Abstract
One of the most important breakthroughs in oceanography in the last 30 years was the discovery that iron (Fe) controls biological production as a micronutrient, and our understanding of Fe and nutrient biogeochemical dynamics in the ocean has significantly advanced. In this review, we looked back both previous and updated knowledge of the natural Fe supply processes and nutrient dynamics in the subarctic Pacific and its impact on biological production. Although atmospheric dust has been considered to be the most important source of Fe affecting biological production in the subarctic Pacific, other oceanic sources of Fe have been discovered. We propose a coherent explanation for the biological response in subarctic Pacific high nutrient low chlorophyll (HNLC) waters that incorporates knowledge of both the atmospheric Fe supplies and the oceanic Fe supplies. Finally, we extract future directions for Fe oceanographic research in the subarctic Pacific and summarize the uncertain issues identified thus far.
Similar content being viewed by others
1 Introduction
1.1 Iron study in the subarctic Pacific
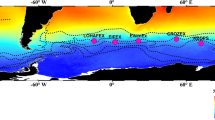
The subarctic Pacific (Fig. 1) is located at the end of the global ocean conveyor belt circulation. A correct understanding of the biogeochemical dynamics in the region is critical for clarifying the global carbon cycle. It has been well-known since the 1980s that there are significant differences in the seasonal cycles of the production processes of the lower trophic level between the subarctic Pacific and the subarctic Atlantic. Parsons and Lalli (1988) indicated that there are clear spring phytoplankton blooms in the subarctic Atlantic, and the seasonal cycle of primary productivity and phytoplankton growth in this area are limited by the depth of mixing (light availability) in spring and nitrate depletion in late summer. In contrast, it was believed that the seasonal cycle of primary productivity in the subarctic Pacific is controlled by low temperature and macro- and microzooplankton grazing in the spring and summer and possibly by an unidentified nutrient (Parsons and Lalli 1988). That is, in the subarctic Pacific, surface waters have much lower biomass at the lower trophic levels than would be expected based on the macronutrient (i.e., nitrate and phosphate) concentrations, similar to the Southern Ocean and the eastern equatorial Pacific Ocean. Oceanographers call these areas “high nutrient low chlorophyll (HNLC) regions”. This unexpected result was one of the main issues in oceanography, and oceanographers debated why very large areas with high nutrients appeared in the ocean throughout the year. In the last three decades, studies to understand the reason for the formation of HNLC water in the global ocean have significantly progressed, and the key to this advancement was understanding “iron (Fe) as a micronutrient” (Martin et al. 1991).
Map of the subarctic Pacific with general circulations and gyres. “WSG” indicates the western subarctic gyre, and “AG” indicates the Alaskan Gyre. The currents and circulations are drawn by referencing Nagata et al. (1992), Harrison et al. (1999), Ohshima et al. (2002), Stabeno et al. (1999) and Hunt et al. (2010). Harrison et al. (1999) indicated that the Subarctic Boundary (Read and Laird 1977) separates the subarctic Pacific region to the north
Many scientific programs have studied Fe in the ocean and atmosphere since the 1980s (Fig. 2). The scientific improvements gained from these Fe studies were recently well-reviewed in Stoll (2020) and Coale et al. (2015), while Takeda (2011) reviewed the research from the subarctic Pacific. Here, we briefly explain “The progress in Fe studies in the ocean”. First, Hart (1934, 1942) reported the idea that phytoplankton growth is limited by Fe availability in the modern ocean. The Geochemical Ocean Sections Study (GEOSECS) project was conducted from 1976 to 1979, and we obtained the first comprehensive global dataset on the distribution of chemical parameters in the ocean (Craig and Turekian 1980; Moore 1984). However, oceanographers could not correctly measure key trace metals, including Fe, in seawater samples because of contamination problems (Moore 1984). Therefore, at that time, the importance of Fe as a limited micronutrient had not been well-described.
Timeline of Fe-related studies, SEAREX project (Duce 1989), GEOSECS (Craig and Turekian 1980), Fe hypothesis (Martin 1990), GEOTRCES (Anderson 2020), OPES (Takeda et al. 1999), SEEDS (Tsuda et al. 2003), SERIES (Boyd et al. 2004), SEEDSII (Tsuda et al. 2007), W-PASS (Uematsu et al. 2014), Amur-Okhotsk project (Shiraiwa et al. 2012), and OMIX project (this study)
In the 1980s, John H. Martin, Director of the Moss Landing Marine Laboratory, and his group first determined dissolved Fe concentrations in oceanic seawater by using a rigorous clean sampling technique (Bruland et al. 1979) and reported that the dissolved Fe in the eastern subarctic Pacific had nutrient-like vertical profiles and that the surface concentrations seemed to be low enough to limit phytoplankton growth (Gordon et al. 1982; Martin et al. 1989). Martin’s group conducted onboard bottle incubation experiments, controlling for extremely low Fe concentrations using a clean technique, and these experimental results demonstrated significant phytoplankton growth upon the addition of Fe to HNLC surface waters (e.g., Martin and Fitzwater 1988; Martin et al. 1989, 1990). Then, he first argued in the part of his “Iron hypothesis” published in Paleoceanography (Martin 1990) that Fe is another nutrient that limits biological production at the lower trophic level, and he claimed that surface phytoplankton growth in HNLC waters, i.e., in the Southern Ocean around Antarctica, the eastern equatorial Pacific and the subarctic Pacific, is limited by Fe availability.
After that, oceanographers accelerated to debate “what controls phytoplankton production in the HNLC area” (Chisholm and Morel 1991). Some oceanographers claimed that “HNLC water is caused by natural overgrazing of algae by zooplankton” (Cullen 1991). To understand “the roles of Fe in regulating biological processes in the marine environment”, it was necessary to clarify the response of the entire planktonic ecosystem community to Fe-enrichment. To tackle this issue, Watson et al. (1991) proposed the “in situ mesoscale experiment”, which artificially manipulated the Fe concentration in seawater to investigate the response of the whole phytoplankton ecosystem to in situ Fe additions to surface water. These experiments were called the “mesoscale iron fertilization experiment (IFE)”, and they have been performed more than 13 times in HNLC waters worldwide (Boyd et al. 2007; Martin et al. 2013). Most of these experiments demonstrated that Fe availability strongly influences phytoplankton growth and carbon and nutrient biogeochemistry in all HNLC regions (de Baar et al. 2005; Boyd et al. 2007). In the subarctic Pacific, three IFEs (SEEDS, SEEDSII, SERIES) have also been conducted (Figs. 1, 2), and the results from these experiments have clearly revealed that phytoplankton biomass is limited by Fe availability in both the western and eastern subarctic Pacific (Tsuda et al. 2003; Boyd et al. 2004). One of the IFEs also demonstrated the simultaneous occurrence of Fe limitation and grazing control on phytoplankton responses in the western subarctic Pacific (Tsuda et al. 2007). In modern oceanography, it has become common knowledge that Fe is an essential nutrient that plays an important role in the control of phytoplankton growth and oceanic biogeochemistry. Since this discovery, many oceanographers have been dedicated to investigating the biogeochemical cycles of Fe in the natural environment.
In the last three decades, backed by Martin’s Fe hypothesis, marine geochemists have also made significant progress in the sampling and analytical techniques used to study the biogeochemistry of trace metals in the ocean (Boyd and Ellwood 2010; Anderson 2020). Our ability to study trace metals in seawater has been largely improved with contamination-free sampling techniques (clean techniques) (e.g., Patterson 1965; Boyle and Edmond 1975; Bruland et al. 1979; de Baar et al. 2008; Measures et al. 2008; Cutter and Bruland 2012) and increased sensitivity, accuracy and precision of analytical methods (e.g., Landing and Bruland 1987; Obata et al. 1993; Measures et al. 1995; Bowie et al. 1998; Wu and Boyle 2002; Bruland and Rue 2001; Sohrin et al. 2008; Sohrin and Bruland 2011). These advances have made it possible to measure Fe concentrations in seawater over a broad range in the field (e.g., Bruland et al. 1994).
Oceanographers called the decade of the 1990s the “Iron age in oceanography” (Coale et al. 1999), and oceanographers realized that determining the distribution of Fe in the global ocean, including the processes involved in biogeochemical cycles, was important for understanding the biological production of the ocean and its impact on the carbon cycle and climate (Coale et al. 1999). Oceanographers discussed “what controls dissolved Fe concentrations in the world ocean? (Johnson et al. 1997)”. To answer this question, a comprehensive dataset of Fe distributions in the global ocean is necessary. This became one motivation for conducting basin-scale transect observations to investigate the marine biogeochemical cycles of trace metals and their isotopes. In 2006, an international GEOTRACES program (GEOTRACES Planning Group 2006) was launched and is ongoing worldwide by marine geochemists (Anderson 2020) (Fig. 2). The Japanese-GEOTRACES program has conducted three extensive transect observations of trace metals, including dissolved Fe, in the Pacific Ocean, and two of these have been conducted across the subarctic Pacific from west to east (Nishioka and Obata 2017; Kim et al. 2017; Zheng et al. 2017; Zheng and Sohrin 2019; Nishioka et al. 2020). These studies clearly revealed the distribution of dissolved Fe in the subarctic Pacific, and the results improved our knowledge of the biogeochemical cycle of Fe and its roles in phytoplankton ecosystems in that area.
1.2 Physical water structure and phytoplankton production in the subarctic Pacific
At the wind-driven circulation depth, the subarctic Pacific consists of the western subarctic gyre (WSG) and the Alaskan gyre (AG) (Nagata et al. 1992; Harrison et al. 1999) (Fig. 1). The WSG is formed by the East Kamchatka Current (EKC), the Oyashio Current and the Subarctic Current (Nagata et al. 1992). The Oyashio Current and the EKC, which are western boundary current, are strongly influenced by origin water that is discharged from the Bering Sea and the Sea of Okhotsk (Nagata et al. 1992), and this discharged water has cold, fresh, nutrient-rich characteristics. The Kuroshio, as the western boundary current of the subtropical North Pacific, transports warm, saline, nutrient-poor water into the midlatitudes of the North Pacific. On the other hand, the AG is a large cyclonic upwelling gyre that is formed by the eastward Subarctic Current and the westward Alaskan Stream (AS), which recirculate near 170°W into the Subarctic Current (Bograd et al. 1999) (Fig. 1). To the east, the gyre is bounded by the Alaska Current (AC) along the continental slope of Alaska (Favorite et al. 1976; Bograd et al. 1999). These two gyres are connected by the eastward Subarctic Current and westward AS (Fig. 1).
Comparisons of biogeochemical properties between the two subarctic gyres have been made in previous studies and have been reviewed by Harrison et al. (1999, 2004). They compared the biology and biogeochemistry between two gyres and indicated that despite their general similarities, such as Fe deficiency, there were clear differences between the two gyres, especially in the lower trophic level. In the WSG, seasonal amplitudes in biogeochemical parameters, e.g., nutrient concentrations, and pCO2, were greater than those in the AG (Shiomoto et al. 1998; Shiomoto and Asami 1999; Harrison et al. 1999, 2004), even though both gyres had HNLC characteristics. Time series studies in both gyres (Whitney and Freeland 1999; Tsurushima et al. 2002) and basin-scale data-mapping analyses (Whitney 2011; Yasunaka et al. 2014, 2020) have demonstrated that higher nutrient concentrations accumulate in the surface water of the WSG than in the AG in winter. Yasunaka et al. (2014) and Yasunaka et al. (2020) indicated that higher seasonal drawdowns of nitrate, phosphate, silicate and dissolved inorganic carbon concentrations occurred in the Oyashio and Oyashio–Kuroshio transition zones than in the AG. Moreover, the effect of biological drawdown on the seasonal amplitude of pCO2 in the surface water was higher in the WSG than in the AG (Takahashi et al. 2002; Chierici et al. 2006). A higher photochemical quantum yield (Fv/Fm), which responds to the Fe supply (Suzuki et al. 2002; Fujiki et al. 2014), and higher chlorophyll a concentrations (1–2 mg/m3) (e.g., Shiomoto et al. 1998; Imai et al. 2002) were observed in the WSG waters than in the AG waters.
In addition, a massive spring phytoplankton bloom was observed every year in the Oyashio waters at the southwestern edge of the WSG, and the massive bloom had significantly higher chlorophyll a concentrations (over 5 mg/m3) than those in the WSG waters (Saito et al. 2002; Yoshie et al. 2010; Okamoto et al. 2010; Sugie et al. 2010; Hattori-Saito et al. 2010; Shiozaki et al. 2014; Isada et al. 2010, 2019; Kuroda et al. 2019).
The increase in the phytoplankton biomass was mainly caused by diatoms (Mochizuki et al. 2002; Fujiki et al. 2014), and this increase led to an effective biological pump for the extensive transport of organic carbon to the deep ocean (Buesseler et al. 2007; Honda 2003; Kawakami et al. 2015). Therefore, the WSG is an important region in the global carbon cycle (Longhurst et al. 1995; Honda 2003; Schlitzer 2004; Buesseler et al. 2007; Boyd et al. 2008).
Similar to the Oyashio region, massive spring phytoplankton blooms were also observed around coastal boundary currents, the AS and AC at the edge of the AG (Whitney et al. 2005; Henson 2007). In contrast to the WSG, blooms in the AS and AC are basically confined to the nearshore area because the boundary current passes the eastern side of the subarctic cyclonic gyre along the west coast of Canada and Alaska.
The differences in the Fe supply between the WSG and AG are needed to explain the eastward decrease in the seasonal amplitude of biogeochemical parameters in the subarctic Pacific. The high primary productivity of the western subarctic region should be fueled by supplies of Fe into the euphotic zone. Wealthy fisheries (Sakurai 2007) in the subarctic region should be sustained by the higher primary productivity, which is caused by the Fe supply.
1.3 The OMIX project and biogeochemical study in the project
In July 2015, the Ocean Mixing Processes (OMIX, PI: I. Yasuda) project was launched and conducted as a Japanese oceanographic scientific program and continued until March 2020. The aim of the project was to develop an efficient system to observe ocean diapycnal mixing with biogeochemical parameters, which can quantify the maintenance mechanism of deep water circulation and biogeochemical cycles in the North Pacific. Diapycnal mixing is a fundamental physical process that regulates ocean vertical circulation of water, nutrients, carbon and heat. One of the goals of the program was to understand “why the western North Pacific has one of the largest seasonal amplitudes of biogeochemical parameters, such as the biological CO2 drawdown (Takahashi et al. 2002, 2009), among the world oceans”. To achieve this goal, we addressed “the natural Fe supply processes and nutrient dynamics in the subarctic Pacific”. Our understanding of Fe and nutrient biogeochemical dynamics in the subarctic Pacific significantly advanced in this decade, which included the OMIX project era. In addition, the OMIX project promoted collaborative research with global international research programs, such as GEOTRACES (https://www.geotraces.org) and SOLAS (https://www.solas-int.org).
The following points (1) and (2) were the remaining major issues related to our understanding of Fe supply processes and nutrient dynamics relevant to biological production in the subarctic Pacific when we started the OMIX project.
-
1.
What are the Fe sources by which seasonal biogeochemical variability is controlled in the subarctic Pacific?
Atmospheric dust, which had been well-studied prior to interior oceanic Fe (e.g., SEAREX project (Duce 1989), Fig. 2), was once thought to be the major source of Fe in many oceanic regions (e.g., Duce and Tindale 1991; Jickells et al. 2005). In addition to atmospheric dust deposition, recent trace metal measurements have highlighted the importance of other sources of external Fe, such as river discharge, shelf sediment load, hydrothermal input and sea ice melting (e.g., Johnson et al. 1999; Elrod et al. 2004; Boyd and Ellwood 2010; Conway and Seth 2014; Tagliabue et al. 2014, 2017; Resing et al. 2015; Lam et al. 2006, 2012; Nishioka et al. 2007; Lam and Bishop 2008; Lam et al. 2012; Lannuzel et al. 2007; Kanna et al. 2014). In the North Pacific, the sedimentary Fe supplied from continental shelves, which is transported by intermediate water circulations, has been highlighted in recent studies (e.g., Lam et al. 2006; Nishioka et al. 2007). Knowledge of the Fe supply processes in the subarctic Pacific was partly compiled and reviewed in Takeda (2011); however, the progress over the last 10 years related to our understanding of the Fe supply processes that control biological production in nutrient-rich waters has been remarkable.
-
2.
Why are there high-nutrient waters at the surface of the subarctic Pacific?
In the subarctic Pacific, as described above, high-nutrient surface water exists, and nutrients also accumulate in old deep water (Broecker et al. 1982; Matsumoto 2007; Matsumoto and Kay 2004). In previous 14C observations in the North Pacific, the oldest water was clearly observed at depths of approximately 2000–2500 m, and the deep water returned southward below the intermediate water (Broecker et al. 1982; Kawabe and Fujio 2010). These facts indicate that high-nutrient deep water does not directly affect the surface layer in the subarctic Pacific. Previous studies implied that the path of nutrient return to the surface existed in the northwest corner of the Pacific around the Kuril Island chain (Sarmiento and Gruber, 2006; Nishioka et al. 2013). However, the processes by which high-nutrient waters are maintained in the subarctic Pacific surface are not understood due to a lack of knowledge of the whole and detailed mechanisms by which nutrients return to the surface layer.
In this review, we look back on both previous and updated knowledge, including new findings from the OMIX project, and describe reasonable explanations of the natural Fe supply processes and macronutrient dynamics in the subarctic Pacific. Then, we discuss the natural Fe supply impact on biological production.
2 Fe sources and transport in the subarctic Pacific
2.1 Definition of Fe terminology in seawater in this review
As described in the Sect 1, the development of trace metal clean techniques and sensitive analytical methods has led to increasing Fe data in seawater over the past three decades. Bruland and Rue (2001) reviewed detailed steps for the determination of Fe and the operational definition of Fe in seawater. In most recent measurements of Fe in seawater, the use of conventional filtration to define the traditional categories of “dissolved” (< 0.2 μm) and “particulate” (> 0.2 μm) fractions was employed for the first physical separation step performed prior to any further chemical analyses. The unfiltered sample and filtrate are acidified (pH ~ 2) by adding ultraclean acid before analysis, and then samples are determined by various analytical methods (e.g., Landing and Bruland 1987; Obata et al. 1993; Measures et al. 1995; Bowie et al. 1998; Wu and Boyle 2002; Bruland and Rue 2001; Sohrin et al. 2008; Sohrin and Bruland 2011). In this review, we use the terms “dissolved Fe”, “total dissolvable Fe”, “labile particulate Fe” and “bioavailable Fe”. The definition of “dissolved Fe” in this review is leachable and detectable Fe in the “dissolved” fraction (in filtrate < 0.2 μm) at pH ~ 2 (Fig. 3). The definition of “total dissolvable Fe” in this review is leachable and detectable Fe in unfiltered seawater at pH ~ 2, which includes “dissolved Fe” and “labile particulate Fe” (Fig. 3). The “labile particulate Fe” is leachable Fe in the “particulate” fraction (> 0.2 μm) at pH ~ 2 (Fig. 3). Some studies conducted additional ultrafiltration to determine the “soluble Fe” and “colloidal Fe” fraction by using smaller pore size filter (0.02 μm, 0.03 μm, 200 kDa, 1000 kDa, e.g., Kuma et al. 1996; Nishioka et al. 2001; Wu and Boyle, 2001; Fitzsimmons and Boyle 2014; Fitzsimmons et al. 2015) before acidifying the sample, and the size-fractionated Fe in the “dissolved” fraction revealed that there are significant portions of “colloidal Fe” [0.02 (0.03) μm–0.2 μm, or 200 (1000) kDa–0.2 μm], which can be separated from “soluble Fe” [< 0.02 (0.03) μm, or < 200 (1000) kDa]. We used this definition of “colloidal Fe” in this review (Fig. 3). We also use the term “bioavailable Fe” for Fe that can be taken up by phytoplankton in seawater in this review.
2.2 Fe distribution in the North Pacific
Data on Fe from the North Pacific collected during the last decade, which were mostly collected in the summer season, clearly show significant progress in our knowledge of Fe sources and distribution. Anderson (2020) reviewed and highlighted the findings of the first decade of the international GEOTRACES program, indicating that “an unexpected finding is the widespread plumes of elevated dissolved Fe concentrations spreading seaward from continental slopes in the marginal seas in the North Pacific”.
In the last decade, the Japanese-GEOTRACES program conducted extensive transect observations of the dissolved Fe in the Pacific Ocean, and two of those (conducted from August–October in 2012 and June–August 2017 by the R/V Hakuho Maru, KH-12-4 and KH-17-3 cruises) were conducted across the subarctic Pacific, which covers the full west–east section of the subarctic Pacific along 47°N (GEOTRCES line ID: GP02). The results of the basin-scale zonal high-resolution transect profile of trace metals along 47°N have been reported in several published papers (Kim et al. 2017; Nishioka and Obata 2017; Zheng and Sohrin 2019; Nishioka et al. 2020) and provide a comprehensive picture of the dissolved Fe distribution (Nishioka and Obata 2017; Zheng and Sohrin 2019; Nishioka et al. 2020) (Fig. 4a). The observations showed very low dissolved Fe concentrations ranging from 0.05 to 0.12 nM in surface water (Fig. 4b), with a significant amount of macronutrients, through the transect. These studies confirmed that the surface waters in both gyres were typical HNLC waters, as indicated by previous studies in the AG (Martin and Gordon 1988; Martin et al. 1989). Prior to the GEOTRACES observations, Lam et al. (2006) first reported that the eastern side of the subarctic Pacific has a continental shelf source of dissolved Fe along the AS, and water with high concentrations of dissolved Fe was observed along the eastern edge and northern edge of the AG by several studies (Cullen et al. 2009; Wu et al. 2009; Zheng and Sohrin 2019; Nishioka and Obata 2017; Nishioka et al. 2020). This water with high concentrations of dissolved Fe in the AC and the AS was confined to the nearshore area on the west coast of Canada and Alaska (Fig. 4c) because boundary currents (the AC and AS) pass along the coast. Several previous studies also indicated that at the western edge of the AG, the Fe-rich waters were transported by the AS, which detached from the Alaskan margin toward center of the AG at approximately 165°–170°W (Martin et al.1989; Lam et al. 2006; Takata et al. 2006; Lippiatt et al. 2010; Nishioka and Obata 2017; Zheng and Sohrin 2019; Nishioka et al. 2020).
a Constructed diagrams showing the 3D distribution of dissolved Fe. The diagrams are cited and modified from Nishioka et al. 2020, Proc Natl Acad Sci, www.pnas.org/cgi/doi/10.1073/pnas.2000658117, under a CC BY license. Ocean Data View (R. Schlitzer, Ocean Data View, https://odv.awi.de/) was used to produce plots for the vertical section profiles of dissolved Fe in these figures. b Horizontal distribution of surface dissolved Fe. This figure is cited and modified from Nishioka et al. 2020, Proc Natl Acad Sci, www.pnas.org/cgi/doi/10.1073/pnas.2000658117, under a CC BY 4.0 license. c Vertical section profile of dissolved Fe along the GP02 line in the map of b. d same as c but along Okh-155 in the map of b. The section profiles are cited and modified from Nishioka et al. 2013, Glob Biogeochem Cycle, https://doi.org/10.1002/gbc.20088, under AGU permissions policy, Nishioka et al. 2020, Proc Natl Acad Sci, www.pnas.org/cgi/doi/10.1073/pnas.2000658117, under a CC BY 4.0 license
On the other hand, on the western edge of the WSG, the dissolved Fe concentration was well-studied in the Oyashio water and reported by Nakayama et al. (2010) and Nishioka et al. (2011). Both studies indicated that surface dissolved Fe concentrations were controlled by the Coastal Oyashio water (COW) distribution. The COW had a higher dissolved Fe concentration (up to ~ 3 nM) than other Oyashio waters, which had moderate dissolved Fe concentrations (0.3–0.5 nM). Nishioka et al. (2011) also reported the results of multiyear (2003–2008) time series observations along the A line, which crosses the Oyashio flow (Fig. 1), and indicated that there was an annual cycle in the concentration of surface dissolved Fe in the Oyashio water, which is similar to the annual cycle of macronutrients controlled by mixing and phytoplankton utilization (Fig. 5a–c). Nishioka et al. (2007) measured vertical profile of dissolved Fe in open oceanic water in the WSG (44°N, 155°E, station KNOT in Fig. 1) in winter season (16 March 2003). They reported higher dissolved Fe concentration (~ 0.3 nM) in the winter surface mixed layer than that of other season (Fig. 6). However, there is still a lack of knowledge in terms of our understanding of the seasonal Fe variation in open oceanic water, such as in the WSG. Time series Fe observations obtained from the open oceanic water of the WSG are still needed.

modified from Nishioka et al. 2011, J Geophys Res-Ocean, https://doi.org/10.1029/2010JC006321, under AGU permissions policy
Monthly compiled data by multiyear observations (2003–2008) for a mixed layer depth, b surface dissolved Fe concentration, and c surface nitrate concentration along the A line in the Oyashio region in Fig. 1, Error bars represent ± 1 SD value. These figures are cited and

modified from Nishioka et al. 2007, J Geophys Res-Ocean, https://doi.org/10.1029/2006JC004055, under AGU permissions policy
Vertical profiles of dissolved Fe and salinity in open oceanic water in the WSG (44 °N, 155 °E, Station KNOT in Fig. 1) in March, April and May. These figures are cited and
In the WSG, the most prominent feature of the dissolved Fe distribution is the existence of water masses with high-dissolved Fe concentration (~ 1.8 nM) in the intermediate layer, as seen along both the meridional and latitudinal dissolved Fe sections reported by Nishioka et al. (2013), Nishioka and Obata (2017), Zheng and Sohrin (2019) and Nishioka et al. (2020) (Fig. 4c, d). From these observation data, it was clear that water masses with high-dissolved Fe concentrations existed from the bottom of the surface mixed layer to the intermediate layer across the WSG over 2000 km (Fig. 4c, d). Judging from the horizontal dissolved Fe distributions at the isopycnal surface, the Fe-rich intermediate water likely reflected the location of the Fe source region, the direction of the intermediate water circulation, the strong western boundary current, and the long-distance lateral Fe transport within the North Pacific (Fig. 7a, b) (we discuss the “long-distance lateral Fe transport” in Sect. 2.3.3).
a Horizontal distribution of dissolved Fe on the 27.0 σθ isopycnal surface. b Same as a) but on the 27.5 σθ isopycnal surface. These figures are constructed using data from Nishioka et al. 2020, Proc Natl Acad Sci, www.pnas.org/cgi/doi/10.1073/pnas.2000658117, under a CC BY 4.0 license. c Section distribution of the vertical dissolved Fe gradient (ddissFe/dz) obtained from vertical profiles of dissolved Fe along GP02 (plus value: dissolved Fe increase with depth). d Section distribution of the vertical diffusive coefficient Kρ (logarithm) along GP02
Zheng and Sohrin (2019) determined the importance of lithogenic particulate Fe for understanding the distribution and budget of Fe in the North Pacific Ocean. Based on their measurements of Fe, Al and Mn, they demonstrated that waters near the continental shelf and slope had high labile particulate Fe concentrations. They inferred that boundary scavenging occurred for labile particulate Fe within 500 km off the Aleutian shelf. They also reported that the dissolved Fe was enhanced in a depth range of 400–2000 m at the center of the WSG. They also suggested, with their Mn data, that continental shelf and slope sediments produced the observed unique distribution of the dissolved Fe, and they claimed that the maxima in the intermediate water were a general feature of dissolved Fe because the feature was also observed at the eastern edge of the AG along the west coast of Canada.
Zheng et al. (2017) also observed continental margins and hydrothermal sources of Fe around the Juan de Fuca Ridge on the eastern side of the Pacific near the west coast of Canada. The influence of Fe from the continental margin appeared from the surface to a depth of ~ 2000 m. Iron from a high-temperature hydrothermal plume was observed at a depth of 2300 m off the west coast of Vancouver Island.
In the GEOTRACES program, other trace metals have been measured, and these results helped advance our understanding of the Fe biogeochemical cycle in the subarctic Pacific. Kim et al. (2017) measured dissolved zinc (Zn) in seawater along section GP02 and indicated that high-dissolved Zn concentrations and high Zn* index anomalies (Zn and Si relationship) existed at the boundary between the intermediate water and deep water in the western and central subarctic Pacific. From the results, they suggested that simple vertical remineralization and accumulation from the particulate form are not the only processes for controlling the dissolved Zn distributions, and there are other biogeochemical controls acting on the dissolved Zn in the North Pacific intermediate water (NPIW) to the deep water. They inferred that the influence of the water from the Bering Sea might provide important information for understanding the Zn cycle in the North Pacific.
As these reports indicate, the remarkable feature of chemical element distribution in the North Pacific probably appears to be linked to the formation of intermediate waters. Therefore, it is necessary to understand the detailed relationship between intermediate water formation and chemical properties.
2.3 Fe transport from subpolar marginal seas
The study of the subpolar marginal seas (the Sea of Okhotsk and the Bering Sea) is indispensable for understanding the intermediate water circulation in the North Pacific. Compared with open ocean areas, the marginal seas have high productivity and active biogeochemical cycling, which are controlled by separate local processes, such as freshwater discharge, interior current systems, tidal mixing, local upwelling, interactions with the continental shelf, and sea ice production and melting. Water exchange occurs between the North Pacific and the marginal seas through straits. These marginal seas have been shown to have a strong influence on physical and biogeochemical processes in the North Pacific. Therefore, it is important to describe the role of the marginal seas in linking with outer oceanic regions to clarify the whole North Pacific biogeochemical system. Following previous projects, such as CREST (1996–2001) and the Amur-Okhotsk project (2004–2009), the OMIX project (Fig. 2) continued collaborative research with the Russian scientist Dr Y. N. Volkov, Director of the Far Eastern Regional Hydrometeorological Research Institute (FERHRI), and conducted joint observational research expeditions in the Sea of Okhotsk, around the EKC, and the western part of the Bering Sea. The dataset from the collaborative cruises, together with the data from the Japanese research cruises, allowed us to collectively create a borderless dataset for the North Pacific, including the marginal seas.
2.3.1 The Sea of Okhotsk
The Sea of Okhotsk is a marginal sea located on the northwest rim of the Pacific Ocean. The water circulation and ventilation system in this region was well-studied by physical oceanographers in 1997–2002 during the CREST project (PI: M. Wakatsuchi; e.g., Ohshima and Martin 2004). After that, based on the obtained physics knowledge, biogeochemical studies were conducted and continued from the Amur-Okhotsk project (2005–2010) (PI: T. Shiraiwa; e.g., Shiraiwa et al. 2012; Nishioka et al. 2014a) to the OMIX project. We provide an overview of the results of the findings relevant to Fe and nutrient dynamics in this review. The Sea of Okhotsk is the seasonal sea ice area at the lowest latitude in the world (Alfultis and Martin 1987; Kimura and Wakatsuchi 2000). Every winter, large amounts of sea ice are produced along the Siberian coast on the northwestern continental shelf of the Sea of Okhotsk as a result of the cold winter winds that blow from East Siberia coupled with the freshwater discharge from the Amur River. Nagao et al. (2007) reported that the water from the Amur River in summer contained approximately 5.3 μM dissolved Fe, which was four to five orders of magnitude greater than that in coastal seawater. Nishioka et al. (2014b) found that a large amount of dissolved Fe in Amur River waters was lost (more than 99%) through estuarine processes, such as flocculation and precipitation. However, dissolved Fe, which probably bound to organic ligands, remained at the surface and was transported by the southward boundary current. Ohshima et al. (2002) conducted near-surface observations with ARGOS drifters and revealed the existence of a southward boundary current, the East Sakhalin Current (hereafter the ESC), off the Sakhalin coast. Nishioka et al. (2014b) observed the Fe concentrations along the ESC in the late summer season (August to September) and estimated that the amount of total dissolvable Fe transported at the surface layer by the ESC along the northern Sakhalin coast was 1.6 × 107 – 2.3 × 107 mol year−1, and this source of Fe stimulated diatom growth (Suzuki et al. 2014).
During the winter, the formation of sea ice produces a large volume of cold brine. The brine subsequently settles on the bottom of the northwestern continental shelf and forms dense shelf water (DSW 26.8–27.0 σθ) (Kitani 1973; Nagata et al. 1992; Martin et al. 1998; Gladyshev et al. 2000). Nakatsuka et al. (2002, 2004) found that the DSW had an extremely high turbidity caused by sediment resuspension from the bottom. After that, Nishioka et al. (2013, 2014b) showed the vertical profiles of dissolved Fe and total dissolvable Fe (including labile particulate Fe) concentrations in the northwestern continental shelf area in late summer and reported that extremely high Fe concentrations were observed in the DSW layer with high turbidity and negative temperature waters. By using the N* index (calculated from the nitrate and phosphate concentrations; Gruber and Sarmiento 1997; Yoshikawa et al. 2006), which behaves as a suitable tracer for the influence of hypoxic bottom sediment, Nishioka et al. (2014b) concluded that Fe was introduced to the DSW by sediment resuspension (Fig. 8a).
a Horizontal distribution of N* [N* = ([Nitrate + Nitrite] − 16[Phosphate] + 2.9) × 0.87] on the 27.0 σθ isopycnal surface. b Horizontal distribution of nitrate + nitrite on the 27.0 σθ isopycnal surface. c Horizontal distribution of phosphate on the 27.0 σθ isopycnal surface. d Horizontal distribution of regenerated phosphate percentage on the 27.0 σθ isopycnal surface. These figures are constructed using data from Nishioka et al. 2020, Proc Natl Acad Sci, www.pnas.org/cgi/doi/10.1073/pnas.2000658117, under a CC BY 4.0 license
The DSW-sourced sedimentary Fe was transported by an intermediate water ventilation system in the Sea of Okhotsk and the North Pacific (Fig. 7a). Fukamachi et al. (2004) reported that the region off the east coast of Sakhalin is an important pathway of the DSW from its production in the northwestern shelf region to the southern Sea of Okhotsk. Observations of chlorofluorocarbons as passive tracers for water masses have also indicated the paths of ventilated DSW water masses (Wong et al. 1998; Yamamoto-Kawai et al. 2004). The DSW tends to penetrate the upper layer of the Okhotsk Sea Intermediate Water (OSIW) (Fukamachi et al. 2004; Itoh et al. 2003) at a water density of approximately 27.0 σθ. The OSIW flows southward along the East Sakhalin coast. Nakatsuka et al. (2002, 2004) also noted that an efficient system of sediment material transport exists from the northwestern continental shelf to the open sea via OSIW transportation. Nishioka et al. (2014b), Yamashita et al. (2020) and Shigemitsu et al. (2013) reported that total dissolvable Fe and dissolved Fe were transported from the continental shelf by the OSIW, and Nishioka et al. (2014b) estimated that 20% of the total dissolvable Fe obtained from DSW on the shelf reached the southern part of the Sea of Okhotsk, Kuril Basin.
Finally, the OSIW is discharged through the Bussol’ Strait into the subarctic Pacific (Katsumata et al. 2004; Katsumata and Yasuda 2010) after strong vertical diapycnal mixing in the Kuril Straits (Itoh et al. 2010, 2011; Yagi and Yasuda 2012; Ono et al. 2013). Then, the discharged intermediate waters from the Sea of Okhotsk contribute to the formation of the NPIW (Talley 1991; Yasuda 1997; Nakamura and Awaji 2004; Nakamura et al. 2006). The water properties of the Oyashio region are strongly influenced by the intermediate water that originates in the Sea of Okhotsk (Yasuda 1997; Yasuda et al. 2001), and high-dissolved Fe concentrations have been observed in the intermediate water in the Oyashio region and in the NPIW (Nishioka et al. 2011, 2013) (Figs. 4d, 7a). Other studies have indicated that injections of large amounts of dissolved organic matter (DOM) from the Sea of Okhotsk lead to increased DOM concentrations in the NPIW (Hansell et al. 2002; Hernes and Benner 2002; Yamashita and Tanoue 2008). Nishioka et al. (2007) hypothesized that the intermediate water circulation processes in this region control the transportation of Fe, and the hypothesis was validated by observations in this region (Nishioka et al. 2011, 2013, 2014b, 2020).
Yasuda et al. (2001) used a potential vorticity to identify water masses coming from the Sea of Okhotsk or WSG in the Oyashio region, indicating that the upper intermediate water density range (26.6–27.0 σθ) is strongly influenced by the OSIW, and the lower intermediate water density range (27.0–27.5 σθ) is influenced mainly by the WSG and the EKC. This knowledge from physics is consistent with the explanation of the dissolved Fe distribution in the WSG and its surrounding waters. Nishioka et al. (2020) reported the results of the isopycnal analysis for dissolved Fe with the compiled Fe dataset. The dissolved Fe-rich water in the upper intermediate water exists mainly west of 155°E (Fig. 7a; 27.0 σθ isopycnal surface) with higher dissolved oxygen (DO) than that in the surrounding water, indicating that the waters are derived from the OSIW and propagate along the upper intermediate layer isopycnal surface to the western North Pacific.
2.3.2 The Bering Sea
In the OMIX project era, from June to July 2014 and August to September 2018, observational studies using a Russian research vessel were conducted around the EKC and the western Bering Sea, which included the Bering Sea Basin and Gulf of Anadyr [the cruise in 2018 was a collaborative expedition with the Arctic Challenge for Sustainability (ArCS) project]. In the OMIX project, the aim of our observational study was to investigate the biogeochemistry in the source waters of the upper stream of the Oyashio Current and the EKC to understand how to set the chemical properties of the intermediate water in the North Pacific. For that, observations of the EKC and the western Bering Sea were essential (Fig. 1).
The physical background of the Bering Sea has been well-reported by Nagata et al. (1992) and Stabeno et al. (1999) and is briefly explained from their study as follows. The Bering Sea is a semi-enclosed, high-latitude sea that is bounded on the north and west by Russia, on the east by Alaska, USA, and the south by the Aleutian Islands. The Bering Sea is divided between a deep basin (maximum depth 3500 m) and continental shelves (< 200 m). Cyclonic gyre circulation exists in the basin, with the southward-flowing western boundary current, the EKC, and the northward-flowing eastern boundary current, “the Bering Slope Current”. Even though only three passes, Amchitka Pass, Near Strait, and Kamchatka Strait, extend deeper than 700 m, the AS enters the Bering Sea through the many passes in the Aleutian Island chain, and the inflow is balanced by outflow through the Kamchatka Strait. Therefore, the circulation in the Bering Sea basin may be more aptly described as a continuation of the North Pacific subarctic gyre. The flow into the Bering Sea through the major passes in the Aleutian Island chain strongly influences the chemical water properties of the basin, and the basin intermediate water flows out from the Kamchatka Strait and Near Strait, the two most western straits along the Aleutian Island chain, to the Pacific side (Stabeno et al. 1999).
Aguilar-Islas et al. (2007) reported surface Fe concentrations in the southeastern region of the Bering Sea. They observed Fe-limited HNLC surface waters in the deep basin of the Bering Sea and nitrate-limited and Fe-replete surface waters over the shelf. They also observed high biomass at the shelf break “Green Belt”, and the dominant diatoms at the Green Belt were stressed by low Fe availability. Hurst and Bruland (2007) and Hurst et al. (2010) reported that particulate Fe in resuspended sediment on the Bering Sea shelf contains a high concentration of the labile particulate Fe fraction, and Buck and Bruland (2007) reported that the concentration of dissolved Fe in the southeastern Bering Sea was strongly correlated with the organic ligand concentrations. Cid et al. (2011) also reported dissolved Fe and labile particulate Fe concentrations on the Bering Sea shelf, and they indicated that Yukon river input, sedimentary reduction and biogeochemical cycles influence to the dissolved Fe distribution. Uchida et al. (2013) reported vertical profiles of nutrient and Fe concentrations in the basin area. Sea ice formation occurs in the northern Bering Sea shelf area. In contrast to the Sea of Okhotsk, however, there is no clear evidence that the sedimentary Fe on the shelf has been transported via the water ventilation process to the basin water.
The lower intermediate water density range (27.0–27.5 σθ) in the WSG is influenced mainly by the EKC and western Bering Sea (Yasuda et al. 2001). In the lower intermediate water density range (27.0–27.5 σθ), the dissolved Fe is high across a wide area in the WSG, including the basin in the Bering Sea and around the eastern Aleutian Islands (Nishioka et al. 2020) (Fig. 7b). The depth of high-Fe water in the lower intermediate water was consistent with the DO minimum and nutrient maximum water in the WSG (Fig. 8b, c). Therefore, the high-Fe properties in the lower intermediate water are probably distributed throughout the circulation of the lower intermediate water in the WSG, including the Bering Sea basin (Nishioka et al. 2020) (Fig. 7b). The source of high dissolved Fe in the lower intermediate water is still under debate.
2.3.3 Long-distance lateral Fe transport
To evaluate the Fe supply by oceanic processes in the North Pacific, long-distance transport mechanisms of sedimentary Fe have become the key for understanding its impact on biological production in remote oceanic areas.
In the AG, the role of eddies in transporting Fe has been well-studied. It was indicated that coastal water and the Fe trapped in the eddy, which has high total labile particulate and dissolved Fe captured during eddy formation, were transported to the open ocean in the AG (e.g., Johnson et al. 2005; Brown et al. 2012). The Fe content of the eddies decreased rapidly during the first year after the eddy formed; however, the high total dissolvable Fe signature in the eddy could be tracked until 16 months after its formation. Xiu et al. (2011) calculated an averaged upwelling dissolved Fe flux based on observations and models, indicating that the eddy-derived Fe supply rate was comparable to the pulsed Fe input from volcanic ash deposition. In the OMIX study, the roles of the eddy in the WSG, south of the western Aleutian Islands (Aleutian eddy), were investigated by Dobashi et al. (2021), indicating that the observed eddy did not have high Fe concentrations in either the dissolved or the particulate fractions, probably because the age of the eddy was old enough for Fe to be removed.
In the WSG, as described above, the water ventilation in the Sea of Okhotsk and intermediate water formation processes transported dissolved Fe at the basin scale. Similar Fe transport was reported in the western Arctic Ocean by Hioki et al. (2014) and Kondo et al. (2016); however, the Fe transport processes by water ventilation from the Sea of Okhotsk observed in the WSG occurred over a significantly larger scale (more than a few thousand kilometers) than those in the western Arctic Ocean. In addition, the waters that had persistently high Fe concentration properties with extremely low DO and high regenerated nutrients in the lower intermediate water in the whole WSG, including the Bering Sea basin, probably have longer residence times. Additional evidence of the long-distance transport of sedimentary Fe has been provided by Conway and John (2015). They presented seawater dissolved Fe isotope ratio profiles in the subtropical Northeast Pacific, indicating that water enriched in sedimentary Fe is likely transported across the Pacific via NPIW.
Inorganic Fe solubility is extremely low in oxic seawater (Byrne and Kester 1976; Kuma et al. 1996). Because the inorganic form of Fe reacts with oxygen to make oxyhydroxide particles and is adsorbed onto particles in seawater, Fe tends to be scavenged from seawater (Lohan and Bruland 2008). However, it is well-known that most dissolved Fe exists in complex form with natural dissolved organic ligands in seawater (van den Berg 1995; Rue and Bruland 1995), and Fe solubility has been found to be controlled by organic ligands (Kuma et al. 1996; Tani et al. 2003; Kitayama et al. 2009). Therefore, the mechanisms that make Fe soluble into seawater by organic ligands and the dynamics of the organic ligands in the subarctic Pacific are critical to understand the external Fe input relevant to biological production.
Siderophores, humic substances, exopolymer substances and phytoplankton cell breakdown ligands are expected to act as natural organic ligands in seawater; each ligand has a different character and chemical structure (e.g., Hassler et al. 2011, 2017). Since the source and sink of organic ligands are variable depending on area and depth, a competitive ligand equilibration-adsorptive cathodic stripping voltammetric (CLE-ACSV) method (van den Berg 1995) has been commonly used to determine the concentration and conditional stability constants of mixtures of natural organic ligands in seawater samples (e.g., Gledhill and van den Berg 1994; Rue and Bruland 1995). Although little is known about the distribution of these natural organic ligands in the subarctic Pacific, the accumulation of organic ligands in the biogeochemical cycle of deep waters (below 3000-m depth) has been observed (Kondo et al. 2012). The concentration of dissolved Fe at depths ranging from 1000 to 2000 m exceeded that of organic ligands in this area, suggesting that excess dissolved Fe existed as organic/inorganic colloidal Fe and/or inorganic complexes that were not detectable by the CLE-ACSV method described in Kondo et al. (2012). The existence of colloidal Fe has also been suggested from the measurement of Fe solubility (Kuma et al. 1996; Nakabayashi et al. 2002; Tani et al. 2003; Kitayama et al. 2009).
Yamashita et al. (2020) measured humic-like florescent dissolved organic matter (FDOMH), which is a quantitative parameter of humic substances, with the dissolved Fe concentration. They distinguished shelf humic substances (allochthonous FDOMH) from in situ produced humic substances (autochthonous FDOMH) using apparent oxygen utilization, and they separated chemical species of dissolved Fe into allochthonous FDOMH-Fe complexes, autochthonous FDOMH-Fe complexes, and colloidal Fe. The spatial distribution of individual chemical species of dissolved Fe from the northwestern shelf of the Sea of Okhotsk to the subtropical Pacific through the WSG clearly shows that the shelf humic substances bound to Fe form complexes and carry dissolved Fe at least 4000 km by the circulation of the upper intermediate water. On the other hand, they also found that in the lower intermediate water at the WSG, Fe bound to autochthonous humic-like substances and colloidal Fe dominated. They concluded that humic substances were probably one of the key organic ligands and controlled the dissolved Fe distribution in the ocean interior, as reported by other previous studies (Kitayama et al. 2009; Laglera and van den Berg 2009; Laglera et al. 2011). Misumi et al. (2013) used numerical modeling to represent humic substances as organic ligands to control the factor of dissolved Fe distribution in seawater.
Colloidal Fe supplied from the shelf of the Sea of Okhotsk seemed to be transported long distances (at least 3000 km) through the WSG by the circulation of the upper and lower intermediate waters (Yamashita et al. 2020). In addition, Nishioka et al. (2003) conducted size-fractionated Fe measurements and indicated that colloidal Fe existed at significant amounts in the intermediate water in the western North Pacific and that it is also possible that this Fe form has a longer residence time in seawater. Kondo et al. (2021) investigated size-fractionated dissolved Fe and ligand distributions in the WSG (47°N, 160°E) and AG (50°N, 145°W), and indicated that the concentration of dissolved organic ligands around the lower intermediate water in the WSG was higher than that of eastern intermediate water and that Fe in organic and inorganic colloid formations are potentially essential for Fe transport mechanisms in the subarctic Pacific. Fitzsimmons et al. (2017) measured the chemical speciation and isotopic composition of Fe in a hydrothermal plume and revealed that hydrothermally derived dissolved Fe in the plume is best explained by reversible exchange onto slowly sinking particles, and this process transports Fe up to 4000 km from the vent source. Similar process which includes interaction between colloidal Fe and slowly sinking particles is important for controlling the long-distance sedimentary Fe transport from the Sea of Okhotsk to the North Pacific (Misumi et al. 2021).
To fully understand the mechanism of the long-distance transport of Fe, it is necessary to reveal the chemical and physical speciation of Fe and its behavior, including the dynamics of organic ligands, the role of colloidal Fe and the particulate form of Fe, in each source and in the water column. The bioavailability of each Fe form, including particulate Fe (e.g., Sugie et al. 2013; Kanna and Nishioka 2016), is also important for understanding the impact of transported Fe on biological production. Although several numerical modeling studies that represent Fe in the North Pacific, including sedimentary Fe sources from the continental shelf, have been conducted (Misumi et al. 2011; Nakanowatari et al. 2017), further collaborative studies with numerical modeling that incorporate the Fe behavior of each chemical form into ocean biogeochemical cycles are essential. These subjects still require future research.
3 Subarctic intermediate nutrient pool
Phytoplankton (a part of biogenic particles) uptake nutrients in the surface layer, and the biogenic particles finally sink toward deep water. The sinking biogenic particles are remineralized and release inorganic nutrients into intermediate/deep water. Since the processes export nutrients (NO3, PO4) from the surface to intermediate/deep water, nutrient supply mechanisms for compensating for nutrient export are required to maintain high nutrients in the surface water in the subarctic region. Broecker (1991) proposed the concept of global deep ocean thermohaline circulation and the great ocean conveyor belt. To date, the North Pacific is recognized as the area where the deep ocean conveyor belt circulation terminates and has been perceived vaguely as the location where deep high-nutrient water rises and where a high-nutrient area is established at the surface. Recent physical oceanographic research has revealed more details about the global water circulation in the North Pacific (Fig. 9). Kawabe and Fujio (2010) indicated that most of the lower circumpolar deep water (Antarctic Bottom Water; AABW) upwells to the upper deep layer in the North Pacific and transforms into Pacific Deep Water (PDW), which shifts southward in the upper deep layer of 2000–2500 m and is modified by mixing with upper circumpolar deep water (Fig. 9). Talley (2013) indicated that the intermediate water meridional overturning cell subsystem in the North Pacific (which is constructed by the NPIW formation and the surface water circulation from subtropical to subarctic region including the Bering Sea and Sea of Okhotsk; see Fig. 9) is mostly unconnected to AABW cells, directly. Broecker and Peng (1982), Matsumoto and Key (2004) and Matsumoto (2007) clearly indicated with the chemical measurement dataset of the global 14C distribution in seawater that the water that had the oldest 14C age was distributed in the depths of the PDW, below the intermediate water meridional overturning cell subsystem (below the NPIW) (Fig. 9). Thus, the present physical and chemical oceanographic research indicates that deep nutrient-rich water does not directly outcrop to the surface of the subarctic Pacific. Therefore, the dynamics of the nutrients and the mechanisms by which the nutrients in deep water return to the surface layer to establish high-nutrient surface waters in the subarctic Pacific are not clearly described at the end of the conveyor belt circulation.
Schematic of Pacific meridional overturning circulation modified by Sigman et al. (2021) with additional information on the overturning cell subsystem containing NPIW described in Talley (2013) and Uehara et al. (2014). The dashed square, which covers the NPIW, indicates “the intermediate water meridional overturning cell subsystem in the North Pacific”. NPIW North Pacific intermediate water, PDW Pacific deep water, AAIW Antarctic intermediate water, SAMW sub-Antarctic mode water, AABW Antarctic bottom water, ITF Indonesian through flow, UCDW upper circumpolar deep water, LCDW lower circumpolar deep water. The dashed arrows indicate where the detailed processes are still unknown. The color indicates the nutrient (N or P) level (red > orange > blue > purple > gray). SINP; Subarctic intermediate water nutrient pool indicated by Nishioka et al. 2020 (colour figure online)
One of the water masses with the most persistent low dissolved oxygen concentrations and the richest nutrient repository in the world ocean is being observed in the intermediate water in the North Pacific (Keeling 2010; Whitney et al. 2013). As described in Sect. 2.3.2, water that is extremely rich in nitrate and phosphate but low in DO was observed in the wide density range of the intermediate layer (26.8–27.6 σθ) in the WSG, including the Aleutian Basin, and the water also propagated in the AG (Fig. 8b, c). Nishioka et al. (2020) proposed the formation of a subarctic intermediate water nutrient pool (SINP) and indicated that a high macronutrient water pool formed in the intermediate water was key for understanding the high-nutrient surface water in the subarctic Pacific. They estimated that the total mass of nitrate accumulated in the whole SINP was 4.2 ± 0.4 × 1014 mol (calculated by volume of the intermediate water × average nitrate concentration). They also calculated regenerated (reg)-phosphate (PO4) in the SINP. First, oxygen solubility was calculated, and the apparent oxygen utilization (AOU) was then calculated as the difference between the solubility and the measured DO concentration. Then, they calculated reg-PO4 with “AOU × RPO:4DO (PO4DO ratio = 170) (Anderson and Sarmiento 1994)”. The definition of preformed PO4 is “Preformed PO4 = observed PO4 − reg-PO4”. The percentage of reg-PO4 out of the total PO4 in the intermediate water indicated that more than half of the total PO4 in the intermediate water masses was reg-PO4 in the WSG and the Bering Sea basin (Fig. 8d). As described in the section below, upward turbulent fluxes of nutrients were reported around the marginal sea island chains, indicating that nutrients were uplifted from the SINP to the surface, which fueled surface organisms and were returned to the SINP by sinking particulate organic matter decomposition during intermediate water transport (Nishioka et al. 2020). In other words, a large proportion of nutrients were repeatedly recycled within the SINP and surface water in the intermediate water meridional overturning cell subsystem in the North Pacific (Fig. 9). Whereas, the SINP formation cannot be explained by upwelled PDW, which contains higher dissolved oxygen and lower NO3 and PO4 concentrations. Nishioka et al. (2020) also indicated that the Sea of Okhotsk ventilation transports newly formed water, which has relatively low PO4 and high DO (with a higher preformed-PO4 percentage), onto the upper intermediate water in the North Pacific (a low percentage of reg-PO4 water is distributed in the Sea of Okhotsk in Fig. 8d). These aspects of the nutrient dynamics in the North Pacific are consistent with the meridional overturning cell subsystem of the NPIW, which is mostly unconnected to AABW cells, as Talley (2013) suggested. Simultaneously, however, the SINP needs to be supplied nutrients to compensate for the amounts that are laterally exported from the intermediate water to low latitudes. The most likely candidate process for compensating for these amounts is that they are fueled from nutrient-rich deep water in the marginal seas. Long et al. (2019) estimated the amount of nitrate that was lost from the SINP by advection of the NPIW as 3.5 × 1012 mol/year, and the amount of nitrate supplied from the deep layer to the intermediate layer should be complementary to this loss (Nishioka et al. 2020). It also remains unclear how the silicate cycle, which is decoupled from the NO3 the PO4 cycles, maintains high concentrations in the meridional overturning cell subsystem of the NPIW.
4 Nutrient return process from the intermediate layer nutrient pool to the surface
Vertical mixing is a key process for understanding nutrient return paths from the intermediate water to the surface. There are some candidate processes reported around the subarctic Pacific that can control nutrient return, such as strong mixing around the Kuril and Aleutian Island chains and along the continental shelf slope in the Bering Sea.
First, we review previous studies on vertical mixing around the Kuril and Aleutian Island chains. The diapycnal mixing caused by interactions of tidal currents with the rough topography around the straits strongly affects the temperature, salinity, and dissolved oxygen properties from the surface to the deep layer (Ono et al. 2013). A previous observational study by Yamamoto-Kawai et al. (2004) showed that strong vertical tidal mixing occurs around the Bussol’ Strait, the deepest strait along the Kuril Island chain, and reaches the OSIW. Sarmiento et al. (2004) used the combined concentration of silicic acid and nitrate as a tracer, Si*, for intermediate water nutrient transportation, and they indicated that the NPIW needs to be the main nutrient return path from the deep water to above the thermocline to maintain high Si* water. They implied that tidal mixing at the Kuril Island chain is one of the candidate physical processes that uplifts nutrients from deep water.
Over the last two decades, direct observations of the island chain around the Sea of Okhotsk (the Kuril Island chain) and around the Bering Sea (the Aleutian Island chain) were conducted, and turbulence mixing parameters were measured in several studies. Itoh et al. (2010, 2011) and Yagi and Yasuda (2012) conducted direct observations of the turbulence parameter Kρ (= 0.2ε/N2; where ε is the turbulent kinetic energy dissipation rate, where N2 is squared buoyancy frequency) using a free-fall vertical microstructure profiler (VMP2000 Rockland Scientific International Co.). Goto et al. (2016, 2018) also measured Kρ by using CTD-attached fast-response thermistors (AFPO7, Rockland Scientific International Co.) for open water in the North Pacific. Goto et al. (2018) conducted a comparison study for both measurement methods and confirmed that the ε values from both measurement methods were comparable and within a factor of 3 (where ε is valid for 10–10 < ε < 10–8 W/kg). Figure 7c, d show the vertical section distribution of the dissolved Fe gradient (plus value: dissolved Fe increase with depth) and the vertical diffusive coefficient, Kρ (logarithm), along GP02. Interestingly, the vertical flux of dissolved Fe from the subsurface to the surface calculated by these two parameters was clearly higher in the WSG than in the AG.
Previous studies (Itoh et al. 2010, 2011; Yagi and Yasuda 2012; Goto et al. 2016, 2018) indicated that the Kρ values observed in the Kuril and Aleutian Island chains were two to four orders of magnitude higher than those observed in the open ocean in the subarctic Pacific. Nishioka et al. (2020) also measured the nitrate and dissolved Fe concentrations at the surrounding station where Kρ was measured and calculated the dissolved Fe and nitrate upward fluxes from the intermediate water to the surface layer (Fig. 10a, b), indicating that the order of dissolved Fe and nitrate vertical flux returning from the intermediate layer to the surface layer by turbulent diapycnal mixing around the island chains was several orders greater than that in the open ocean. They concluded that the Kuril and Aleutian Island chains are hot spots that return nutrients from the SINP to the surface layer, and are probably very important areas for maintaining surface HNLC water in the subarctic Pacific.
a Vertical nitrate upward fluxes from intermediate water to sea surface estimated by in situ observation. b Vertical dissolved Fe upward fluxes from intermediate water to sea surface estimated by in situ observation. The surface to subsurface gradients of the dissolved Fe gradient (ddissFe/dz) and nitrate gradient (dN/dz) were evaluated at all stations located around the straits. To estimate fluxes, we used the gradients and multiplied the average of the measured vertical diffusivity Kρ for depths of 100–500 m (see details in Nishioka et al. 2020). These figures are cited from Nishioka et al. 2020, Proc Natl Acad Sci, www.pnas.org/cgi/doi/10.1073/pnas.2000658117, under a CC BY 4.0 license
Another possible candidate area for the hot spots that return nutrients from the SINP to the subarctic Pacific surface is along the shelf break in the southeastern Bering Sea. Tanaka et al. (2012a, b, 2017) reported the results of direct measurement of turbulence mixing parameters, and Tanaka et al. (2012a, b, 2013) conducted a numerical modeling study on tidal mixing along a shelf break in the southern eastern Bering Sea, indicating that the strong vertical mixing along the shelf break induced by diurnal and semidiurnal tides played important roles in maintaining the Fe and nutrient supply along the high productive area at the shelf break surface, called the “Green Belt”. Large vertical diffusivity was observed over the shelf break (Kρ = 10–4 − 10–2 m2/s), and strong vertical mixing contributed to the formation of a thick layer over the outer shelf, which induced high vertical fluxes of nitrate and Fe to the surface.
These turbulent mixing processes in the subarctic Pacific are very important for setting the chemical properties just below the surface mixed layer to the intermediate layer and are critical for maintaining the high-nutrient surface water accompanying surface winter mixing processes. To understand the nutrient dynamics and biological production in the intermediate water meridional overturning cell subsystem in the North Pacific, we need more quantitative studies to evaluate the whole nutrient budget of the SINP.
5 Impact on the phytoplankton ecosystem
Previously, atmospheric dust has been considered to be the most important source of Fe in the North Pacific affecting biological production. Studies by Uematsu et al. (1983), Duce and Tindale (1991) and Mahowald et al. (2005) indicated that there is a longitudinal dust supply gradient across the North Pacific, and the flux of dust Fe over the WSG is an order of magnitude higher than that in the AG. This difference is due to the close proximity to the Gobi Desert, and this has been believed to be the leading cause of the longitudinal differences in biological production between the WSP and the AG (Harrison et al. 2004). In addition, aeolian dust from Alaska is important for the eastern subarctic Pacific (Boyd et al. 1998). However, now that other oceanic sources of Fe have been discovered, as explained in this review, a coherent explanation of the biological response in HNLC waters must be made by incorporating both the knowledge of the atmospheric Fe supplies and the oceanic Fe supplies.
Since satellite observations have been developed, we have observed several different patterns and phenologies of phytoplankton increases in the subarctic Pacific. These different patterns and phenologies are probably caused by different Fe sources. For instance, Shiozaki et al. (2014) analyzed satellite imagery and oceanographic data collected from 2003 to 2009, and they suggested that the factor that determined the onset of the spring bloom (phytoplankton increase) varied among subregions in the subarctic Pacific and that the magnitude of the bloom was probably controlled by Fe availability. From the present knowledge of Fe supply processes, we can categorize the biological response into three cases. First-case, as we mentioned in the Sect 1, phytoplankton growth which causes seasonal amplification of biogeochemical parameters in open oceanic waters in the two subarctic gyres, which occur every year (e.g., Fujiki et al. 2014; Matsumoto et al. 2014; Shiozaki et al. 2014). Second-case, there are massive spring phytoplankton blooms that occur around the coastal boundary current, such as the Oyashio at the edge of the WSG (Saito et al. 2002; Okamoto et al. 2010; Shiozaki et al. 2014; Isada et al. 2019; Kuroda et al. 2019) and in the AS and AC at the edge of the AG (Whitney et al. 2005; Henson 2007). Third-case, there are evidences that sporadic and patchy phytoplankton production is occasionally observed in both spring and summer. (e.g., Bishop et al. 2002; Hamme et al. 2010). Each biological phenomenon in subarctic waters has a different timing, magnitude and phenology, probably driven by different sources and supply processes of Fe and is accompanied by different physical conditions, such as light and temperature.
Fe supply processes related to the third-case phenomena have been well-discussed in many previous reports and were reviewed in Takeda (2011). Previous studies to date conclude that this third-case biological production is mainly due to the atmospheric dust Fe supply. Briefly, the first clear evidence of a sporadic phytoplankton response was reported by Bishop et al. (2002), who observed an increase in carbon biomass after a dust storm from the Gobi Desert in the surface mixed layer in the AG by robotic profiling float observations. Additional evidence of phytoplankton growth stimulated by volcanic ash supply was reported by Hamme et al. (2010). They captured the volcanic ash spread from a volcanic eruption at the Aleutian Islands in August 2008 and compared it to the biological production obtained by satellite images and shipboard observations, indicating that volcanic ash induced a large-scale diatom bloom in the AG, with decreasing pCO2 and nutrients (silicate) in the surface waters. These examples clearly demonstrate that atmospheric Fe input episodically induces phytoplankton production in surface water in the subarctic Pacific. Many other studies have observed atmospheric Fe deposits in the surface ocean by observations and estimated them by numerical models. Previously, for instance, Uematsu et al. (2003) reported that a numerical model simulation successfully reproduced the variation in mineral aerosol concentrations and total deposition flux over the western North Pacific. Measures et al. (2005) estimated the dust fluxes based on dissolved Al concentrations in surface water. Iwamoto et al. (2011) observed Fe deposition of Asian dust in the semi-pelagic region of the western North Pacific, and they estimated the amount of bioavailable Fe deposits from the dust event. Ito et al. (2016) developed numerical modeling that represented the delivery of bioavailable Fe from mineral dust and combustion aerosols to the ocean, indicating that anthropogenic aerosols had significant roles in stimulating biological production in the ocean surface. However, overall, Boyd et al. (2010) conducted a re-examination of evidence from ocean observations, indicating spatial and temporal mismatches between dust inputs and biological activities, and they noted that dust-mediated phytoplankton blooms were rare in the modern ocean. Jickells et al. (2005) also indicated that the role of the Fe dust supply in stimulating biological production has not been quantitatively evaluated well due to a lack of information on the fraction of atmospheric Fe that is bioavailable. Aerosol chemistry is also important for controlling the bioavailability of Fe from atmospheric sources (Landing and Paytan 2010). To estimate the impact of the supply of atmospheric Fe on phytoplankton growth, the dust storm and aerosol transportation scale, frequency, and deposition area, residence time and dissolution rate of atmospheric Fe, and fraction of bioavailable Fe under natural conditions are still uncertain issues. Fe isotope measurements and isotope mass balance analyses are powerful tools to tackle this issue. Kurisu et al. (2016) reported that very low isotope ratio in fine aerosol particles, which probably signifies evaporation of Fe at high temperatures, indicating the contribution of anthropogenic Fe to the surface of the North Pacific. Therefore, Fe isotope measurements with other trace metal isotopes will be very important for quantitative understanding of the relative importance of different sources of Fe to the ocean (Conway and John 2014, 2015; Kurisu et al. 2016; Pinedo-González et al. 2020). Future research is needed to understand the atmospheric Fe supply.
To explain the second-case of massive spring phytoplankton blooms, which cause nutrient depletion, the Fe input into the surface coastal boundary current is a key process. As mentioned in Sect. 2.2, the AC and AS along the Alaskan coast contain large amounts of Fe originating from coastal shelf areas (Cullen et al. 2009). In addition, the AS receives freshwater from the continent, precipitation and glacier meltwater, which contain high Fe (Wu et al. 2009). The Fe-rich coastal water has massive spring phytoplankton blooms (Whitney et al. 2005; Henson et al. 2007). These coastal waters fuel not only coastal biological production but also offshore open ocean production because the coastal water is detached from the northern coast of Alaska toward the open ocean. On the western side, massive spring blooms in the Oyashio area have been well-studied (Saito et al. 2002; Sugie et al. 2010), and Kuroda et al. (2019) and Isada et al. (2019) indicated that the COW is a key factor affecting the magnitude of phytoplankton blooms (Fig. 11a). The COW clearly has lower temperatures (Fig. 11b), lower salinities (Nishioka et al. 2011; Kuroda et al. 2019) and higher Fe concentrations (Fig. 11c) than the surrounding waters (Nakayama et al. 2010; Nishioka et al. 2011), which are probably influenced by the winter surface ESC (including the influence of the Amur River discharge) and by sea ice melt water from the Sea of Okhotsk, which contains high amounts of Fe (Kanna et al. 2014, 2018) (Fig. 11c). These Fe sources possibly fuel the massive phytoplankton blooms in the COW (Fig. 11a); however, we need clearer evidence to connect the water and biogeochemical material circulation between the COW and the Sea of Okhotsk (Mizuno et al. 2018). As described above, Fe that enters the surface layer laterally with fresh water (including sea ice melt water) directly from terrestrial origin is probably important for stimulating the second-case of biological production in the subarctic Pacific.
a Satellite chlorophyll data obtained by MODIS/Terra on 31 March 2020; massive phytoplankton blooms were observed in COW. b Satellite sea surface temperature data obtained on 31 March 2020; the COW, which has very low temperature, was observed in the coastal area of east Hokkaido. c Fe supply from the southern Sea of Okhotsk to coastal Oyashio water (COW). The black arrow indicates an image of high Fe water discharge from the Sea of Okhotsk to the coastal Oyashio region (colour figure online)
Finally, we would like to discuss the first-case of biological production. This biological production is not like the massive spring blooms that occur in coastal currents, such as the AC, AS, and COW, in the subarctic area. The first-case, which we focus on here, is the biological production that causes the seasonal drawdown of the pCO2 and nutrient concentrations in the open oceanic area in HNLC waters, which are especially high in the WSG (e.g., Fujiki et al. 2014; Matsumoto et al. 2014; Shiozaki et al. 2014; Yasunaka et al. 2020). This phenomenon is a regular biological production process that occurs every year at the basin-scale range in the western to central subarctic Pacific. This basin-scale steady biological production caused by the steady growth of phytoplankton in the surface layer starts in mid-April in spring, producing organic carbon while consuming nutrients until August in the summer. Fujiki et al. (2014) observed that the potential photosynthetic activity, quantified in terms of Fv/Fm, is controlled by Fe availability, and the termination of the phytoplankton increase is caused by Fe limitations in the WSG. Matsumoto et al. (2014) revealed that the mixed layer depth and light availability exerted an important control on the seasonal variability of primary production and phytoplankton biomass in the WSG. Shiozaki et al. (2014) indicated that in the formation regions of dense central mode water (D-CMW) and transition region mode water (TRMW) (area 1 in Fig. 12a), bloom onset coincided with possible turbulence weakening but not with MLD shoaling. The observed peak of chlorophyll a in the mode water formation regions was 0.44–0.58 mg/m3, and the values were ca. five times lower than those in Oyashio, although nitrate remained and MLDs became shallow enough at the bloom peak in the regions. They also reported that the “bloom magnitude west of 150°E and in the north Kuroshio extension was increased relative to that in the eastern region, suggesting a chemical property in the water delivered from the Sea of Okhotsk that would influence the western bloom”. These observational results probably indicate that through several physical processes, such as eddy diffusion, turbulent mixing, and winter surface mixing, the mixed layer was enriched with macronutrients and Fe (Fig. 12d) brought up from below the surface (area 1 in Fig. 12a), and either the Fe and light availability or solo Fe availability controlled the primary productivity at the mixed layer depth (Matsumoto et al. 2014). Therefore, the Fe and nutrient supply from intermediate water driven by ocean mixing (winter mixing and eddy diffusive mixing) possibly contribute to maintaining biological production and dissolved inorganic carbon drawdowns in area 1 in Fig. 12b (Yasunaka et al. 2014, 2020) from spring to summer in the western subarctic Pacific. Interestingly, higher continuous primary production was observed around the Aleutian and Kuril Island chains (areas 2 and 3 in Fig. 12a). Although Fe and light co-limitation was observed for microsized diatoms in the vicinity of these island chains (Yoshimura et al. 2010; Suzuki et al. 2014), the continuous higher primary productivity is explained by the continuous Fe and nutrient supply from intermediate water driven by strong diapycnal water mixing around the straits of the island chains, as indicated in Sect. 4 (Fig. 10a, b, Nishioka et al. 2020). In addition, a higher dissolved Fe-to-nitrate ratio in the uplifted waters in the Kuril Island chain than in the Aleutian Island chain was observed (Fig. 12c); therefore, biological production around the Kuril Island chain and downstream region in the WSG (area 1 in Fig. 12a, b) might be higher than that around the Aleutian Island chain (Fig. 12a). Nutrient consumption and organic carbon production in the WSG are approximately twice as large as those in the AG. To explain this quantitatively, the Fe flux in the WSP should be a “moderate” value that is higher than that on the eastern side but not sufficient to cause nutrient depletion. Therefore, there is considerable interest in the sources and processes of Fe input that lead to the occurrence of the higher amplitude of biogeochemical parameters in the WSG. The intermediate water Fe transport processes (Fig. 13) are able to explain the significant, twice-as-high and steady increases in phytoplankton biomass in the WSP as a result of several mixing processes that increase the surface Fe concentrations (Nishioka et al. 2013). As described above, the sedimentary Fe supplied from the continental margin circulated laterally through the intermediate layer and upwelled to the surface by several mixing processes, and this Fe is important for controlling the first-case of biological production in the subarctic Pacific.
a Satellite-observed annual integrated primary production in the subarctic Pacific and marginal seas. Climatological data for the period between 1998 and 2012. Formation regions of the transition region mode water (TRMW), dense central mode water (D-CMW) and shallow central mode water (S-CMW) described in Shiozaki et al. 2014 are indicated. b Seasonal drawdown of dissolved inorganic carbon from March to August. This figure is cited from Yasunaka et al. 2014, JGR-Ocean, https://doi.org/10.1002/2014JC010318, under the AGU permissions policy. c Plots of dissolved Fe to nitrate ratio (nM/μM) vs water density (Sigma theta) around the Kuril Island chain (red circles) and the Aleutian Island chain (open circles). d Horizontal distributions of dissolved Fe to nitrate ratio (nM/μM) at isopycnal surface 26.6 σθ in the North Pacific. This figure is cited and modified from Nishioka et al. 2020, Proc Natl Acad Sci, www.pnas.org/cgi/doi/10.1073/pnas.2000658117, under a CC BY 4.0 license. We define “area 1”, “area 2”, and “area 3” as the black circles in panels a, b, and d (colour figure online)
Figure 14 shows a schematic diagram of a coherent explanation of the external Fe supply to the western subarctic Pacific and the relevant biological response that incorporates the knowledge of the atmospheric Fe supplies and the oceanic Fe supplies from this review.
6 Conclusion and future directions
As we described in the Sect 1, Parsons and Lalli (1988) previously indicated that there are significant differences in the seasonal cycles and production processes of the lower trophic level between the subarctic Pacific and the subarctic Atlantic. Over the last 30 years, led by John H. Martin’s “Iron hypothesis”, it has become apparent that Fe, previously an unidentified nutrient, is the limiting factor for phytoplankton in the subarctic Pacific, and this nutrient causes the observed differences. The scientific oceanographic projects carried out in the last 10 years, including OMIX, revealed an overview of the different Fe supply processes in the HNLC waters of the subarctic Pacific and their relevance to biological production. Based on all knowledge from this review, we have made significant progress in understanding Fe and nutrient biogeochemistry and phytoplankton ecosystems in the subarctic Pacific.
To explain the higher biogeochemical amplitude on the western side of the subarctic Pacific basin, it is important to consider the existence of the subpolar marginal seas, which are located upstream of the western boundary current, the ventilation and the intermediate water circulation system, the formation of the SINP, the western boundary current (the Oyashio current) that is released toward the open ocean, the strong mixing hotspots caused by complicated bathymetric topography, and the westerlies winds. For these reasons, it is understandable that when looking at the entire subarctic Pacific, the effects of land are concentrated on the western side of the subarctic Pacific through Fe transport.
The future directions of Fe research are extracted from this review as follows.
-
1.
To quantitatively understand the biogeochemical Fe and nutrient cycle in the intermediate water meridional overturning cell subsystem in the North Pacific, further collaborative research between chemical and physical oceanographers is essential. Additional measurements of turbulent mixing parameters with measurements of Fe and nutrients are needed to estimate the fluxes in several hotspots.
-
2.
We still need a quantitative understanding with a coherent explanation of the biological response in HNLC waters that must be made by incorporating both the knowledge of the atmospheric Fe supplies and the oceanic Fe supplies.
-
3.
Fe isotope measurement and isotope mass balance analysis are powerful tools that could provide a quantitative understanding of the relative importance of different sources of Fe to the ocean. We need more data of Fe isotopic composition at the source regions in the North Pacific.
-
4.
To estimate the impact of the supply of atmospheric Fe (including mineral dust and anthropogenic aerosols) on phytoplankton growth, data on the mineral dust storm and aerosol transportation scale, frequency, deposition area, residence time in surface water and dissolution rate, and fraction of bioavailable Fe under natural conditions are still needed.
-
5.
To fully understand the mechanism of long-distance oceanic transport of Fe, it is necessary to understand the chemical and physical speciation of Fe and its behavior, including the dynamics of organic ligands, the role of colloidal Fe and the particulate form of Fe, in each source and in the water column. Fe isotope measurements will be a powerful tool for revealing transport processes.
-
6.
The bioavailability of each Fe form, including particulate Fe, is still uncertain in terms of understanding the impact of transported Fe on biological production.
-
7.
We need seasonal time series data for surface Fe concentrations in the WSG and AG to understand the details of how Fe controls biogeochemistry.
-
8.
We need clearer evidence for connecting fresh water (the Amur River and sea ice meltwater) and biogeochemical material in the surface circulation between the COW and Sea of Okhotsk systems.
-
9.
Further collaborative research with numerical modeling that incorporates the Fe behavior of chemical and physical species into ocean biogeochemical cycles is essential. Additionally, numerical modeling that represents the biogeochemical Fe and nutrient cycle in the intermediate water meridional overturning cell subsystem in the North Pacific is needed.
References
Aguilar-Islas A, Hurst MP, Buck KN, Sohst B, Smith GJ, Lohan MC, Bruland KW (2007) Micro- and macronutrients in the southeastern Bering Sea: insight into iron-replete and iron-depleted regime. Prog Oceanogr 73:99–126. https://doi.org/10.1016/j.pocean.2006.12.002
Alfultis MA, Martin S (1987) Satellite passive microwave studies of the Sea of Okhotsk ice cover and its relation to oceanic processes 1978–1982. J Geophys Res Oceans 92:13013–13028
Anderson RF (2020) GEOTRACES: accelerating research on the marine biogeochemical cycles of trace elements and their isotopes. Annu Rev Mar Sci 12:49–85. https://doi.org/10.1146/annurev-marine-010318-095123
Anderson LA, Sarmiento JL (1994) Redfield ratios of remineralization determined by nutrient data analysis. Global Biogeochem Cycles 8:65–80
Bishop JKB, Davis RE, Sherman JT (2002) Robotic observations of dust storm enhancement of carbon biomass in the North Pacific. Science 298:817–821
Bograd SJ, Thomson RE, Rabinovich AB, Leblond PH (1999) Near-surface circulation of the northeast Pacific Ocean derived from WOCE-SUP satellite-tracked drifters. Deep Sea Res II 46:2371–2403
Bowie AR, Achterberg EP, Mantoura RFC, Worsfold PJ (1998) Determination of sub-nanomolar levels of iron in seawater using flow injection with chemiluminescence detection. Anal Chim Acta 361:189–200
Boyd PW, Ellwood MJ (2010) The biogeochemical cycle of iron in the ocean. Nature Geo 3:675–682
Boyd PW, Wong CS, Merrill J, Whitney F, Snow J, Harrison PJ, Gower J (1998) Atmospheric iron supply and enhanced vertical carbon flux in the NE subarctic Pacific: is there a connection? Global Biogeochem Cycles 12:429–441
Boyd PW, Law CS, Wong CS, Nojiri Y, Tsuda A, Levasseur M, Takeda S, Rivkin R, Harrison PJ, Strzepek R, Gower J, McKay RM, Abraham E, Arychuk M, Barwell-Clarke J, Crawford W, Hale M, Harada K, Johnson K, Kiyosawa H, Kudo I, Marchetti A, Miller W, Needoba J, Nishioka J, Ogawa H, Page J, Robert M, Saito H, Sastri A, Sherry N, Soutar T, Sutherland N, Taira Y, Whitney F, Wong SE, Yoshimura T (2004) The decline and fate of an iron-induced subarctic phytoplankton bloom. Nature 428:549–553
Boyd PW, Jickells T, Law CS, Blain S, Boyle EA, Busseler KO, Coale KH, Cullen JJ, de Baar HJW, Flows M, Harvey M, Lancelot C, Levasseur M, Owens NPJ, Pollard R, Rivkin RB, Sarmiento J, Schoemann V, Smetacek V, Takeda S, Tsuda A, Turner S, Watson AJ (2007) Mesoscale iron enrichment experiments 1993–2005: synthesis and future directions. Science 315:612–617
Boyd PW, Gall MP, Silver MW, Coale SL, Bidigare RR, Bishop JLKB (2008) Quantifying the surface subsurface biogeochemical coupling during the VERTIGO ALOHA and K2 studies. Deep Sea Res Part II 55:1578–1593
Boyd PW, Mackie DS, Hunter KA (2010) Aerosol iron deposition to the surface ocean-Modes of iron supply and biological responses. Mar Chem 120:128–143. https://doi.org/10.1016/j.marchem.2009.01.008
Boyle E, Edmond JM (1975) Copper in surface seawaters south of New Zealand. Nature 253:107–109
Broecker WS (1991) The great ocean conveyor. Oceanogr 4(2):79–89
Broecker WS, Peng T-H (1982) Tracers in the sea. Lamont-Doherty Geological Observatory, Columbia University, 690 pp. https://doi.org/10.2307/1309641
Brown MT, Lippiatt SM, Lohan MC, Bruland KW (2012) Trace metal distributions withn a Sitka eddy in the northern Gulfof Alaska. Limnol Oceanogr 57(2):503–518. https://doi.org/10.4319/lo.2012.57.2.0503
Bruland KW, Rue EL (2001) Analytical methods for thedetermination of concentrations and speciation of iron. In: Turner DR, Hunter KA (eds) Biogeochemistry of Fe in Seawater. SCOR-IUPAC series. J Wiley, Baltimore, pp 255–289 (Chapter 6)
Bruland KW, Franks RP, Knauer GA, Martin JH (1979) Sampling and analytical methods for the determination of copper, cadmium, zinc, and nickel at the nanogram per liter level in sea-water. Anal Chim Acta 105:233–245
Bruland KW, Orians KJ, Cowen JP (1994) Reactive trace metal in the stratified central North Pacific. Geochim Cosmochim acta 58(15):3171–3182
Buck NK, Bruland KW (2007) The physicochemical speciation of dissolved iron in the Bering Sea. Alaska Limnol Oceanogr 52(5):1800–1808
Buesseler KO, Lamborg CH, Boyd PW, Lam PJ, Trull TW, Bidigare RR, Bishop JKB, Casciotti KL, Dehairs F, Elskens M, Honda M, Karl DM, Siegel DA, Silver MW, Steinberg DK, Valdes J, Van Mooy B, Wilson S (2007) Revisiting carbon flux through the ocean’s twilight zone. Science 316:567–570. https://doi.org/10.1126/science.1137959
Byrne RH, Kester DR (1976) Solubility of hydrous ferric oxide and iron speciation in seawater. Mar Chem 4:255–274
Chierici M, Fransson A, Nojiri Y (2006) Biogeochemical processes as drivers of surface fCO2 in contrasting provinces in the subarctic North Pacific Ocean. Global Biogeochem Cycles 20:GB1009. https://doi.org/10.1029/2004GB002356
Chisholm SW, Morel, MM (1991) What controls phytoplankton production in nutrient-rich areas of the open sea? Limnol Oceanogr 36(8): preface
Cid AP, Urushihara S, Minami T, Norisuye K, Sohrin Y (2011) Stoichiometry among bioactive trace metals in seawater on the Bering Sea shelf. J Oceanogr 67(6):747–764
Coale KH, Worsfold P, de Baar HJW (1999) Iron age in oceanography. Eos 80(34):377–382
Coale KH et al (2015) John Holland Martin from picograms to petagrams and copepods to climate. Bulletin Limol Oceanogr 24:1–17
Conway TM, John SG (2014) Quantification of dissolved iron sources to the North Atlantic Ocean. Nature 511:212–215
Conway TM, John SG (2015) The cycling of iron, zinc and cadmium in the North East pacific Ocean-insight from stable isotopes. Geochem Cosmochim Acta 164:262–283. https://doi.org/10.1016/j.gca.2015.05.023
Craig H, Turekian KK (1980) The GEOSECS program: 1976–1979. Earth Planet Sci Let 49:263–265
Cullen JJ (1991) Hypotheses to explain high-nutrient conditions in the open sea. Limno Oceanogr 36(8):1578–1599
Cullen JT, Chong M, Ianson D (2009) British Columbian continentalshelf as a source of dissolved iron to the subarcticnortheast Pacific Ocean. Global Biogeochem Cycles 23:GB4012. https://doi.org/10.1029/2008GB003326
Cutter GA, Bruland KW (2012) Rapid and non-contaminating sampling system for trace elements in global ocean surveys. Limnol Oceanogr Methods 10:425–436. https://doi.org/10.4319/lom.2012.10.425
de Baar HJW, Boyd PW, Coale KH, Landry MR, Tsuda A, Assmy P, Bakker DCE, Bozec Y, Barbar RT, Brzezinski MA, Buesseler KO, Boye M, Croot PL, Gervais F, Gorbunov MY, Harrison PJ, Hiscock W, Laan P, Lancelot C, Law C, Levasseur M, Marchetti A, Millero FJ, Nishioka J, Nojiri Y, van Oijen T, Riebesell U, Rijkenberg MJA, Saito H, Takeda S, Timmermans KR, Veldhuis MJW (2005) Synthesis of iron fertilization experiments: from the iron age in the age of enlightenment. J Geophys Res Ocean. https://doi.org/10.1029/2004JC002601
de Baar HJW, Timmermans KR, Laan P, De Porto HH, Ober S, Blom JJ, Bakker MC, Schilling J, Sarthou G, Smit MG, Klunder M (2008) Titan: a new facility for ultraclean sampling of trace elements and isotopes in the deep oceans in the international Geotraces program. Mar Chem 111:4–21. https://doi.org/10.1016/j.marchem.2007.07.009
Dobashi R, Ueno H, Okada Y, Tanaka T, Nishioka J, Hirawake T, Ooki A, Itoh S, Hasegawa D, Sasai Y, Sasaki H, Yasuda I (2021) Observations of anticyclonic eddies in the western subarctic North Pacific. J Oceanogr. https://doi.org/10.1007/s10872-020-00586-y
Duce RA (1989) SEAREX; The sea/air exchange program. Chem Oceanogr 10. Academic Press, London, p 404
Duce RA, Tindale NW (1991) Atmospheric transport of iron and its deposition in the ocean. Limnol Oceanogr 36:1715–1726
Elrod VA, Berelson WM, Coale K, Johnson KS (2004) The flux of iron from continental shelf sediment: a missing source for global budget. Geophys Res Lett 31:L12307. https://doi.org/10.1029/2004GL020216
Favorite F, Dodimead AJ, Nasu K (1976) Oceanography of the subarctic Pacific region. 1960–1971. Bull Int North Pac Fish Comm 33:1–187
Fitzsimmons JN, Boyle EA (2014) Both soluble and colloidal iron phases control dissolved iron variability in the tropical north Atlantic ocean. Geochim Cosmochim Acta 125:539–550. https://doi.org/10.1016/j.gca.2013.10.032
Fitzsimmons JN, Carrasco GG, Wu J, Roshan S, Hatta M, Measures CI, Conway TM, John SG, Boyle EA (2015) Partitioning of dissolved iron and iron isotopes into soluble and colloidal phases along the GA03 GEOTRACES North Atlantic Transect. Deep Sea Res II 116:130–151. https://doi.org/10.1016/j.dsr2.2014.11.014
Fitzsimmons JN, John SG, Marsay CM, Hoffman CL, Nicholas SL, Toner BM, German CR, Sherrell RM (2017) Iron persistence in a distal hydrothermal plume supported by dissolved-particulate exchange. Nature Geo 10:195–201. https://doi.org/10.1038/ngeo2900
Fujiki T, Matsumoto K, Mino Y, Sasaoka K, Wakita M, Kawakami H, Honda MC, Watanabe S, Saino T (2014) Seasonal cycle of phytoplankton community structure and photophysiological state in the western subarctic gyre of the North Pacific. Limnol Oceanogr 59(3):887–900
Fukamachi Y, Mizuta G, Ohshima KI, Talley LD, Riser SC, Wakatsuchi M (2004) Transport and modification processes of dense shelf water revealed bylong-term moorings off Sakhalin in the Sea of Okhotsk. J Geophys Res Oceans 109:C09S10. https://doi.org/10.1029/2003JC001906
GEOTRACES Planning Group (2006) GEOTRACES Science Plan. Baltimore, Maryland: Scientific Committee on Oceanic Research. https://geotracesold.sedoo.fr/libraries/documents/Science_plan.pdf
Gladyshev S, Martin S, Riser S, Figureurkin A (2000) Dense water production on the northern Okhotsk shelves: comparison of ship-based spring-summer observations for 1996 and 1997 with satellite observations. J Geophys Res Oceans 105:26281–26299
Gledhill M, van den Berg CMG (1994) Determination of complexation of iron (III) with natural organic complexing ligands in seawater using cathodic stripping voltammetry. Mar Chem 47:41–54. https://doi.org/10.1016/0304-4203(94)90012-4
Gordon RM, Martin JM, Knauer GA (1982) Iron in north-east Pacific waters. Nature 299:611–612
Goto Y, Yasuda I, Nagasawa M (2016) Turbulence estimation using fast-response thermistors attached to a free-fall vertical microstructure profiler. J Atmos Ocean Tech 33:2065–2078. https://doi.org/10.1175/JTECH-D-15-0220.1
Goto Y, Yasuda I, Nagasawa M (2018) Comparison of turbulence intensity from CTD-attached and free-fall microstructure profilers. J Atmos Ocean Tech 35:147–162. https://doi.org/10.1175/JTECH-D-17-0069.1
Gruber N, Sarmiento JL (1997) Global patterns of marine nitrogen fixation and denitrification. Glob Biogeochem Cycles 11:235–266
Hamme RC, Webley PW, Crawford WR, Whitney FA, DeGrandpre MD, Emerson SR, Eriksen CC, Giesbrecht KE, GowerJFR KMT, Pena MA, Sabine CL, Batten SD, Coogan LA, Grundle DS, Lockwood D (2010) Volcanic ash fuels anomalous plankton bloom in subarctic northeast Pacific. Geophys Res Lett 37:L19604. https://doi.org/10.1029/2010GL044629
Hansell DA, Carlson CA, Suzuki Y (2002) Dissolved organic carbon export with North Pacific Intermediate Water formation. Global Biogeochem Cycles. https://doi.org/10.1029/2000GB001361
Harrison PJ, Boyd PW, Varela DE, Takeda S, Shiomoto A, Odate T (1999) Comparison of factors controlling phytoplankton productivity in the NE and NW subarctic Pacific. Prog Oceanogr 43:205–234
Harrison PJ, Whitney FA, Tsuda A, Saito H, Tadokoro K (2004) Nutrient and plankton dynamics in the NE and NW gyres of the subarctic Pacific Ocean. J Oceanogr 60:93–117
Hart TJ (1934) On the phytoplankton of the southeast Atlantic and the Bellingshausen Sea, 1929–1931. Discovery Reports 8:1–268
Hart TJ (1942) Phytoplankton periodicity in Antarctic surface waters. Discovery Report 21:261–365
Hassler CS, Schoemann V, Nichols CM, Butler ECV, Boyd PW (2011) Saccharides enhance iron bioavailability to Southern Ocean phytoplankton. Proc Natl Acad Sci USA 108:1076–1081. https://doi.org/10.1073/pnas.1010963108
Hassler CS, van den Berg CMG, Boyd PW (2017) Toward a regional classification to provide a more inclusive examination of the ocean biogeochemistry of iron-binding ligands. Front Mar Sci. https://doi.org/10.3389/fmars.2017.00019
Hattori-Saito A, Nishioka J, Ono T, McKay RML, Suzuki K (2010) Iron deficiency in micro-sized diatom in the Oyashio region of the western subarctic Pacific during spring. J Oceanogr 66:105–115
Henson SA (2007) Water column stability and spring bloom dynamics in the Gulf of Alaska. J Mar Res 65:715–736
Hernes PJ, Benner R (2002) Transport and diagenesis of dissolved and particulate terrigenous organic matter in the North Pacific Ocean. Deep Sea Res I 49:2119–2132
Hioki N, Kuma K, Morita Y, Sasayama R, Ooki A, Kondo Y, Obata H, Nishioka J, Yamashita Y, Nishino S, Kikuchi T, Aoyama M (2014) Laterally spreading iron, humic-like dissolved organic matter and nutrients in cold, dense subsurface water of the Arctic Ocean. Sci Rep 4:6765
Honda MC (2003) Biological pump in northwestern North Pacific. J Oceanogr 59:671–684
Hunt GL Jr, Allen BM, Angliss RP, Baker T, Bond N, Buck G, Byrd GV, Coyle KO, Devol A, Eggers DM, Eisner L, Feely R, Fitzgerald S, Fritz LW, Gritsay EV, Ladd C, Lewis W, Mathis J, Mordy CW, Mueter F, Napp J, Sherr E, Shull D, Stabeno P, Stepanenko MA, Strom S, Whitledge TE (2010) Status and trends of the Bering Sea region, 2003–2008, pp 196–267 in S.M. McKinnell and M.J. Dagg [Eds.] Marine Ecosystems of the North Pacific Ocean, 2003–2008. PICES Special Publication 4:393
Hurst MP, Bruland KW (2007) An investigation into the exchange of iron and zinc between soluble, colloidal, and particulate size-fractions in shelf waters using low-abundance isotopes as tracers in shipboard incubation experiments. Mar Chem 103:211–226
Hurst MP, Aguila-Islas AM, Bruland KW (2010) Iron in the southeast Bering Sea: elevated leachable particulate Fe in shelf bottom waters as an important source for surface waters. Cont Shelf Res 30:467–480. https://doi.org/10.1016/j.csr.2010.01.001
Imai K, Nojiri Y, Tsurushima N, Saino T (2002) Time series of seasonal variation of primary productivity at station KNOT (44° N, 155° E) in the sub-arctic western North Pacific. Deep Sea Res II 49:5395–5408
Isada T, Hattori-Saito A, Saito H, Ikeda T, Suzuki K (2010) Primary productivity and its bio-optical modeling in the Oyashio region, NW Pacific during the spring bloom 2007. Deep Sea Res II 57:1653–1664
Isada T, Hattori-Saito A, Saito H, Kondo Y, Nishioka J, Kuma K, Hattori H, McKay RML, Suzuki K (2019) Responses of phytoplankton assemblages to iron availability and mixing water masses during the spring bloom in the Oyashio region, NW Pacific. Limnol Oceanogr 64:197–216. https://doi.org/10.1002/lno.11031
Ito A, Shi Z (2016) Delivery of anthropogenic bioavailable iron from mineral dust and combustion aerosols to the ocean. Atmos Chem Phys 16:85–99. https://doi.org/10.5194/acp-16-85-2016
Itoh M, Ohshima KI, Wakatsuchi M (2003) Distribution and formation of Okhotsk Sea intermediate water. An analysis of isopycnal climatological data. J Geophys Res 108(C8):3258. https://doi.org/10.1029/2002JC001590
Itoh S, Yasuda I, Nakatsuka T, Nishioka J, Volkov YN (2010) Fine- and microstructure observation in the Urup Strait, Kuril Islands, during August 2006. J Geophys Res 115(C8):C08004. https://doi.org/10.1029/2002JC005629
Itoh S, Yasuda I, Yagi M, Osafune S, Kaneko H, Nishioka J, Nakatsuka T, Volkov YN (2011) Strong vertical mixing in the Urup Strait. Geophys Res Lett 38:L16607. https://doi.org/10.1029/2011GL048507
Iwamoto Y, Yumimoto K, Toratani M, Tsuda A, Miura K, Uno I, Uematsu M (2011) Biogeochemical implications of increased mineral particle concentrations in surface waters of the northwestern North Pacific during an Asia dust event. Geophys Res Lett 38:L01604. https://doi.org/10.1029/2010GL045906
Jickells TD, An ZS, Andersen KK, Baker AR, Bergametti G, Brooks N, Cao JJ, Boyd PW, Duce RA, Hunter KA, Kawahata H, Kubilay N, LaRoche J, Liss PS, Mahowald N, Prospero JM, Ridgwell AJ, Tegen I, Torres R (2005) Global iron connections between desert dust, ocean biogeochemistry. Science 308:67–71
Johnson KS, Gordon RM, Coale KH (1997) What controls dissolved iron concentrations in the world ocean? Mar Chem 57:137–161
Johnson KS, Chavez FP, Friederich GE (1999) Continental-shelf sediment as a primary source of iron for coastal phytoplankton. Nature 398:697–700
Johnson WK, Millar LA, Sutherland NE, Wong CS (2005) Iron transport by mesoscale Haida eddies in the Gulf of Alaska. Deep-Sea Res II 52:933–953
Kanna N, Nishioka J (2016) Bio-availability of iron derived from subarctic first-year sea ice. Mar Chem 186:189–197. https://doi.org/10.1016/j.marchem.2016.09.009
Kanna N, Toyota T, Nishioka J (2014) Iron and macro-nutrient concentrations in sea ice and their impact on the nutritional status of surface waters in the southern Okhotsk Sea. Prog Oceanogr 126:44–57. https://doi.org/10.1016/j.pocean.2014.04.012
Kanna N, Sibano Y, Toyota T, Nishioka J (2018) Winter iron supply processes fueling spring phytoplankton growth in a sub-polar marginal sea, the Sea of Okhotsk: importance of sea ice and the East Sakhalin current. Mar Chem 206:109–120. https://doi.org/10.1016/j.marchem.2018.08.006
Katsumata K, Yasuda I (2010) Estimate of non-tidal exchange transport between the Sea of Okhotsk and the North Pacific. J Oceanogr 66:489–504
Katsumata K, Ohshima KI, Kono T, Itoh M, Yasuda I, Volkov YN, Wakatsuchi M (2004) Water exchange and tidal currents through the Bussol’ Strait revealedby direct current measurements. J Geophys Res Oceans 117:C09S06. https://doi.org/10.1029/2003JC001864
Kawabe M, Fujio S (2010) Pacific Ocean circulation based on observation. J Oceanogr 66:389–403
Kawakami H, Honda MC, Matsumoto K, Wakita M, Kitamura M, Fujiki T, Watanabe S (2015) POC fluxes estimated from 234Th in late spring-early summer in the western subarctic North Pacific. J Oceanogr 71:311–324
Keeling RF, Körtzinger A, Gruber N (2010) Ocean deoxygenation in a warming world. Annu Rev Mar Sci 2:199–229. https://doi.org/10.1146/annurev.marine.010908.163855
Kim T, Obata H, Nishioka J, Gamo T (2017) Distribution of dissolved zinc in the western and central subarctic North Pacific. Glob Biogeochem Cycle 31:1454–1468. https://doi.org/10.1002/2017GB005711
Kimura N, Wakatsuchi M (2000) Relationship between sea-ice motion and geostrophic wind in the Northen hemisphere. Geophys Res Lett 27:3735–3738
Kitani K (1973) An oceanographic study of the Sea of Okhotsk, particularly in regard to cold waters. Bullet Far Seas Fisheries Res Laboratory 9:45–47
Kitayama S, Kuma K, Manabe E, Sugie K, Takata H, Isoda Y, Toya K, Saito S, Takagi S, Kamei Y, Sakaoka K (2009) Controls on iron distributions in the deep water column of the North Pacific Ocean: Iron(III) hydroxide solubility and marine humic-type dissolved organic matter. J Geophys Res 114:C08019. https://doi.org/10.1029/2008JC004754
Kondo Y, Takeda S, Furuya K (2012) Distinct trends in dissolved Fe speciation between shallow and deep waters in the Pacific Ocean. Mar Chem 134–135:18–28. https://doi.org/10.1016/j.marchem.2012.03.002
Kondo Y, Obata H, Hioki N, Ooki A, Nishino S, Kikuchi T, Kuma K (2016) Transport of trace metals (Mn, Fe, Ni, Zn and Cd) in the western Arctic Ocean (Chukchi Sea and Canada Basin) in late summer 2012. Deep Sea Res I 116:236–252
Kondo Y, Bamba R, Obata H, Nishioka J, Takeda S (2021) Distinct profiles of size-fractionated iron-binding ligands between the eastern and western subarctic Pacific. Sci Rep 11:2053. https://doi.org/10.1038/s41598-021-81536-6
Kuma K, Nishioka J, Matsunaga K (1996) Controls on Iron(III) hydroxide solubility in seawater: the influence of pH and natural organic chelators. Limnol Oceanogr 41:396–407
Kurisu M, Takahashi Y, Iizuka T, Uematsu M (2016) Very low isotope ratio of iron in fine aerosols related to its contribution to the surface ocean. J Geophys Res 121:11119–11136. https://doi.org/10.1002/2016JD024957
Kuroda H, Toya Y, Watanabe T, Nishioka J, Hasegawa D, Taniuchi Y, Kuwata A (2019) Influence of Coastal Oyashio water on massive spring diatom blooms in the Oyashio area of the North Pacific Ocean. Prog Oceanogr 175:328–344. https://doi.org/10.1016/j.pocean.2019.05.004
Laglera LM, van den Berg CMG (2009) Evidence for geochemical control of iron by humic substances in seawater. Limnol Oceanogr 54:610–619
Laglera LM, Battaglia G, van den Berg CMG (2011) Effect of humic substances on the iron speciation in natural waters by CLE/CSV. Mar Chem 127:134–143
Lam PJ, Bishop JKB (2008) The continental margin is a key source of iron to the HNLC North Pacific Ocean. Geophys Res Lett 35:L07608. https://doi.org/10.1029/2008GL033294
Lam PJ, Bishop JKB, Henning CC, Marcus MA, Waychunas GA, Fung IY (2006) Wintertime phytoplankton bloom in the subarctic Pacific supported by continental margin iron. Global Biogeochem Cycle 20:GB1006. https://doi.org/10.1029/2005GB002557
Lam PJ, Ohnemus DC, Marcus MA (2012) The speciation of marine particulate iron adjacent to active and passive continental margins. Geochim Cosmochim Acta 80:108–124
Landing WM, Bruland KW (1987) The contrasting biogeochemistry of iron and manganese in the Pacific Ocean. Geochim Cosmochim Acta 51:29–43
Landing WM, Paytan A (2010) Aerosol chemistry and impact on the ocean. Mar Chem 120:1–3
Lannuzel D, de Jong J, Schoemann V, Trevena A, Tison J-L, Chou L (2007) Development of a sampling and flow injection analysis technique for iron determination in the sea ice environment. Anal Chim Acta 556:476–483
Lippiatt SM, Lohan MC, Bruland KW (2010) The distribution of reactive iron in northern Gulf of Alaska coastal waters. Mar Chem 121:187–199. https://doi.org/10.1016/j.marchem.2010.04.007
Lohan MC, Bruland KW (2008) Elevated Fe (II) and dissolved Fe in hypoxic shelf waters off Oregon and Washington: an enhanced source of iron to coastal upwelling regimes. Environ Sci Technol 42:6462–6468. https://doi.org/10.1021/es800144j
Long Y, Zhou X-H, Guo X (2019) The Oyashio nutrient stream and its nutrient transport to the mixed water region. Geophys Res Lett 46:1513–1520. https://doi.org/10.1029/2018GL081497
Longhurst A, Sathyendranath S, Platt T, Caverhill C (1995) An estimate of global primary production in the ocean from satellite radiometer data. J Plankton Res 17:1245–1271
Mahowald NM, Baker AR, Bergametti G, Brooks N, Duce A, Jickells TD, Kubliay N, Prospero JM, Tegen I (2005) Atmosphericglobal dust cycle and iron inputs to the ocean. Global Biogeochem Cycles 19:GB4025. https://doi.org/10.1029/2004GB002402
Martin JH (1990) Glacial-Interglacial CO2 Change: the iron hypothesis. Paleoceanography 5(1):1–13
Martin JH, Fitzwater SE (1988) Iron deficiency limits phytoplankton growth in the north-east Pacific subarctic. Nature 331:341–343
Martin JH, Gordon RM (1988) Northeast Pacific iron distributions in relation to phytoplankton productivity. Deep Sea Res I 35(2):177–196
Martin JH, Gordon RM, Fitzwater S, Broenkow WW (1989) VERTEX: phytoplankton/iron studies in the Gulf of Alaska. Deep Sea Res I 36(5):649–680. https://doi.org/10.1016/0198-0149(89)90144-1
Martin JH, Fitzwater SE, Gordon RM (1990) Iron deficiency limits phytoplankton growth in Antarctic waters. Global Biogeochem Cycles. https://doi.org/10.1029/GB004i001p00005
Martin JH, Gordon RM, Fitzwater SE (1991) The case for iron. Limnol Oceanogr 36(8):1793–1802
Martin S, Drucker R, Yamashita K (1998) The production of ice and dense shelfwater in the Okhotsk Sea polynyas. J Geophys Res Oceans 103:27771–27782
Martin P et al (2013) Iron fertilization enhanced net community production but not downward particle flux during the Southern Ocean iron fertilization experiment LOHAFEX. Global Biogeochem Cycle 27:871–881. https://doi.org/10.1002/gbc.20077
Matsumoto K (2007) Radiocarbon-based circulation age of the world oceans. J Geophys Res 112:C09004. https://doi.org/10.1029/2007JC004095
Matsumoto K, Key RM (2004) Natural radiocarbon distribution in the deep Ocean. In: M Shiyomi et al. (Eds.). Global environmental change in the ocean and on land. Terapub, pp 45–58
Matsumoto K, Honda MC, Sasaoka K, Wakita M, Kawakai H, Watanabe S (2014) Seasonal variability of primary production and phytoplankton biomass in the western Pacific subarctic gyre: control by light availability within the mixed layer. J Geophys Res Oceans 119:6523–6534. https://doi.org/10.1002/2014JC009982
Measures CI, Yuan J, Resing JA (1995) Determination of iron in seawater by flow injection analysis using in-line preconcentration and spectrophotometric detection. Mar Chem 50:3–12
Measures CI, Brown MT, Vink S (2005) Dust deposition to thesurface waters of the western and central North Pacific inferred fromsurface water dissolved aluminium concentrations. Geochem Geophys Geosyst 6:Q09M03. https://doi.org/10.1029/2005GC000922
Measures CI, Landing WM, Brown MT, Burck CS (2008) A commercially available rosette system for trace metal-clean sampling. Limnol Oceanogr Methods 6:384–394. https://doi.org/10.4319/lom.2008.6.384
Misumi K, Tsumune D, Yoshida Y, Uchimoto K, Nakamura T, Nishioka J, Mitsudera H, Bryan F, Lindsay K, Moore J, Doney SC (2011) Mechanisms controlling dissolved iron distribution in the North Pacific: a model study. J Geophys Res. https://doi.org/10.1029/2010JG001541
Misumi K, Lindsay K, Moor JK, Doney S, Tsumune D, Yoshida Y (2013) Humic substances may control dissolved iron distributions in the global ocean: implications from numerical simulations. Glob Biogeochem Cycle 27:450–462. https://doi.org/10.1002/gbc.20039
Misumi K, Nishioka J, Obata H, Tsumune D, Tsubono T, Long MC, Lindsay K, Moor JK (2021) Slowly sinking particles underlie dissolved iron transport across the Pacific Ocean. Glob Biogeochem Cycle. https://doi.org/10.1029/2020GB006823
Mochizuki M, Shiga N, Saito M, Imai K, Nojiri Y (2002) Seasonal changes in nutrients, chlorophyll a and the phytoplankton assemblage of the western subarctic gyre in the Pacific Ocean. Deep Sea Res II 49:5241–5439
Moore WS (1984) Review of the GEOSECS project. Nuclear Instrum Method Phys Res 223:459–465
Nagao S, Terashima M, Kodama H, Kim VI, Shesterkin PV, Makhinov AN (2007) Migration behavior of Fe in the Amur River basin. Report Amur-Okhotsk Project 4:37–48
Nagata Y, Ohtani K, Kashiwai M (1992) Subarctic gyre in the North Pacific Ocean. Umino-Kenkyu 1(3):75–104
Nakabayashi S, Kuma K, Sasaoka K, Saito S, Mochizuki M, Shiga N (2002) Variation in iron(III) solubility and iron concentration in the northwestern North Pacific Ocean. Limnol Oceanogr 47(3):885–892
Nakamura T, Awaji T (2004) Tidally induced diapycnal mixing in the Kuril Straitsand its role in water transformation and transport: a three-dimensionalnon-hydrostatic model experiment. J Geophys Res Oceans 109:C09S07. https://doi.org/10.1029/2003JC001850
Nakamura T, Toyoda T, Ishikawa Y, Awaji T (2006) Effect of tidal mixing at the Kuril Straits on North Pacific ventilation: adjustment of the intermediate layer revealed from numerical experiments. J Geophys Res 111(C4):C04003. https://doi.org/10.1029/2005JC003142
Nakanowatari T, Nakamura T, Uchimoto K, Nishioka J, Mitsudera H, Wakatsuchi M (2017) Importance of Ekman transport and gyre circulation change on seasonal variation of surface dissolved iron in the western subarctic North Pacific. J Geophys Res- Ocean 122(5):4364–4391. https://doi.org/10.1002/2016JC012354
Nakatsuka T, Yoshikawa C, Toda M, Kawamura K, Wakatsuchi M (2002) An extremely turbid intermediate water in the Sea of Okhotsk: implication for the transport of particulate organic matter in a seasonally ice-bound sea. Geophys Res Lett 29(16):1757. https://doi.org/10.1029/2001GL014029
Nakatsuka T, Toda M, Kawamura K, Wakatsuchi M (2004) Dissolved andparticulate organic carbon in the Sea of Okhotsk: transport from continental shelf to ocean interior. J Geophys Res 109:C09S14. https://doi.org/10.1029/2003JC001909
Nakayama Y, Kuma K, Fujita S, Sugie K, Ikeda T (2010) Temporal variability and bioavailability of iron and other nutrients during the spring phytoplankton bloom in the Oyashio region. Deep Sea Res II 57:1618–1629. https://doi.org/10.1016/j.dsr2.2010.03.006
Nishioka J, Obata H (2017) Dissolved iron distribution in the western and central subarctic Pacific: HNLC water formation and biogeochemical processes. Limnol Oceanogr 62(5):2004–2022
Nishioka J, Takeda S, Wong CS, Johnson K (2001) Size-fractionated iron concentrations in the northeast Pacific Ocean: distribution of soluble and small colloidal iron. Mar Chem 74:158–179
Nishioka J, Takeda S, Kudo I, Tsumune D, Yoshimura T, Kuma K, Tsuda A (2003) Size fractionated iron distributions and iron-limitation processes in the subarctic NW Pacific. Geophys Res Lett 30:1730. https://doi.org/10.1029/2002GL016853
Nishioka J, Ono T, Saito H, Nakatsuka T, Takeda S, Yoshimura T, Suzuki K, Kuma K, Nakabayashi S, Tsumune D, Mitsudera H, Johnson WK, Tsuda A (2007) Iron supply to the western subarctic Pacific: importance of iron export from the Sea of Okhotsk. J Geophys Res 112:C10012. https://doi.org/10.1029/2006JC004055
Nishioka J, Ono T, Saito H, Sakaoka K, Yoshimura T (2011) The annual cycle of surface iron and the source of iron supporting the spring diatom bloom in the Oyashio region, western subarctic Pacific. J Geophys Res 116:C02021. https://doi.org/10.1029/2010JC006321
Nishioka J, Nakatsuka T, Watanabe YW, Yasuda I, Kuma K, Ogawa H, Ebuchi N, Scherbinin A, Volkov YN, Shiraiwa T, Wakatsuchi M (2013) Intensive mixing along an island chain controls oceanic biogeochemical cycles. Global Biogeochem Cycle 27:920–929
Nishioka J, Mitsudera H, Yasuda I, Liu H, Nakatsuka T, Volkov YN (2014a) Biogeochemical and physical processes in the Sea of Okhotsk and the linkage to the Pacific Ocean. Prog Oceanogr 126:1–7. https://doi.org/10.1016/j.pocean.2014.04.027
Nishioka J, Nakatsuka T, Ono K, Volkov YN, Scherbinin A, Shiraiwa T (2014b) Quantitative evaluation of iron transport processes in the Sea of Okhotsk. Prog Oceanogr 126:180–193
Nishioka J, Obata H, Ogawa H, Ono K, Yamashita Y, Lee KJ, Takeda S, Yasuda I (2020) Sub-polar marginal seas fuel the North Pacific through the intermediate water at the termination of the global ocean circulation. Proc Natl Acad Sci USA 117(23):12665–12673. https://doi.org/10.1073/pnas.2000658117
Obata H, Karatani H, Nakayama E (1993) Automated determination of iron in seawater by chelating resin concentration and chemiluminescence detection. Anal Chem 65:1524–1528. https://doi.org/10.1021/ac00059a007
Ohshima KI, Martin S (2004) Introduction to special section: oceanography of theOkhotsk Sea. J Geophys Res 109:C09S01. https://doi.org/10.1029/2004JC002604
Ohshima KI, Wakatsuchi M, Fukamachi Y, Mizuta G (2002) Near-surfacecirculation and tidal currents of the Okhotsk Sea observed with satellite-trackeddrifters. J Geophys Res. https://doi.org/10.1029/2001JC001005
Okamoto S, Hirawake T, Saito S (2010) Internal variability in the magnitude and timing of the spring bloom in the Oyashio region. Deep Sea Res II 57:1608–1617. https://doi.org/10.1016/j.dsr2.2010.03.005
Ono K, Ohshima KI, Kono T, Katsumata K, Yasuda I, Wakatsuchi M (2013) Distribution of vertical diffusivity in the Bussol’ Strait: a mixing hot spot in the North Pacific. Deep Sea Res I 79:62–73
Parsons TR, Lalli CM (1998) Comparative oceanic ecology of the plankton communities of the subarctic Atlantic and Pacific oceans. Oceanogr Mar Biol Annu Rev 26:317–359
Patterson CC (1965) Contaminated and natural lead environments of man. Arch Environ Health 11:344–363
Pinedo-González P, Hawco NJ, Bundy RM, Armbrust EV, Follows MJ, Cael BB, White AE, Ferron S, Karl DM, John SG (2020) Anthropogenic Asian aerosol provide Fe to the North Pacific Ocean. Proc Natl Acad Sci USA. https://doi.org/10.1073/pnas.2010315117
Read RK, Laird NP (1977) A study of subarctic boundary region in the western North Pacific. J Oceanogr Society of Japan 33:247–253
Resing JA, Sedwick PN, German CR, Jenkins WJ, Moffett JW, Sohst BM, Tagliabue A (2015) Basin-scale transport of hydrothermal dissolved metals across the South Pacific Ocean. Nature 523:200–203. https://doi.org/10.1038/nature14577
Rue EL, Bruland KW (1995) Complexation of iron(III) by natural organic ligands in the Central North Pacific as determined by a new competitive ligand equilibration/ adsorptive cathodic stripping voltammetric method. Mar Chem 50:117–138
Saito H, Tsuda A, Kasai H (2002) Nutrient and plankton dynamics in the Oyashio region of the western subarctic Pacific Ocean. Deep Sea Res II 49:5463–5486
Sakurai Y (2007) An overview of Oyashio ecosystem. Deep Sea Res Part II 54:2526–2542
Sarmiento JL, Gruber N (2006) Ocean biogeochemical dynamics. Princeton University Press, Princeton
Sarmiento JL, Gruber N, Brzezinski MA, Dunne JP (2004) High-latitude controls of thermocline nutrients and low latitude biological productivity. Nature 427:56–60
Schlitzer R (2004) Export production in equatorial and North Pacific derived from dissolved oxygen, nutrient and carbon data. J Oceanogr 60:53–62
Shigemitsu M, Nishioka J, Watanabe YW, Yamanaka Y, Nakatsuka T, Volkov YN (2013) Fe/Al ratios of suspended particulate matter from intermediate water in the Okhotsk Sea: implications for long-distance lateral transport of particulate Fe. Mari Chem 157:41–48
Shiomoto A, Asami H (1999) High-West and Low-East distribution patterns of chlorophyll a. primary productivity and diatoms in the subarctic North Pacific surface waters, mid winter 1996. J Oceanogr 55:493–503
Shiomoto A, Ishida Y, Tamaki M, Yamanaka Y (1998) Primary production and chlorophyll a in the NW pacific ocean in summer. J Geophys Res 103:24651–24661
Shiozaki T, Ito S, Takahashi K, Saito H, Nagata T, Furuya K (2014) Regional variability of factors controlling the onset timing and magnitude of spring algal blooms in the northwestern North Pacific. J Geophys Res 119:1–13. https://doi.org/10.1002/2013JC009187
Shiraiwa T (2012) “Giant Fish-Breeding Forest”: a new environmental system linking continental watershed with open water. Global Environmental Studies book series 77–85
Sigman DM, Fripiat F, Studer AS, Kemeny PC, Martínez-García A, Hain MP, Ai X, Wang X, Ren H, Hang GH (2021) The Southern Ocean during the ice ages: a review of the Antarctic surface isolation hypothesis, with comparison to the North Pacific. Quatern Sci Rev 254:106732. https://doi.org/10.1016/j.quascirev.2020.106732
Sohrin Y, Bruland KW (2011) Global status of trace elements in the ocean. Trends Anal Chem 30(8):1291–1307. https://doi.org/10.1016/j.trac.2011.03.006
Sohrin Y, Urushihara S, Nakatsuka S, Kono T, Higo E, Minami T, Norisuye K, Umetani S (2008) Multielemental determination of GEOTRACES key trace metals in seawater by ICPMS after preconcentration using an ethylenediaminetriacetic acid chelation resin. Anal Chem 80:6267–6273. https://doi.org/10.1021/ac800500f
Stabeno PJ, Schumacher JD, Ohtani K (1999) The physical oceanography of the Bering Sea. Dynamics of the Bering Sea 1–59
Stoll H (2020) 30 years of the iron hypothesis of ice ages. Nature 578:370–371
Sugie K, Kuma K, Fujita S, Nakayama Y, Ikeda T (2010) Nutrient and diatom dynamics during late winter and spring in the Oyashio region of the western subarctic Pacific Ocean. Deep Sea Res II 57:1630–1642
Sugie K, Nishioka J, Kuma K, Volkov YN, Nakatsuka T (2013) Availability of particulate Fe to phytoplankton in the Sea of Okhotsk. Mar Chem 152:20–31. https://doi.org/10.1016/j.marchem.2013.03.005
Suzuki K, Liu H, Saino T, Obata H, Takano M, Okamura K, Sohrin Y, Fujishima Y (2002) East-west gradients in the photosynthetic potential of phytoplankton and iron concentration in the subarctic Pacific Ocean during early summer. Limnol Oceanogr 47(6):1581–1594
Suzuki K, Hattori-Saito A, Sekiguchi Y, Nishioka J, Sigemitsu M, Isada T, Liu H, McKay RM (2014) Spatial variability in iron nutritional status of large diatoms in the Sea of Okhotsk with special reference to the Amur river discharge. Biogeosciences 11:2503–2517. https://doi.org/10.5194/bg-11-2503-2014
Tagliabue A, Aumont O, Bopp L (2014) The impact of different external sources of iron in the global carbon cycle. Geophys Res Lett 41:920–926. https://doi.org/10.1002/2013GL059059
Tagliabue A, Bowie AR, Boyd PW, Buck KN, Johnson KS, Saito M (2017) The integral role of iron in ocean biogeochemistry. Nature 543:51–59
Takahashi T, Sutherland SC, Sweeney C, Poisson A, Metzl N, Tilbrook B, Bates N, Wanninkhof R, Feely RA, Sabine C, Olafsson J, Nojiri Y (2002) Global sea-air CO2 flux based on climatological surface ocean pCO2, and seasonal biological and temperature effects. Deep Sea Res II 49:1601–1622
Takahashi T, Sutherland SC, Wanninkhof R, Sweeney C, Feely RA, Chipman DW, Hales B, Friederich G, Chavez F, Sabine C, Watson A, Bakker DCE, Schuster U, Metzl N, Yoshikawa-Inoue H, Ishii M, Midorikawa T, Nojiri Y, Körtzingerm A, Steinhoff T, Hoppema M, Olafsson J, Arnarson TS, Tilbrook B, Johannessen T, Olsen A, Bellerby R, Wong CS, Delille B, Bates NR, de Baar HJW (2009) Climatological mean and decadal change in surface ocean pCO2, and net sea–air CO2 flux over the global oceans. Deep Sea Res II 56:554–577
Takata H, Kuma K, Saito Y, Chikira M, Saito S, Isoda Y, Takagi S, Sakaoka K (2006) Comparing the vertical distribution of iron in the eastern and western North Pacific Ocean. Geophys Res Lett 33:L02613. https://doi.org/10.1029/2005GL024538
Takeda S (2011) Iron and phytoplankton growth in the subarctic North Pacific. Aqua BioSci Monogr 4(2):41–93. https://doi.org/10.5047/absm.2011.00402.0041
Takeda S, Nishioka J, Wong CS, Whitney FA, Johnson WK, Sotuar TJ (1999) Application of open-ocean enclosures to study the control of biological CO2 Pump in the subarctic North Pacific Ocean. Proceeding, CO2 in the Ocean, Tsukuba 1999
Talley LD (1991) An Okhotsk Sea Water anomaly: Implications for ventilation in the North Pacific. Deep Sea Res I 38:S171–S190
Talley LD (2013) Closure of the global overturning circulation through the Indian, Pacific, and Southern Oceans. Oceanogr 26(1):80–97. https://doi.org/10.5670/oceanog.2013.07
Tanaka T, Yasuda I, Kuma K, Nishioka J (2012a) Vertical turbulent iron flux sustains the Green Belt along the shelf break in the southeastern Bering Sea. Geophys Res Lett 39:L08603. https://doi.org/10.1029/2012GL051164
Tanaka Y, Yasuda I, Hasumi H, Tatebe H, Osafune S (2012b) Effects of the 18.6-year modulation of tidal mixing on the North Pacific bidecadal climate variability in a coupled climate model. J Climate 25:7625–7642
Tanaka T, Yasuda I, Tanaka Y, Carter GS (2013) Numerical study on tidal mixing along the shelf break in the Green Belt in the southeastern Bering Sea. J Geophys Res 118:6525–6542. https://doi.org/10.1002/2013JC009113
Tanaka T, Yasuda I, Kuma K, Nishioka J (2017) Evaluation of the biogeochemical impact of iron-rich shelf water to the Green Belt in the southeastern Bering Sea. Conti Shelf Res 143:130–138. https://doi.org/10.1016/j.csr.2016.11.008
Tani H, Nishioka J, Kuma K, Takata H, Yamashita Y, Tanoue E, Midorikawa T (2003) Iron (III) hydroxide solubility and humic-type fluorescent organic matter in the deep water column of the Okhotsk Sea and the northwestern North Pacific Ocean. Deep Sea Res I 50:1063–1078. https://doi.org/10.1016/S0967-0637(03)00098-0
Tsuda A, Takeda S, Saito H, Nishioka J, Nojiri Y, Kudo I, Kiyosawa H, Shiomoto A, Imai K, Ono T, Shimamoto A, Tsumune D, Yoshimura T, Aono T, Hinuma A, Kinugasa M, Suzuki K, Sohrin Y, Noiri Y, Tani H, Deguchi D, Tsurushima N, Ogawa H, Fukami K, Kuma K, Saino T (2003) A mesoscale iron enrichment in the western subarctic Pacific induces large centric diatom bloom. Science 300:958–961
Tsuda A, Takeda S, Saito H, Nishioka J, Kudo I, Nojiri Y, Suzuki K, Uematsu M, Wells ML, Tsumune D, Yoshimura T, Aono T, Aramaki T, Cochlan WP, Hayakawa M, Imai K, Isada T, Iwamoto Y, Johnson WK, Kameyama S, Kato S, Kiyosawa H, Kondo Y, Levasseur M, Machida R, Nagao I, Nakagawa F, Nakanishi T, Nakatsuka S, Narita A, Noiri Y, Obata H, Ogawa H, Oguma K, Ono T, Sakuragi T, Sasakawa M, Sato M, Shimamoto A, Takada H, Trick CG, Watanabe YW, Wong CS, Yoshie N (2007) Evidence for the grazing hypothesis: Grazing reduces phytoplankton responses of the HNLC ecosystem to iron enrichment in the western subarctic Pacific (SEEDS II). J Oceanogr 63:983–994
Tsurushima N, Nojiri Y, Imai K, Watanabe S (2002) Seasonal variations of carbon dioxide system and nutrients in the surface mixed layer at station KNOT (44°N, 155°E) in the subarctic western North Pacific. Deep Sea Res II 49:5377–5394
Uchida R, Kuma K, Omata A, Ishikawa S, Hioki N, Ueno H, Isoda Y, Sakaoka K, Kamei Y, Takagi S (2013) Water column iron dynamics in the subarctic North Pacific Ocean and the Bering Sea. J Geophys Res 118:1257–1271. https://doi.org/10.1002/jgrc.20097
Uehara H, Kruts AA, Mitsudara H, Nakamura T, Volkov YN, Wakatsuchi M (2014) Remotely propagating salinity anomaly varies the source of North Pacific ventilation. Prog Oceanogr 126:80–97. https://doi.org/10.1016/j.pocean.2014.04.016
Uematsu M, Duce RA, Prospero JM, Chen L, Merrill JT, McDonald RL (1983) Transport of mineral aerosol from Asia over the North Pacific Ocean. J Geophys Res 88:5343–5352
Uematsu M, Wand Z, Uno I (2003) Atmospheric input of mineral dust to the western North Pacific region based on direct measurement and regional chemical transport model. Geophys Res Lett 30(6):1342. https://doi.org/10.1029/2002GL016645
Uematsu M, Yokouchi Y, Watanabe TW, Takeda S, Yamanaka Y (edit) (2014) Western Pacific Air-Sea Interaction Study. TERRAPUB, 284 pp
van den Berg CMG (1995) Evidence for organic complexation of iron in seawater. Mar Chem 50:139–157. https://doi.org/10.1016/0304-4203(95)00032-M
Watson A, Liss P, Duce R (1991) Design of a small-scale in situ iron fertilization experiment. Limnol Oceanogr 36(8):1960–1965
Whitney FA (2011) Nutrient variability in the mixed layer of the subarctic Pacific Ocean, 1987–2010. J Oceanogr. https://doi.org/10.1007/s10872-011-0051-2
Whitney FA, Freeland HJ (1999) Variability in upper-ocean water properties in the NE Pacific Ocean. Deep Sea Res II 46:2351–2370
Whitney FA, Crawfoard WR, Harrison PJ (2005) Physical processes that enhance nutrient transport and primary productivity in the coastal and open ocean of the subarctic NE Pacific. Deep Sea Res II 52:681–706
Whitney FA, Bograd SJ, Ono T (2013) Nutrient enrichment of the subarctic Pacific Ocean pycnocline. Geophys Res Lett 40:2200–2205. https://doi.org/10.1002/grl.50439
Wong CS, Matear RJ, Freeland HJ, Whitney FA, Bychkov AS (1998) WOCE line P1W in the sea of Okhotsk: 2. CFCs and the formation rate of intermediate water. J Geophys Res Oceans 103:15625–15642
Wu J, Boyle E (2002) Determination of iron in seawater by high-resolution isotope dilution inductively coupled mass spectrometry after Mg(OH)2 coprecipitation. Anal Chim Acta 367:183–191
Wu J, Boyle E, Sunda W, Wen L-S (2001) Soluble and colloidal iron in the oligotrophic North Atlantic and North Pacific. Science 293:847–849. https://doi.org/10.1126/science.1059251
Wu J, Aguilar-Islas A, Rember R, Weingartner T, Danielson S, Whitledge T (2009) Size-fractionated iron distribution on the northern Gulf of Alaska. Geophys Res Lett 36:L11606. https://doi.org/10.1029/2009GL038304
Xiu P, Palacz AP, Chai F, Roy EG, Wells ML (2011) Iron flux induced by Hida eddies in the Gulf of Alaska. Geophys Res Lett 38:L13607. https://doi.org/10.1029/2011GL047946
Yagi M, Yasuda I (2012) Deep intense vertical mixing in the Bussol’ Strait. Geophys Res Lett 39:L01602. https://doi.org/10.1029/2011GL050349
Yamamoto-Kawai M, Watanabe S, Tsunogai S, Wakatsuchi M (2004) Chlorofluorocarbons in the Sea of Okhotsk: ventilation of the intermediatewater. J Geophys Res Oceans 109:C09S11. https://doi.org/10.1029/2003JC001919
Yamashita Y, Tanoue E (2008) Production of bio-refractory fluorescent dissolved organic matter in the ocean interior. Nat Geosci 1:579–582. https://doi.org/10.1038/ngeo279
Yamashita Y, Nishioka J, Obata H, Ogawa H (2020) Shelf humic substances as carriers for basin-scale iron transport in the North pacific. Sci Rep 10:4505. https://doi.org/10.1038/s41598-020-61375-7
Yasuda I (1997) The origin of the North Pacific intermediate water. J Geophys Res 102(C1):893–909
Yasuda I, Hiroe Y, Komatsu K, Kawasaki K, Joyce TM, Bahr F, Kawasaki Y (2001) Hydrographic structure and transport of the Oyashio south of Hokkaido and the formation of North pacific Intermediate Water. J Geophys Res 106(C4):6931–6942
Yasunaka S, Nojiri Y, Nakaoka S, Ono T, Whitney FA, Telszewski M (2014) Mapping of sea surface nutrients in the North Pacific: basin-wide distribution and seasonal to interannual variability. J Geophys Res 119:7756–7771. https://doi.org/10.1002/2014JC010318
Yasunaka S, Mitsudera H, Whitney F, Nakaoka S (2020) Nutrient and dissolved inorganic carbon variability in the North pacific. J Oceanogr. https://doi.org/10.1007/s10872-020-00561-7
Yoshie N, Suzuki K, Kuwata A, Nishioka J, Saito H (2010) Temporal and spatial variations in photosynthetic physiology of diatoms during the spring bloom in the western subarctic Pacific. Mar Ecol Prog Ser 309:39–52
Yoshikawa C, Nakatsuka T, Wakatsuchi M (2006) Distribution of N* in the Sea of Okhotsk and its use as a biogeochemical tracer of the Okhotsk Sea Intermediate Water formation process. J Mar Syst 63:49–62
Yoshimura T, Nishioka J, Nakatsuka T (2010) Iron nutritional status of the phytoplankton assemblage in the Okhotsk Sea during summer. Deep Sea Res I 57:1454–1464
Zheng L, Sohrin Y (2019) Major lithogenic contributions to the distribution and budget of iron in the North Pacific Ocean. Sci Rep 9:11652. https://doi.org/10.1038/s41598-019-48035-1
Zheng L, Minami T, Takano S, Minami H, Sohrin Y (2017) Distribution and stoichiometry of Al, Mn, Fe Co, Ni, Cu, Zn, Cd, and Pb in seawater around the Juan de Fuca Ridge. J Oceanogr 73:669–685. https://doi.org/10.1007/s10872-017-0424-2
Acknowledgements
The author is grateful to the captain, crew, and scientists on board the previous expeditions that were related to the projects in this review. We thank to Drs. T. Nakatsuka, T. Shiraiwa, N. Ebuchi, H. Mitsudera, Y. Fukamachi, K.I. Ohshima and M. Wakatsuchi for their support with the marginal seas observational study. Thanks to Dr. Y.N. Volkov and Mr. A. Shcherbinin from the Far Eastern Regional Hydrometeorological Research Institute for their cooperation with the Japanese-Russian joint research program. The authors thank A. Murayama and K. Ono for their support in the laboratory and onboard. This work was supported by the Ministry of Education, Science, and Culture KAKEN grants B “26281002”, “25121501”, and A “17H00775”. This work was supported by Grant-in-Aid for Scientific Research on Innovative Areas, the Ocean Mixing Processes: OMIX Project (JP15H05820, JP15H05817, JP15H05818, JP16H01592, JP16H01595, JP18H04910, JP18H04919 JP18H04922, JP20H05598), Grants JP20K21838, JP20H05707 funded to J.N., H.O, T.H., I.Y., Y.Y., Y.K., K.M, the Arctic Challenge for Sustainability Project, and the Grant for Joint Research Program of the Institute of Low Temperature Science, Hokkaido University. The comments and suggestions from the anonymous reviewers are also gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nishioka, J., Obata, H., Hirawake, T. et al. A review: iron and nutrient supply in the subarctic Pacific and its impact on phytoplankton production. J Oceanogr 77, 561–587 (2021). https://doi.org/10.1007/s10872-021-00606-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10872-021-00606-5