces in a nonpolar liquid. J Chem Phys 75:1400–1411. https://doi.org/10.1063/1.442146
Christenson HK (1983) Experimental measurements of solvation forces in nonpolar liquids. J Chem Phys 78:6906–6913. https://doi.org/10.1063/1.444639
Horn RG, Israelachvili JN (1988) Molecular organization and viscosity of a thin film of molten polymer between two surfaces as probed by force measurements. Macromolecules 21:2836–2841. https://doi.org/10.1021/ma00187a032
Perkin S, Albrecht T, Klein J (2010) Layering and shear properties of an ionic liquid, 1-ethyl-3-methylimidazolium ethylsulfate, confined to nano-films between mica surfaces. Phys Chem Chem Phys 12:1243–1247. https://doi.org/10.1039/B920571C
Han M, Espinosa-Marzal RM (2018) Electroviscous retardation of the squeeze out of nanoconfined ionic liquids. J Phys Chem C 122:21344–21355. https://doi.org/10.1021/acs.jpcc.8b04778
Carstens T, Gustus R, Höfft O et al (2014) Combined STM, AFM, and DFT study of the highly ordered pyrolytic graphite/1-octyl-3-methyl-imidazolium bis(trifluoromethylsulfonyl)imide interface. J Phys Chem C 118:10833–10843. https://doi.org/10.1021/jp501260t
Fedorov MV, Lynden-Bell RM (2012) Probing the neutral graphene-ionic liquid interface: insights from molecular dynamics simulations. Phys Chem Chem Phys 14:2552–2556. https://doi.org/10.1039/c2cp22730d
Vijayakumar M, Schwenzer B, Shutthanandan V et al (2014) Elucidating graphene-ionic liquid interfacial region: a combined experimental and computational study. Nano Energy 3:152–158. https://doi.org/10.1016/j.nanoen.2012.09.014
Rollins JB, Fitchett BD, Conboy JC (2007) Structure and orientation of the imidazolium cation at the room-temperature ionic liquid/SiO2 interface measured by sum-frequency vibrational spectroscopy. J Phys Chem B 111:4990–4999. https://doi.org/10.1021/jp0671906
Payal RS, Balasubramanian S (2014) Effect of cation symmetry on the organization of ionic liquids near achargedmica surface. J Phys Condens Matter 26: 284101. https://doi.org/10.1088/0953-8984/26/28/284101
Espinosa-Marzal RM, Arcifa A, Rossi A, Spencer ND (2014) Ionic liquids confined in hydrophilic nanocontacts: structure and lubricity in the presence of water. J Phys Chem C 118:6491–6503. https://doi.org/10.1021/jp5000123
Smith AM, Parkes MA, Perkin S (2014) Molecular friction mechanisms across nanofilms of a bilayer-forming ionic liquid. J Phys Chem Lett 5:4032–4037. https://doi.org/10.1021/jz502188g
Sweeney J, Hausen F, Hayes R et al (2012) Control of nanoscale friction on gold in an ionic liquid by a potential-dependent ionic lubricant layer. Phys Rev Lett 109:1–10. https://doi.org/10.1103/PhysRevLett.109.155502
Jiang W, Wang Y, Voth GA (2007) Molecular dynamics simulation of nanostructural organization in ionic liquid/water mixtures. J Phys Chem B 111:4812–4818. https://doi.org/10.1021/jp067142l
Cremer T, Kolbeck C, Lovelock KRJ et al (2010) Towards a molecular understanding of cation-anion interactions-Probing the electronic structure of imidazolium ionic liquids by NMR spectroscopy, X-ray photoelectron spectroscopy and theoretical calculations. Chem Eur J 16:9018–9033. https://doi.org/10.1002/chem.201001032
Uhl B, Cremer T, Roos M et al (2013) At the ionic liquidmetal interface: structure formation and temperature dependent behavior of an ionic liquid adlayer on Au(111). Phys Chem Chem Phys 15:17295–17302. https://doi.org/10.1039/c3cp52184b
Bazant MZ, Storey BD, Kornyshev AA (2011) Double layer in ionic liquids: overscreening versus crowding. Phys Rev Lett 106:6–9. https://doi.org/10.1103/PhysRevLett.106.046102
Krämer G, Hausen F, Bennewitz R (2017) Dynamic shear force microscopy of confined liquids at a gold electrode. Faraday Discuss 199:299–309. https://doi.org/10.1039/c6fd00237d
Lainé A, Niguès A, Bocquet L, Siria A (2020) Nanotribology of ionic liquids: transition to yielding response in nanometric confinement with metallic surfaces. Phys Rev X 10:1–10. https://doi.org/10.1103/physrevx.10.011068
Jurado LA, Kim H, Rossi A et al (2016) Effect of the environmental humidity on the bulk, interfacial and nanoconfined properties of an ionic liquid. Phys Chem Chem Phys 18:22719–22730. https://doi.org/10.1039/c6cp03777a
Espinosa-Marzal RM, Arcifa A, Rossi A, Spencer ND (2014) Microslips to “avalanches” in confined, molecular layers of ionic liquids. J Phys Chem Lett 5:179–184. https://doi.org/10.1021/jz402451v
Smith AM, Lovelock KRJ, Gosvami NN et al (2013) Quantized friction across ionic liquid thin films. Phys Chem Chem Phys 15:15317–15320. https://doi.org/10.1039/c3cp52779d
Elbourne A, Sweeney J, Webber GB et al (2013) Adsorbed and near-surface structure of ionic liquids determines nanoscale friction. Chem Commun 49:6797–6799. https://doi.org/10.1039/c3cc42844c
Sweeney J, Webber GB, Rutland MW, Atkin R (2014) Effect of ion structure on nanoscale friction in protic ionic liquids. Phys Chem Chem Phys 16:16651–16658. https://doi.org/10.1039/c4cp02320j
Kumacheva E, Klein J (1998) Simple liquids confined to molecularly thin layers. II. Shear and frictional behavior of solidified films. J Chem Phys 108:7010–7022. https://doi.org/10.1063/1.476115
Gee ML, McGuiggan PM, Israelachvili JN, Homola AM (1990) Liquid to solidlike transitions of molecularly thin films under shear. J Chem Phys 93:1895–1906. https://doi.org/10.1063/1.459067
Ouyang W, Ramakrishna SN, Rossi A et al (2019) Load and velocity dependence of friction mediated by dynamics of interfacial contacts. Phys Rev Lett 123:116102. https://doi.org/10.1103/PhysRevLett.123.116102
Filippov AE, Klafter J, Urbakh M (2004) Friction through dynamical formation and rupture of molecular bonds. Phys Rev Lett 92:2–5. https://doi.org/10.1103/PhysRevLett.92.135503
Li Z, Szlufarska I (2018) Multiphysics model of chemical aging in frictional contacts. Phys Rev Mater 2:063602. https://doi.org/10.1103/PhysRevMaterials.2.063602
Jacobs TDB, Carpick RW (2013) Nanoscale wear as a stress-assisted chemical reaction. Nat Nanotechnol 8:108–112. https://doi.org/10.1038/nnano.2012.255
Barel I, Urbakh M, Jansen L, Schirmeisen A (2010) Multibond dynamics of nanoscale friction: the role of temperature. Phys Rev Lett 104:1–4. https://doi.org/10.1103/PhysRevLett.104.066104
Atkin R, El Abedin SZ, Hayes R et al (2009) AFM and STM studies on the surface interaction of [BMP]TFSA and [EMIm]TFSA ionic liquids with Au(111). J Phys Chem C 113:13266–13272. https://doi.org/10.1021/jp9026755
Fitchett BD, Conboy JC (2004) Structure of the room-temperature ionic liquid/SiO 2 interface studied by sum-frequency vibrational spectroscopy. J Phys Chem B 108:20255–20262. https://doi.org/10.1021/jp0471251
Matthews RP, Ashworth C, Welton T, Hunt PA (2014) The impact of anion electronic structure: similarities and differences in imidazolium based ionic liquids. J Phys Condens Matter 26:284112. https://doi.org/10.1088/0953-8984/26/28/284112
Liu Z, Cui T, Lu T et al (2016) Anion effects on the solid/ionic liquid interface and the electrodeposition of zinc. J Phys Chem C 120:20224–20231. https://doi.org/10.1021/acs.jpcc.6b07812
Smith AM, Lovelock KRJ, Gosvami NN et al (2013) Monolayer to bilayer structural transition in confined pyrrolidinium-based ionic liquids. J Phys Chem Lett 4:378–382. https://doi.org/10.1021/jz301965d
Smith AM, Lovelock KRJ, Perkin S (2013) Monolayer and bilayer structures in ionic liquids and their mixtures confined to nano-films. Faraday Discuss 167:279–292. https://doi.org/10.1039/c3fd00075c
Hayes R, Borisenko N, Tam MK et al (2011) Double layer structure of ionic liquids at the Au(111) electrode interface: an atomic force microscopy investigation. J Phys Chem C 115:6855–6863. https://doi.org/10.1021/jp200544b
Li H, Endres F, Atkin R (2013) Effect of alkyl chain length and anion species on the interfacial nanostructure of ionic liquids at the Au(111)-ionic liquid interface as a function of potential. Phys Chem Chem Phys 15:14624–14633. https://doi.org/10.1039/c3cp52421c
Li H, Wood RJ, Endres F, Atkin R (2014) Influence of alkyl chain length and anion species on ionic liquid structure at the graphite interface as a function of applied potential. J Phys Condens Matter 26:284115. https://doi.org/10.1088/0953-8984/26/28/284115
Fedorov MV, Kornyshev AA (2014) Ionic liquids at electrified interfaces. Chem Rev 114:2978–3036. https://doi.org/10.1021/cr400374x
Hayes R, Warr GG, Atkin, (2010) At the interface: solvation and designing ionic liquids. Phys Chem Chem Phys 12:1709–1723. https://doi.org/10.1039/c001176m
Cheng HW, Stock P, Moeremans B et al (2015) Characterizing the influence of water on charging and layering at electrified ionic-liquid/solid interfaces. Adv Mater Interfaces 2:1–9. https://doi.org/10.1002/admi.201500159
Cheng HW, Dienemann JN, Stock P et al (2016) The effect of water and confinement on self-assembly of imidazolium based ionic liquids at mica interfaces. Sci Rep 6:1–9. https://doi.org/10.1038/srep30058
Cooper PK, Li H, Rutland MW et al (2016) Tribotronic control of friction in oil-based lubricants with ionic liquid additives. Phys Chem Chem Phys 18:23657–23662. https://doi.org/10.1039/c6cp04405k
Li H, Rutland MW, Atkin R (2013) Ionic liquid lubrication: influence of ion structure, surface potential and sliding velocity. Phys Chem Chem Phys 15:14616–14623. https://doi.org/10.1039/c3cp52638k
Li H, Wood RJ, Rutland MW, Atkin R (2014) An ionic liquid lubricant enables superlubricity to be “switched on” in situ using an electrical potential. Chem Commun 50:4368–4370. https://doi.org/10.1039/c4cc00979g
Hjalmarsson N, Wallinder D, Glavatskih S et al (2015) Weighing the surface charge of an ionic liquid. Nanoscale 7:16039–16045. https://doi.org/10.1039/c5nr03965g
David A, Fajardo OY, Kornyshev AA et al (2017) Electrotunable lubricity with ionic liquids: the influence of nanoscale roughness. Faraday Discuss 199:279–297. https://doi.org/10.1039/c6fd00244g
Mendonça ACF, Pádua AAH, Malfreyt P (2013) Nonequilibrium molecular simulations of new ionic lubricants at metallic surfaces: prediction of the friction. J Chem Theory Comput 9:1600–1610. https://doi.org/10.1021/ct3008827
Sheehan A, Jurado LA, Ramakrishna SN et al (2016) Layering of ionic liquids on rough surfaces. Nanoscale 8:4094–4106. https://doi.org/10.1039/c5nr07805a
Nalam PC, Sheehan A, Han M, Espinosa-marzal RM (2020) Effects of nanoscale roughness on the lubricious behavior of an ionic liquid. Adv Mater Interfaces 7:2000314
Li H, Atkin R, Page AJ (2015) Combined friction force microscopy and quantum chemical investigation of the tribotronic response at the propylammonium nitrate-graphite interface. Phys Chem Chem Phys 17:16047–16052. https://doi.org/10.1039/c5cp01952d
Jacobs TDB, Greiner C, Wahl KJ, Carpick RW (2019) Insights into tribology from in situ nanoscale experiments. MRS Bull 44:478–486. https://doi.org/10.1557/mrs.2019.122
Vakis AI, Yastrebov VA, Scheibert J et al (2018) Modeling and simulation in tribology across scales: an overview. Tribol Int 125:169–199. https://doi.org/10.1016/j.triboint.2018.02.005
Pivnic K, Fajardo OY, Bresme F et al (2018) Mechanisms of electrotunable friction in friction force microscopy experiments with ionic liquids. J Phys Chem C 122:5004–5012. https://doi.org/10.1021/acs.jpcc.8b00516
Di Lecce S, Kornyshev AA, Urbakh M, Bresme F (2020) Electrotunable lubrication with ionic liquids: the effects of cation chain length and substrate polarity. ACS Appl Mater Interfaces 12:4105–4113. https://doi.org/10.1021/acsami.9b19283
Tran CD, De Paoli Lacerda SH, Oliveira D (2003) Absorption of water by room-temperature ionic liquids: effect of anions on concentration and state of water. Appl Spectrosc 57:152–157. https://doi.org/10.1366/000370203321535051
Fazio B, Triolo A, Di Marco G (2007) Local organization of water and its effect on the structural heterogeneities in room-temperature ionic liquid/H2O mixtures. J Raman Spectrosc 38:1538–1553. https://doi.org/10.1002/jrs
Annapureddy HVR, Hu Z, Xia J, Margulis CJ (2008) How does water affect the dynamics of the room-temperature ionic liquid 1-hexyl-3-methylimidazolium hexafluorophosphate and the fluorescence spectroscopy of coumarin-153 when dissolved in it? J Phys Chem B 112:1770–1776. https://doi.org/10.1021/jp077623k
Schröder C, Rudas T, Neumayr G et al (2007) On the collective network of ionic liquid/water mixtures. I Orientational structure J Chem Phys 127:234503. https://doi.org/10.1063/1.2805074
Sakai K, Okada K, Uka A et al (2015) Effects of water on solvation layers of imidazolium-type room temperature ionic liquids on silica and mica. Langmuir 31:6085–6091. https://doi.org/10.1021/acs.langmuir.5b01184
Horn RG, Evans DF, Ninham BW (1988) Double-layer and solvation forces measured in a molten salt and its mixtures with water. J Phys Chem 92:3531–3537. https://doi.org/10.1021/j100323a042
Wang Z, Li H, Atkin R, Priest C (2016) Influence of water on the interfacial nanostructure and wetting of [Rmim][NTf2] ionic liquids at mica surfaces. Langmuir 32:8818–8825. https://doi.org/10.1021/acs.langmuir.6b01790
Fajardo OY, Bresme F, Kornyshev AA, Urbakh M (2017) Water in ionic liquid lubricants: friend and foe. ACS Nano 11:6825–6831. https://doi.org/10.1021/acsnano.7b01835
Han M, Espinosa-Marzal RM (2019) Influence of water on structure, dynamics, and electrostatics of hydrophilic and hydrophobic ionic liquids in charged and hydrophilic confinement between mica surfaces. ACS Appl Mater Interfaces 11:33465–33477. https://doi.org/10.1021/acsami.9b10923
Perez-Martinez CS, Perkin S (2019) Interfacial structure and boundary lubrication of a dicationic ionic liquid. Langmuir 35:15444–15450. https://doi.org/10.1021/acs.langmuir.9b01415
Han M, Espinosa-marzal RM (2018) Molecular mechanisms underlying lubrication by ionic liquids : activated slip and flow. Lubricants 6:64. https://doi.org/10.20944/preprints201807.0248.v3
Arcifa A, Rossi A, Ramakrishna SN et al (2018) Lubrication of Si-based tribopairs with a hydrophobic ionic liquid: the multiscale influence of water. J Phys Chem C 122:7331–7343. https://doi.org/10.1021/acs.jpcc.8b01671
Freire MG, Neves CMSS, Marrucho IM et al (2010) Hydrolysis of tetrafluoroborate and hexafluorophosphate counter ions in imidazolium-based ionic liquids. J Phys Chem A 114:3744–3749. https://doi.org/10.1021/jp903292n
Gusain R, Gupta P, Saran S, Khatri OP (2014) Halogen-free bis(imidazolium)/bis(ammonium)-di[bis(salicylato)borate] ionic liquids as energy-efficient and environmentally friendly lubricant additives. ACS Appl Mater Interfaces 6:15318–15328. https://doi.org/10.1021/am503811t
Petkovic M, Seddon KR, Rebelo LPN, Pereira CS (2011) Ionic liquids: A pathway to environmental acceptability. Chem Soc Rev 40:1383–1403. https://doi.org/10.1039/c004968a
Adams H, Miller BP, Furlong OJ et al (2017) Modeling mechanochemical reaction mechanisms. ACS Appl Mater Interfaces 9:26531–26538. https://doi.org/10.1021/acsami.7b05440
Tysoe W (2017) On stress-induced tribochemical reaction rates. Tribol Lett 65:1–16. https://doi.org/10.1007/s11249-017-0832-x
Adams H, Miller BP, Kotvis PV et al (2016) In situ measurements of boundary film formation pathways and kinetics: dimethyl and diethyl disulfide on copper. Tribol Lett 62:1–9. https://doi.org/10.1007/s11249-016-0664-0
Furlong OJ, Manzi SJ, Martini A, Tysoe WT (2015) Influence of potential shape on constant-force atomic-scale sliding friction models. Tribol Lett 60:1–9. https://doi.org/10.1007/s11249-015-0599-x
Spikes H, Tysoe W (2015) On the commonality between theoretical models for fluid and solid friction, wear and tribochemistry. Tribol Lett 59:1–14. https://doi.org/10.1007/s11249-015-0544-z
Adams HL, Garvey MT, Ramasamy US et al (2015) Shear induced mechanochemistry: pushing molecules around. J Phys Chem C 119:7115–7123. https://doi.org/10.1021/jp5121146
Furlong O, Miller B, Kotvis P et al (2014) Shear and thermal effects in boundary film formation during sliding. RSC Adv 4:24059–24066. https://doi.org/10.1039/c4ra03519d
Furlong OJ, Miller BP, Kotvis P, Tysoe WT (2011) Low-temperature, shear-induced tribofilm formation from dimethyl disulfide on copper. ACS Appl Mater Interfaces 3:795–800. https://doi.org/10.1021/am101149p
Felts JR, Oyer AJ, Hernández SC et al (2015) Direct mechanochemical cleavage of functional groups from graphene. Nat Commun 6:6467. https://doi.org/10.1038/ncomms7467
Raghuraman S, Elinski MB, Batteas JD, Felts JR (2017) Driving surface chemistry at the nanometer scale using localized heat and stress. Nano Lett 17:2111–2117. https://doi.org/10.1021/acs.nanolett.6b03457
Gosvami NN, Bares JA, Mangolini F et al (2015) Mechanisms of antiwear tribofilm growth revealed in situ by single-asperity sliding contacts. Science 348:102–106. https://doi.org/10.1126/science.1258788
Dorgham A, Azam A, Morina A, Neville A (2018) On the transient decomposition and reaction kinetics of zinc dialkyldithiophosphate. ACS Appl Mater Interfaces 10:44803–44814. https://doi.org/10.1021/acsami.8b08293
Mohammadtabar K, Eder SJ, Dörr N, Martini A (2019) Heat-, load-, and shear-driven reactions of di- tert-butyl disulfide on Fe(100). J Phys Chem C 123:19688–19692. https://doi.org/10.1021/acs.jpcc.9b05068
Khajeh A, He X, Yeon J et al (2018) Mechanochemical association reaction of interfacial molecules driven by shear. Langmuir 34:5971–5977. https://doi.org/10.1021/acs.langmuir.8b00315
He X, Kim SH (2018) Surface chemistry dependence of mechanochemical reaction of adsorbed molecules - an experimental study on tribopolymerization of α-pinene on metal, metal oxide, and carbon surfaces. Langmuir 34:2432–2440. https://doi.org/10.1021/acs.langmuir.7b03763
Boscoboinik A, Olson D, Adams H et al (2020) Measuring and modelling mechanochemical reaction kinetics. Chem Commun 56:7730–7733. https://doi.org/10.1039/d0cc02992k
Sawyer WG, Wahl KJ (2008) Accessing inaccessible interfaces: in situ approaches to materials tribology. MRS Bull 33:1145–1150. https://doi.org/10.1557/mrs2008.244
Khare HS, Gosvami NN, Lahouij I et al (2018) Nanotribological printing: a nanoscale additive manufacturing method. Nano Lett 18:6756–6763. https://doi.org/10.1021/acs.nanolett.8b02505
Li Z, Dolocan A, Morales-Collazo O et al (2020) Lubrication mechanism of phosphonium phosphate ionic liquid in nanoscale single-asperity sliding contacts. Adv Mater Interfaces 7:2000426. https://doi.org/10.1002/admi.202000426
Smith AM, Hallett JE, Perkin S (2019) Solidification and superlubricity with molecular alkane films. Proc Natl Acad Sci U S A 116:25418–25423. https://doi.org/10.1073/pnas.1910599116
Dazzi A, Prater CB (2017) AFM-IR: technology and applications in nanoscale infrared spectroscopy and chemical imaging. Chem Rev 117:5146–5173. https://doi.org/10.1021/acs.chemrev.6b00448
Fellows AP, Puhan D, Casford M, Davies P (2020) Understanding the lubrication mechanism of polyvinyl alcohol hydrogels using infrared nanospectroscopy. J Phys Chem C 124:18091–18101. https://doi.org/10.1021/acs.jpcc.0c04782
Acknowledgements
The material is based upon work supported by the Welch Foundation (Grant No. F-2002-20190330), the National Science Foundation Faculty Early Career Development Program (Grant No. 2042304), and the Taiho Kogyo Tribology Research Foundation (Grant No. 20A03). F.M. acknowledges support from the 2018 Ralph E. Powe Junior Faculty Enhancement Award sponsored by the Oak Ridge Associated Universities (ORAU), and from the Walker Department of Mechanical Engineering and the Texas Materials Institute at the University of Texas at Austin.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, Z., Mangolini, F. Recent Advances in Nanotribology of Ionic Liquids. Exp Mech 61, 1093–1107 (2021). https://doi.org/10.1007/s11340-021-00732-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11340-021-00732-7