Abstract
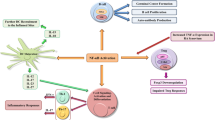
P53 is a transcription factor that regulates many signaling pathways like apoptosis, cell cycle, DNA repair, and cellular stress responses. P53 is involved in inflammatory responses through the regulation of inflammatory signaling pathways, induction of cytokines, and matrix metalloproteinase expression. Also, p53 regulates immune responses through modulating Toll-like receptors expression and innate and adaptive immune cell differentiation and maturation. P53 is a modulator of the apoptosis and proliferation processes through regulating multiple anti and pro-apoptotic genes. Rheumatoid arthritis (RA) is categorized as an invasive inflammatory autoimmune disease with irreversible deformity of joints and bone resorption. Different immune and non-immune cells contribute to RA pathogenesis. Fibroblast-like synoviocytes (FLSs) have been recently introduced as a key player in the pathogenesis of RA. These cells in RA synovium produce inflammatory cytokines and matrix metalloproteinases which results in synovitis and joint destruction. Besides, hyper proliferation and apoptosis resistance of FLSs lead to synovial hyperplasia and bone and cartilage destruction. Given the critical role of p53 in inflammation, apoptosis, and cell proliferation, lack of p53 function (due to mutation or low expression) exerts a prominent role for this gene in the pathogenesis of RA. This review focuses on the role of p53 in different mechanisms and cells (specially FLSs) that involved in RA pathogenesis.


Similar content being viewed by others
Availability of data and material
Not applicable.
Abbreviations
- Ag:
-
Antigen
- AGO2:
-
Argonaute RISC catalytic component 2
- AID:
-
Activation-induced cytidine deaminase
- AP-1:
-
Activator protein 1
- APCs:
-
Antigen-presenting cells
- autoAbs:
-
Autoantibodies
- Bcl2:
-
B cell lymphoma 2
- BRG1:
-
Brahma-related gene 1
- CKI:
-
Cyclin-dependent kinase inhibitor 1
- cyr61:
-
Cysteine-rich angiogenic inducer 61
- DC:
-
Dendritic cells
- DD1α:
-
Death domain1α
- DDRs:
-
DNA damage responses
- DN:
-
Double negative
- DNMTs:
-
DNA methyltransferases
- DUSP:
-
Dual-specificity phosphatases
- ECM:
-
Extracellular matrix
- ERK:
-
Extracellular regulated kinase
- FLSs:
-
Fibroblast-like synoviocytes
- Foxp3:
-
Forkhead box P3
- GADD45A:
-
Growth arrest and DNA damage-inducible alpha
- HATs:
-
Histone acetyltransferases
- HDACs:
-
Histone deacetylases
- HIF-1α:
-
Hypoxia-inducible factor 1-alpha
- IFN-γ:
-
Interferon-γ
- IL:
-
Interleukin
- iNOS:
-
Inducible nitric oxide synthase
- iTreg:
-
Induced Treg
- JNK:
-
C-Jun N-terminal kinase
- MAPK:
-
Mitogen-activated protein kinases
- MDM-2:
-
Mouse double minute 2 homolog
- MIF:
-
Macrophage migration inhibitory factor
- MKP-1:
-
MAPK phosphatase-1
- MLSs:
-
Macrophage-like synoviocytes
- MMPs:
-
Matrix metalloproteinases
- MSH6:
-
MutS Homolog 6
- NF-κB:
-
Nuclear factor-κB
- NO:
-
Nitric oxide
- OA:
-
Osteoarthritis
- P53AIP1:
-
P53-regulated apoptosis-inducing protein 1
- PADI4:
-
Peptidyl arginine deiminase 4
- PBMCs:
-
Peripheral blood mononuclear cells
- PCNA:
-
Proliferating cell nuclear Ag
- PERP:
-
P53 apoptosis effector related to PMP-22
- PGs:
-
Prostaglandins
- PRR:
-
Pattern recognition receptor
- PUMA:
-
P53 upregulated mediator of apoptosis
- REs:
-
Responsive elements
- RA:
-
Rheumatoid arthritis
- RANKL:
-
Receptor activator of nuclear factor-κB ligand
- Rb:
-
Retinoblastoma
- RGS:
-
Regulator of G protein signaling
- RNSs:
-
Reactive nitrogen species
- ROSs:
-
Reactive oxygen species
- SCID:
-
Severe combined immunodeficiency
- SP:
-
Single positive
- TLRs:
-
Toll-like receptors
- TNF-α:
-
Tumor necrosis factor-α
- TP53:
-
P53 tumor suppressor gene
- Treg :
-
T regulatory
- TSP-1:
-
Thrombospondin-1
- VEGF:
-
Vascular endothelial growth factor
- WIP-1:
-
Wild-type p53-induced phosphatase-1
- wt-P53:
-
Wild-type P53
- XIAP:
-
X-linked inhibitor of apoptosis protein
References
Smolen JS, Aletaha D, Barton A, Burmester GR, Emery P, Firestein GS, et al. Rheumatoid arthritis Nature Reviews Disease Primers. 2018;4:18001.
Croia C, Bursi R, Sutera D, Petrelli F, Alunno A, Puxeddu I. One year in review 2019: pathogenesis of rheumatoid arthritis. Clin Exp Rheumatol. 2019;37:347–57.
Smolen JS, Aletaha D, Koeller M, Weisman MH, Emery P. New therapies for treatment of rheumatoid arthritis. The Lancet. 2007;370:1861–74.
Hashimoto M. Th17 in animal models of rheumatoid arthritis. J Clin Med. 2017;6:73.
Yoshida Y, Tanaka T. Interleukin 6 and rheumatoid arthritis. BioMed Res Int. 2014;2014:698313. https://doi.org/10.1155/2014/698313.
Fattahi MJ, Mirshafiey A. Prostaglandins and rheumatoid arthritis. Arthritis. 2012;2012:239310.
Shibuya H, Yoshitomi H, Murata K, Kobayashi S, Furu M, Ishikawa M, et al. TNFα, PDGF, and TGFβ synergistically induce synovial lining hyperplasia via inducible PI3Kδ. Mod Rheumatol. 2015;25:72–8.
Ogata A, Kato Y, Higa S, Yoshizaki K. IL-6 inhibitor for the treatment of rheumatoid arthritis: a comprehensive review. Mod Rheumatol. 2019;29:258–67.
Bottini N, Firestein GS. Duality of fibroblast-like synoviocytes in RA: passive responders and imprinted aggressors. Nat Rev Rheumatol. 2013;9:24.
Tak PP, Zvaifler NJ, Green DR, Firestein GS. Rheumatoid arthritis and p53: how oxidative stress might alter the course of inflammatory diseases. Immunol Today. 2000;21:78–82.
Firestein GS. Invasive fibroblast-like synoviocytes in rheumatoid arthritis Passive responders or transformed aggressors? Arthritis Rheum. 1996;39:1781–90.
Müller-Ladner U, Kriegsmann J, Franklin BN, Matsumoto S, Geiler T, Gay RE, et al. Synovial fibroblasts of patients with rheumatoid arthritis attach to and invade normal human cartilage when engrafted into SCID mice. Am J Pathol. 1996;149:1607.
Arrowsmith CH. Structure and function in the p53 family. Cell Death Differ. 1999;6:1169–73.
Yamanishi Y, Boyle DL, Pinkoski MJ, Mahboubi A, Lin T, Han Z, et al. Regulation of joint destruction and inflammation by p53 in collagen-induced arthritis. Am J Pathol. 2002;160:123–30.
Levine A, Hu W, Feng Z. The P53 pathway: what questions remain to be explored? Cell Death Differ. 2006;13:1027.
Aupperle KR, Boyle DL, Hendrix M, Seftor EA, Zvaifler NJ, Barbosa M, et al. Regulation of synoviocyte proliferation, apoptosis, and invasion by the p53 tumor suppressor gene. Am J Pathol. 1998;152:1091.
Lane DP. Cancer p53 guardian of the genome. Nature. 1992;358:15–6.
Han Z, Boyle DL, Shi Y, Green DR, Firestein GS. Dominant-negative p53 mutations in rheumatoid arthritis. Arthritis Rheum. 1999;42:1088–92.
Malemud C, Haque A, Louis N, Wang J. Immune response and apoptosis–intro-duction. J Clin Cell Immunol Sci. 2012;3:e001.
Tak PP, Smeets TJ, Boyle DL, Kraan MC, Shi Y, Zhuang S, et al. p53 overexpression in synovial tissue from patients with early and longstanding rheumatoid arthritis compared with patients with reactive arthritis and osteoarthritis. Arthritis Rheum. 1999;42:948–53.
Zhang T, Li H, Shi J, Li S, Li M, Zhang L, et al. p53 predominantly regulates IL-6 production and suppresses synovial inflammation in fibroblast-like synoviocytes and adjuvant-induced arthritis. Arthritis Res Ther. 2016;18:271.
Taranto E, Xue JR, Lacey D, Hutchinson P, Smith M, Morand EF, et al. Detection of the p53 regulator murine double-minute protein 2 in rheumatoid arthritis. J Rheumatol. 2005;32:424–9.
Seemayer CA, Kuchen S, Neidhart M, Kuenzler P, Řihošková V, Neumann E, et al. p53 in rheumatoid arthritis synovial fibroblasts at sites of invasion. Ann Rheum Dis. 2003;62:1139–44.
Lee CS, Portek I, Edmonds J, Kirkham B. Synovial membrane p53 protein immunoreactivity in rheumatoid arthritis patients. Ann Rheum Dis. 2000;59:143–5.
Sugiyama M, Tsukazaki T, Yonekura A, Matsuzaki S, Yamashita S, Iwasaki K. Localisation of apoptosis and expression of apoptosis related proteins in the synovium of patients with rheumatoid arthritis. Ann Rheum Dis. 1996;55:442–9.
Mc Gonagle D, Reece R, Green M, Jack A, Veale D, Emery P. p53 Is not demonstrable by immunohistochemistry in early rheumatoid arthritis. Arthritis Rheum. 1997;40:S119-S.
Salvador G, Sanmarti R, Garcia-Peiro A, Rodriguez-Cros J, Munoz-Gomez J, Canete J. p53 expression in rheumatoid and psoriatic arthritis synovial tissue and association with joint damage. Ann Rheum Dis. 2005;64:183–7.
Xiao C, Pan Y, Guo X, Wu Y, Gu J, Cai D. Expression of β-catenin in rheumatoid arthritis fibroblast-like synoviocytes. Scand J Rheumatol. 2011;40:26–33.
Maas K, Westfall M, Pietenpol J, Olsen NJ, Aune T. Reduced p53 in peripheral blood mononuclear cells from patients with rheumatoid arthritis is associated with loss of radiation-induced apoptosis. Arthritis Rheum. 2005;52:1047–57.
Du Y, Deng L, Li Y, Gan L, Wang Y, Shi G. Decreased PERP expression on peripheral blood mononuclear cells from patient with rheumatoid arthritis negatively correlates with disease activity. Clin Dev Immunol. 2013;2013.
Sionov RV and Haupt Y, editors. Apoptosis by p53: mechanisms, regulation, and clinical implications. Springer seminars in immunopathology; 1998: Springer.
Lee S-H, Chang DK, Goel A, Boland CR, Bugbee W, Boyle DL, et al. Microsatellite instability and suppressed DNA repair enzyme expression in rheumatoid arthritis. J Immunol. 2003;170:2214–20.
Yamanishi Y, Boyle DL, Green DR, Keystone EC, Connor A, Zollman S, et al. p53 tumor suppressor gene mutations in fibroblast-like synoviocytes from erosion synovium and non-erosion synovium in rheumatoid arthritis. Arthritis Res Ther. 2004;7:R12.
Firestein GS, Echeverri F, Yeo M, Zvaifler NJ, Green DR. Somatic mutations in the p53 tumor suppressor gene in rheumatoid arthritis synovium. Proc Natl Acad Sci. 1997;94:10895–900.
Altindag O, Karakoc M, Kocyigit A, Celik H, Soran N. Increased DNA damage and oxidative stress in patients with rheumatoid arthritis. Clin Biochem. 2007;40:167–71.
Forrester K, Ambs S, Lupold SE, Kapust RB, Spillare EA, Weinberg WC, et al. Nitric oxide-induced p53 accumulation and regulation of inducible nitric oxide synthase expression by wild-type p53. Proc Natl Acad Sci. 1996;93:2442–7.
Igarashi H, Hashimoto J, Tomita T, Yoshikawa H, Ishihara K. TP53 mutations coincide with the ectopic expression of activation-induced cytidine deaminase in the fibroblast-like synoviocytes derived from a fraction of patients with rheumatoid arthritis. Clin Exp Immunol. 2010;161:71–80.
Goloudina AR, Kochetkova EY, Pospelova TV, Demidov ON. Wip1 phosphatase: between p53 and MAPK kinases pathways. Oncotarget. 2016;7:31563.
Kawauchi K, Araki K, Tobiume K, Tanaka N. Activated p53 induces NF-kappaB DNA binding but suppresses its transcriptional activation. Biochem Biophys Res Commun. 2008;372:137–41.
Son D-S, Kabir SM, Dong Y-L, Lee E, Adunyah SE. Inhibitory effect of tumor suppressor p53 on proinflammatory chemokine expression in ovarian cancer cells by reducing proteasomal degradation of IκB. PLoS ONE. 2012;7:e51116.
Xia Y, Padre RC, De Mendoza TH, Bottero V, Tergaonkar VB, Verma IM. Phosphorylation of p53 by IkappaB kinase 2 promotes its degradation by beta-TrCP. Proc Natl Acad Sci USA. 2009;106:2629–34.
Lim HS, Kim YJ, Kim BY, Jeong SJ. Bakuchiol suppresses inflammatory responses via the downregulation of the p38 MAPK/ERK signaling pathway. Int J Mol Sci. 2019;20.
Yin Y, Liu Y-X, Jin YJ, Hall EJ, Barrett JC. PAC1 phosphatase is a transcription target of p53 in signalling apoptosis and growth suppression. Nature. 2003;422:527.
Takekawa M, Adachi M, Nakahata A, Nakayama I, Itoh F, Tsukuda H, et al. p53-inducible Wip1 phosphatase mediates a negative feedback regulation of p38 MAPK-p53 signaling in response to UV radiation. EMBO J. 2000;19:6517–26.
Cha H, Lowe JM, Li H, Lee J-S, Belova GI, Bulavin DV, et al. Wip1 directly dephosphorylates γ-H2AX and attenuates the DNA damage response. Can Res. 2010;70:4112–22.
Lu X, Ma O, Nguyen T-A, Jones SN, Oren M, Donehower LA. The Wip1 Phosphatase acts as a gatekeeper in the p53-Mdm2 autoregulatory loop. Cancer Cell. 2007;12:342–54.
Perlman H, Bradley K, Liu H, Cole S, Shamiyeh E, Smith RC, et al. IL-6 and matrix metalloproteinase-1 are regulated by the cyclin-dependent kinase inhibitor p21 in synovial fibroblasts. J Immunol. 2003;170:838–45.
van Hamburg JP, Asmawidjaja PS, Davelaar N, Mus AM, Colin EM, Hazes JM, et al. Th17 cells, but not Th1 cells, from patients with early rheumatoid arthritis are potent inducers of matrix metalloproteinases and proinflammatory cytokines upon synovial fibroblast interaction, including autocrine interleukin-17A production. Arthritis Rheum. 2011;63:73–83.
Kimura A, Kishimoto T. IL-6: regulator of Treg/Th17 balance. Eur J Immunol. 2010;40:1830–5.
Yamanishi Y, Boyle DL, Rosengren S, Green DR, Zvaifler NJ, Firestein GS. Regional analysis of p53 mutations in rheumatoid arthritis synovium. Proc Natl Acad Sci U S A. 2002;99:10025–30.
Zheng S-J, Lamhamedi-Cherradi S-E, Wang P, Xu L, Chen YH. Tumor suppressor p53 inhibits autoimmune inflammation and macrophage function. Diabetes. 2005;54:1423–8.
Ameyar M, Wisniewska M, Weitzman J. A role for AP-1 in apoptosis: the case for and against. Biochimie. 2003;85:747–52.
Lin J, Huo R, Xiao L, Zhu X, Xie J, Sun S, et al. A novel p53/microRNA-22/Cyr61 axis in synovial cells regulates inflammation in rheumatoid arthritis. Arthritis Rheumatol. 2014;66:49–59.
Lin J, Zhou Z, Huo R, Xiao L, Ouyang G, Wang L, et al. Cyr61 induces IL-6 production by fibroblast-like synoviocytes promoting Th17 differentiation in rheumatoid arthritis. J Immunol. 2012;188:5776–84.
Paleolog EM. Angiogenesis in rheumatoid arthritis. Arthritis Res. 2002;4(Suppl 3):S81-90.
Dameron KM, Volpert OV, Tainsky MA, Bouck N. Control of angiogenesis in fibroblasts by p53 regulation of thrombospondin-1. Science. 1994;265:1582–4.
Teodoro JG, Parker AE, Zhu X, Green MR. p53-mediated inhibition of angiogenesis through up-regulation of a collagen prolyl hydroxylase. Science. 2006;313:968–71.
Oda S, Oda T, Nishi K, Takabuchi S, Wakamatsu T, Tanaka T, et al. Macrophage migration inhibitory factor activates hypoxia-inducible factor in a p53-dependent manner. PLoS ONE. 2008;3:e2215.
Fingerle-Rowson G, Petrenko O, Metz C, Forsthuber T, Mitchell R, Huss R, et al. The p53-dependent effects of macrophage migration inhibitory factor revealed by gene targeting. Proc Natl Acad Sci. 2003;100:9354–9.
Ravi R, Mookerjee B, Bhujwalla ZM, Sutter CH, Artemov D, Zeng Q, et al. Regulation of tumor angiogenesis by p53-induced degradation of hypoxia-inducible factor 1α. Genes Dev. 2000;14:34–44.
Wymann D, Blüggel M, Kalbacher H, Blesken T, Akdis C, Meyer H, et al. Human B cells secrete migration inhibition factor (MIF) and present a naturally processed MIF peptide on HLA-DRB1* 0405 by a FXXL motif. Immunology. 1999;96:1.
Santos L, Hall P, Metz C, Bucala R, Morand E. Role of macrophage migration inhibitory factor (MIF) in murine antigen-induced arthritis: interaction with glucocorticoids. Clin Exp Immunol. 2001;123:309–14.
Hudson JD, Shoaibi MA, Maestro R, Carnero A, Hannon GJ, Beach DH. A proinflammatory cytokine inhibits p53 tumor suppressor activity. J Exp Med. 1999;190:1375–82.
Liao H, Bucala R, Mitchell RA. Adhesion-dependent signaling by macrophage migration inhibitory factor (MIF). J Biol Chem. 2003;278:76–81.
Mitchell RA, Metz CN, Peng T, Bucala R. Sustained mitogen-activated protein kinase (MAPK) and cytoplasmic phospholipase A2 activation by macrophage migration inhibitory factor (MIF) Regulatory role in cell proliferation and glucocorticoid action. J Biol Chem. 1999;274:18100–6.
Leech M, Lacey D, Xue JR, Santos L, Hutchinson P, Wolvetang E, et al. Regulation of p53 by macrophage migration inhibitory factor in inflammatory arthritis. Arthritis Rheum. 2003;48:1881–9.
Menendez D, Lowe JM, Snipe J, Resnick MA. Ligand dependent restoration of human TLR3 signaling and death in p53 mutant cells. Oncotarget. 2016;7:61630.
Huang QQ, Pope RM. The role of toll-like receptors in rheumatoid arthritis. Curr Rheumatol Rep. 2009;11:357–64.
Wehr P, Purvis H, Law SC, Thomas R. Dendritic cells. T cells and their interaction in rheumatoid arthritis. 2019;196:12–27.
Ade N, Antonios D, Kerdine-Romer S, Boisleve F, Rousset F, Pallardy M. NF-κB plays a major role in the maturation of human dendritic cells induced by NiSO4 but not by DNCB. Toxicol Sci. 2007;99:488–501.
Popa C, van Lieshout AW, Roelofs MF, Geurts-Moespot A, van Riel PL, Calandra T, et al. MIF production by dendritic cells is differentially regulated by Toll-like receptors and increased during rheumatoid arthritis. Cytokine. 2006;36:51–6.
Zhong H, Neubig RR. Regulator of G protein signalingp: novel multifunctional drug targets. J Pharmacol Exp Ther. 2001;297:837.
Wang B, Niu D, Lam T, Xiao Z, Ren E. Mapping the p53 transcriptome universe using p53 natural polymorphs. Cell Death Differ. 2014;21:521–32.
Wang D, Liu Y, Li Y, He Y, Zhang J, Shi G. Gαq regulates the development of rheumatoid arthritis by modulating Th1 differentiation. Mediat Inflamm. 2017;2017.
Wang Y, Li Y, He Y, Sun Y, Sun W, Xie Q, et al. Expression of G protein αq subunit is decreased in lymphocytes from patients with rheumatoid arthritis and is correlated with disease activity. Scand J Immunol. 2012;75:203–9.
Wang Y, Xiao H, Wu H, Yao C, He H, Wang C, et al. G protein subunit α q regulates gastric cancer growth via the p53/p21 and MEK/ERK pathways. Oncol Rep. 2017;37:1998–2006.
McKelvey KJ, Millier MJ, Doyle TC, Stamp LK, Highton J, Hessian PA. Co-expression of CD21L and IL17A defines a subset of rheumatoid synovia, characterised by large lymphoid aggregates and high inflammation. PLoS ONE. 2018;13:e0202135.
Iwaki S, Lu Y, Xie Z, Druey KM. p53 negatively regulates RGS13 protein expression in immune cells. J Biol Chem. 2011;286:22219–26.
Tang BX, You X, Zhao LD, Li Y, Zhang X, Tang FL, et al. p53 in fibroblast-like synoviocytes can regulate T helper cell functions in patients with active rheumatoid arthritis. Chin Med J (Engl). 2011;124:364–8.
Kawashima H, Takatori H, Suzuki K, Iwata A, Yokota M, Suto A, et al. Tumor suppressor p53 inhibits systemic autoimmune diseases by inducing regulatory T cells. J Immunol. 2013;191:3614–23.
Malemud CJ. Defective T-cell apoptosis and T-regulatory cell dysfunction in rheumatoid arthritis. Cells. 2018;7:223.
Shen X-F, Zhao Y, Jiang J-P, Guan W-X, Du J-F. Phosphatase Wip1 in immunity: an overview and update. Front Immunol. 2017;8.
Liu M-F, Yang C-Y, Chao S-C, Li J-S, Weng T-H, Lei H-Y. Distribution of double-negative (CD4− CD8−, DN) T subsets in blood and synovial fluid from patients with rheumatoid arthritis. Clin Rheumatol. 1999;18:227–31.
Samuels J, Ng Y-S, Paget D, Meffre E. Impaired early B-cell tolerance in patients with rheumatoid arthritis. Arthritis Res Ther. 2005;7:1–2.
Tardito S, Martinelli G, Soldano S, Paolino S, Pacini G, Patane M, et al. Macrophage M1/M2 polarization and rheumatoid arthritis: a systematic review. Autoimmun Rev. 2019;18:102397.
Li L, Ng DS, Mah WC, Almeida FF, Rahmat SA, Rao VK, et al. A unique role for p53 in the regulation of M2 macrophage polarization. Cell Death Differ. 2015;22:1081–93.
Oda K, Arakawa H, Tanaka T, Matsuda K, Tanikawa C, Mori T, et al. p53AIP1, a potential mediator of p53-dependent apoptosis, and its regulation by Ser-46-phosphorylated p53. Cell. 2000;102:849–62.
Haupt S, Berger M, Goldberg Z, Haupt Y. Apoptosis-the p53 network. J Cell Sci. 2003;116:4077–85.
Igarashi H, Hirano H, Yahagi A, Saika T, Ishihara K. Anti-apoptotic roles for the mutant p53R248Q through suppression of p53-regulated apoptosis-inducing protein 1 in the RA-derived fibroblast-like synoviocyte cell line MH7A. Clin Immunol. 2014;150:12–21.
Chen S-Y, Shiau A-L, Wu C-L, Wang C-R. P53-derived hybrid peptides induce apoptosis of synovial fibroblasts in the rheumatoid joint. Oncotarget. 2017;8:115413.
Li H, Wan A. Apoptosis of rheumatoid arthritis fibroblast-like synoviocytes: possible roles of nitric oxide and the thioredoxin 1. Mediators Inflamm. 2013;2013:953462.
Xiao P, Hao Y, Zhu X, Wu X. p53 contributes to quercetin-induced apoptosis in human rheumatoid arthritis fibroblast-like synoviocytes. Inflammation. 2013;36:272–8.
Sung M-S, Lee E-G, Jeon H-S, Chae H-J, Park SJ, Lee YC, et al. Quercetin inhibits IL-1β-induced proliferation and production of MMPs, COX-2, and PGE2 by rheumatoid synovial fibroblast. Inflammation. 2012;35:1585–94.
Hou H, Xing W and Li W. Brahma-related gene 1 induces apoptosis in a p53-dependent manner in human rheumatoid fibroblast-like synoviocyte MH7A. Medicine. 2016;95.
Harper JW, Adami GR, Wei N, Keyomarsi K, Elledge SJ. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell. 1993;75:805–16.
El-Deiry WS, Tokino T, Velculescu VE, Levy DB, Parsons R, Trent JM, et al. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993;75:817–25.
Yamanishi Y, Boyle DL, Pinkoski MJ, Mahboubi A, Lin T, Han Z, et al. Regulation of joint destruction and inflammation by p53 in collagen-induced arthritis. Am J Pathol. 2002;160:123–30.
Muñoz-Fontela C, Mandinova A, Aaronson SA, Lee SW. Emerging roles of p53 and other tumour-suppressor genes in immune regulation. Nat Rev Immunol. 2016;16:741.
Nemtsova MV, Zaletaev DV, Bure IV, Mikhaylenko DS, Kuznetsova EB, Alekseeva EA, et al. Epigenetic changes in the pathogenesis of rheumatoid arthritis. Front Genet. 2019;10.
Hodge DR, Peng B, Cherry JC, Hurt EM, Fox SD, Kelley JA, et al. Interleukin 6 supports the maintenance of p53 tumor suppressor gene promoter methylation. Cancer Res. 2005;65:4673–82.
Glant TT, Mikecz K, Rauch TA. Epigenetics in the pathogenesis of rheumatoid arthritis. BMC Med. 2014;12:35.
Grabiec AM. Regulation of inflammation by histone deacetylases in rheumatoid arthritis: beyond. Epigenetics 2012.
Gu W, Roeder RG. Activation of p53 sequence-specific DNA binding by acetylation of the p53 C-terminal domain. Cell. 1997;90:595–606.
Hull EE, Montgomery MR and Leyva KJ. HDAC inhibitors as epigenetic regulators of the immune system: impacts on cancer therapy and inflammatory diseases. BioMed Res Int. 2016;2016.
Horiuchi M, Morinobu A, Chin T, Sakai Y, Kurosaka M, Kumagai S. Expression and function of histone deacetylases in rheumatoid arthritis synovial fibroblasts. J Rheumatol. 2009;36:1580–9.
Shi Y, Lan F, Matson C, Mulligan P, Whetstine JR, Cole PA, et al. Histone demethylation mediated by the nuclear amine oxidase homolog LSD1. Cell. 2004;119:941–53.
Zhai Q, Wang L, Zhao P, Li T. Role of citrullination modification catalyzed by peptidylarginine deiminase 4 in gene transcriptional regulation. Acta Biochim Biophys Sin. 2017;49:567–72.
Bartel DP. MicroRNAs target recognition and regulatory functions. Cell. 2009;136:215–33.
Boominathan L. The tumor suppressors p53, p63, and p73 are regulators of microRNA processing complex. PLoS ONE. 2010;5:e10615.
Mizoguchi F, Hasegawa H and Kohsaka H, editors. Functional screening of micrornas using the inhibitor library identified micrornas to regulate expression of MMP-3 and IL-6 in rheumatoid arthritis synovial fibroblasts. Arthritis Rheumatol; 2016: Wiley
Moran-Moguel MC, Petarra-Del Rio S, Mayorquin-Galvan EE, Zavala-Cerna MG. Rheumatoid arthritis and miRNAs: a critical review through a functional view. J Immunol Res. 2018;2018:2474529.
Niederer F, Trenkmann M, Ospelt C, Karouzakis E, Neidhart M, Stanczyk J, et al. Down-regulation of microRNA-34a* in rheumatoid arthritis synovial fibroblasts promotes apoptosis resistance. Arthritis Rheum. 2012;64:1771–9.
Feng Z, Zhang C, Wu R, Hu W. Tumor suppressor p53 meets microRNAs. J Mol Cell Biol. 2011;3:44–50.
Inazuka M, Tahira T, Horiuchi T, Harashima S, Sawabe T, Kondo M, et al. Analysis of p53 tumour suppressor gene somatic mutations in rheumatoid arthritis synovium. Rheumatology. 2000;39:262–6.
Author information
Authors and Affiliations
Contributions
MT and MA: acquisition of data, drafting the article, designed the figures, final approval of the version to be submitted. EF and MM: the conception and design of the study, revising it critically for important intellectual content, designed the figures, final approval of the version to be submitted. AJ: interpretation of data, revising it critically for important intellectual content, final approval of the version to be submitted.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Mahdi Taghadosi and Mehrnoosh Adib Contributed equally to this paper.
Rights and permissions
About this article
Cite this article
Taghadosi, M., Adib, M., Jamshidi, A. et al. The p53 status in rheumatoid arthritis with focus on fibroblast-like synoviocytes. Immunol Res 69, 225–238 (2021). https://doi.org/10.1007/s12026-021-09202-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12026-021-09202-7