Abstract
Eye movements are inhibited prior to the occurrence of temporally predictable events. This ‘oculomotor inhibition effect’ has been demonstrated with various tasks and modalities. Specifically, it was shown that when intervals between cue and target are fixed, saccade rate prior to the target is lower than when they are varied. However, it is still an open question whether this effect is linked to temporal expectation to the predictable target, or to the duration estimation of the interval preceding it. Here, we examined this question in 20 participants while they performed an implicit temporal expectation and an explicit time estimation task. In each trial, following cue onset, two consecutive grating patches were presented, each preceded by an interval. Temporal expectation was manipulated by setting the first interval duration to be either fixed or varied within each block. Participants were requested to compare either the durations of the two intervals (time estimation), or the tilts of the two grating patches (temporal expectation). Saccade rate, measured prior to the first grating, was lower in the fixed relative to the varied condition of both tasks. This suggests that the inhibition effect is elicited by target predictability and indicates that it is linked to temporal expectation, rather than to time estimation processes. Additionally, this finding suggests that the oculomotor inhibition is independent of motor readiness, as it was elicited even when no response was required. We conclude that the prestimulus oculomotor inhibition effect can be used as a marker of temporal expectation, and discuss its potential underlying mechanisms.
Similar content being viewed by others
Introduction
Our surrounding environment is in constant flux. To make sense of it, our perceptual system engages in the continuous development of expectations, serving to guide our behavior (Clark, 2013). Some of the substantial predictions that our cognitive system makes regard the temporal domain—when is a goal-relevant event most likely to occur (Nobre & Rohenkohl, 2014). By using temporal expectation, our perceptual system can orient attentional resources to maximize its readiness at crucial moments and improve perceptual performance.
Recent studies established a tight link between temporal predictability and the oculomotor system—in anticipation for the onset of predictable targets, oculomotor behaviors, such as saccades, are inhibited. This pretarget oculomotor inhibition effect was demonstrated across multiple temporal structures (Amit et al., 2019; Dankner et al., 2017; Denison et al., 2019; Tal-Perry & Yuval-Greenberg, 2020) as well as in different modalities (Abeles et al., 2020; Amit et al., 2019; Badde et al., 2020), and was shown to covary with target probability (Tal-Perry & Yuval-Greenberg, 2020). In these previous studies, the oculomotor inhibition effect was interpreted as the direct or indirect outcome of implicit processes involved in the development of temporal expectation (i.e., the building up of expectation toward the upcoming target).
However, this interpretation of the oculomotor inhibition effect is still inconclusive, and specifically, the involvement of timing processes in this effect was never investigated. Events are rendered temporally predictable when they occur at regular intervals or when they are preceded by informative temporal cues (Nobre & van Ede, 2017). Importantly, the development of expectations regarding an upcoming temporally predictable event depends on the ability to estimate the duration of the interval preceding it (Zelaznik et al., 2002). Thus, any perceptual gain of predictability reflects not only the development of expectations but also the implicit involvement of timing mechanisms. In fact, tasks that require a perceptual decision, but in which timing is beneficial, are often referred to as ‘implicit timing’ tasks. This is in contrast to ‘explicit timing’ tasks, which require an explicit decision regarding time (Coull & Nobre, 2008). The tasks that we have used in previous studies require implicit timing, and it is, therefore, possible that the oculomotor inhibition effect is the consequence of processes involved in time estimation, rather than in the development of temporal expectation. This hypothesis may be especially appealing because it could imply a central functional role for the oculomotor inhibition effect: previous studies have demonstrated that the estimated duration of a time-interval may be distorted when an oculomotor action occurs during that interval (Morrone et al., 2005; Schütz & Morrone, 2010; Yu et al., 2017). It could be, therefore, suggested that the purpose of the inhibition of eye movements while anticipating a predictable target is to avoid these distortions.
The goal of the present study is to examine whether pretarget oculomotor inhibition is the consequence of temporal expectation or whether it is a consequence of time estimation. In this study, we focus on saccades, being a frequent and easy-to-detect oculomotor behavior. Pretarget inhibition of saccades was tested in 20 participants as they performed either an implicit temporal expectation or an explicit time estimation task, in separate blocks, using an identical trial procedure for both tasks. In each trial, participants were presented with a trial-onset cue followed by two consecutive visual stimuli, each of which was preceded by a fixed or varying interval. In the explicit time estimation task, participants were requested to attend to the duration of the intervals and make a two-alternative forced-choice (2AFC) comparison between them (duration task). In the implicit temporal expectation task, participants were requested to attend to the tilt of the visual targets and make a 2AFC perceptual comparison between them (tilt task). Temporal expectation was manipulated by setting the first interval of both tasks to be either fixed throughout the block or varied within blocks. Prestimulus inhibition of saccades was assessed by measuring saccade rate (SR) prior to the onset of the first stimulus, and contrasting it between conditions.
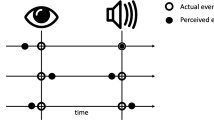
This design resulted in diverging predictions for the two alternative hypotheses. According to the timing hypothesis (see Fig. 1a), oculomotor inhibition occurs whenever time estimation takes place, either implicitly as when preparing for a stimulus onset, or explicitly as when measuring time. This hypothesis predicts different outcomes for the tilt and the duration tasks. In the tilt task, time estimation processes are expected to occur only in the fixed condition, where the first target is fully predictable and can be timed, and not in the varied condition, where it is less predictable. Therefore, in this task, the timing hypothesis predicts stronger inhibition of saccades in the fixed relative to the varied condition. In contrast, in the duration task, time estimation is required for the varied condition, as each new trial brings about a new interval to be timed. Therefore, under the timing hypothesis, the prediction for the duration task will be of similar or even stronger inhibition in the varied relative to the fixed conditions.
Hypotheses. Theoretical predictions made by the timing (a) and the temporal expectation (b) hypotheses for the effects of the fixed and varying conditions of the duration and tilt tasks on saccade rate (SR) prior to the first stimulus. Note that this schematic figure depicts stronger inhibition in the varied relative to the fixed condition in the duration task under the timing hypothesis, whereas the hypothesis predicts stronger or equal inhibition with this task
Alternatively, the temporal expectation hypothesis predicts a similar pattern of results for both tasks (see Fig. 1b). According to this hypothesis, saccade inhibition occurs in preparation for a predictable upcoming event. In both tasks, the first target is more predictable in the fixed condition than in the varied condition. Therefore, the hypothesis predicts stronger oculomotor inhibition for the fixed relative to the varied condition, regardless of the task.
Results showed a prestimulus inhibition of saccades for the fixed but not varied condition, in both tasks. These findings indicate that time estimation is not sufficient to elicit the oculomotor inhibition, supporting the interpretation of the oculomotor inhibition effect as reflecting temporal expectation. We discuss the functionality and underlying mechanism of the oculomotor inhibition in light of these results.
Method
Participants
Participants in this experiment were 20 students of Tel-Aviv University (eight male, 19 right-handed, Mage = 22.25 years, SD = 2.31). The size of this sample was chosen according to prior studies that used similar sample sizes (Amit et al., 2019; Tal-Perry & Yuval-Greenberg, 2020). To ensure that this sample size leads to an adequate power to observe the expected effects, we implemented a power simulation (with the Superpower package; Caldwell & Lakens, 2019) using data collected in a previous study (Tal-Perry & Yuval-Greenberg, 2020). According to the results, we determined that a sample size of 20 participants, each performing 80 trials in every condition, leads to a high probability of observing a disordinal interaction between condition and task if oculomotor inhibition occurs due to time estimation (\( 1-\beta =.89,{\eta}_p^2=.370 \)) and a main effect of condition if oculomotor inhibition occurs solely due to temporal orienting (\( 1-\beta >.99,{\eta}_p^2=.701 \)). Participants were compensated for their participation via payment or course credits. Ethical approval was provided by the ethical committees of Tel-Aviv University and the School of Psychological Sciences. Prior to participation, participants signed informed consent forms.
Stimuli
A black cross (0.4° × 0.4°) was used as a fixation target. The stimuli (S1 and S2) were tilted Gabor grating patches (both 2° in diameter, with a spatial frequency of 5 cycles per degree [cpd] and 30% contrast). All stimuli in this experiment were presented at the center of the screen, using a mid-gray background.
Procedure
Setup
Participants were seated in a dimly lit room, with the computer monitor placed 100 cm in front of them (24-in. LCD ASUS VG248QE, 1,920 × 1,080 pixels resolution, 120 Hz refresh rate, mid-gray luminance was measured to be 110 cd/m2). During the experiment, participants rested their heads on a chin rest.
Trial procedure
A central black fixation-cross was presented during the intertrial interval (ITI). The trial started after 500 ms of stable fixation (gaze located within a 1.5° radius from screen center, verified by online gaze sampling) plus a jittered period of 200–700 ms. At trial onset, the fixation-cross changed color from black to blue, signaling the onset of the first interval (I1). After I1 has elapsed, a tilted Gabor patch (S1) was presented for 33 ms and was followed by a blank screen (300 ms) to prevent backward masking. This was followed by a central blue fixation-cross indicating the beginning of the second interval (I2). After I2 has elapsed, a second tilted Gabor patch (S2) was presented for 33 ms and was followed by a blank screen, during which participants were requested to respond by pressing one of two keys on the keyboard. Once a response was detected, a green or red fixation-cross was presented for 500 ms to provide feedback on a correct or an incorrect response, respectively. After feedback, the fixation-cross changed to black, indicating the start of the next ITI. The trial procedure is depicted in Fig. 2.
Trial procedure. Tasks (duration and tilt) were performed in separate blocks. Following response, a feedback (green/red fixation cross for correct/incorrect response, respectively) was displayed for 500 ms (omitted from figure). All stimuli are shown for illustration purposes only and are not to scale. S1, S2 represent Stimuli 1 and 2; I1, I2 represent Interval 1 and 2
Duration and tilt manipulations
Temporal expectation for S1 was manipulated between blocks, by setting I1 to be either fixed at 1 s (fixed condition) or varied between 1 and 3 s in 0.5-s increments (varied condition). In both types of blocks, I2 was set to be slightly shorter or slightly longer than the current trial’s I1, with the percentage difference between I1 and I2 set individually per participant via a staircase procedure (mean ∣I1 − I2∣ = 19.65%, SD = 6.56%, range: 8%–30%). Therefore, I2 was never fixed, and S2 onset could not be predicted by cue onset. S1 was tilted 25°–65° from vertical. S2 was tilted slightly clockwise or counter-clockwise from S1, with the difference in tilt between S1 and S2 determined individually per participant via staircase procedure (mean ∣S1 − S2∣ = 6.8°, SD = 2.5°, range: 3°–13°).
Tasks
At the beginning of each block participants were asked to perform one of two tasks: (1) to report which of the Gabor patches (S1 or S2) was tilted more horizontally (tilt task); or (2) to report which of the two intervals (I1 or I2) was longer in duration (duration task). Note that the comparison of duration is not between the duration of the Gabor stimuli (as both are always presented for 33 ms), but between the durations of the intervals between the fixation color change and the subsequent Gabor onset. There was no limit on response time and participants were encouraged to focus on accuracy rather than on reaction time. Participants were also requested to maintain fixation whenever the fixation cross was presented. Participants were not informed regarding the fixed or varied conditions, thus, any learning of interval duration that occurred was incidental.
Experimental blocks and sessions
The experiment consisted of three sessions with participants completing, in addition to a practice and an adaptive staircase procedure, a total of 12 blocks of the main experiment (80 trials each, ~10 minutes): one block of each task in the fixed condition, and five blocks of each task in the varied condition, in order to equate the number of 1-s trials in the fixed and the varied conditions. The first session included a practice block, an adaptive staircase block for each task, and two blocks of the varied condition (one from each task). The second and third sessions started with one block from the fixed condition and then four blocks from the varied condition. The two tasks alternated between blocks, with task order counterbalanced between participants.
Practice and staircase
The practice block, consisting of 20 trials from each task, was administered at the beginning of the first session, with tilt-difference between S1 and S2 set to ±20° and percentage difference between I1 and I2 set to ±25%. The order of the tasks in the practice block matched the participant’s order of experimental conditions in the main experiment. Following the practice session, participants completed two adaptive staircase procedures (one for each task) to determine individual thresholds. For all participants, the staircase of the tilt task was completed first, with the percentage difference between I1 and I2 set to its starting value throughout the procedure. The staircase of the duration task was completed next, with the tilt difference between S1 and S2 set to the value converged in the preceding staircase procedure. Each procedure followed a one-up three-down regime, which should yield an accuracy of approximately 79%, and with step-size changing logarithmically. The stopping rule of the staircase procedure was either four consecutive direction reversals or the completion of 100 trials, whichever came first. In both the practice session and the staircase procedures, I1 was set randomly to one of five intervals in each trial as in the varied condition.
Eye tracking
Eye tracking was done binocularly using EyeLink 1000 Plus infrared video-oculographic desktop mounted system (SR Research Ltd., Oakville, ON, Canada), with a sampling rate of 1000 Hz. This system has <0.01° spatial resolution and an average accuracy of 0.25°–0.5° when a chin rest is used, according to the manufacturer. A 9-point calibration and drift correction were performed prior to each block. Calibration accuracy, as measured through a validation procedure, was kept at <1° for each participant in each eye (mean average error 0.60°, range: 0.26°–0.91°).
The detection of saccadic events was based on a published algorithm (Engbert, 2006; Engbert & Kliegl, 2003) and the protocol used in previous publications (Abeles et al., 2020; Amit et al., 2019; Tal-Perry & Yuval-Greenberg, 2020). Binocular gaze position data were first smoothed using a 60 Hz low-pass filter, then converted to velocity and segmented to −300 ms relative to I1 onset and +300 ms relative to S1 onset (analyses of other intervals are included in the Supplementary Material). Saccade onsets were defined as the sample which deviated by six or more standard deviations from the segment’s median velocity, for six or more consecutive samples of both eyes. A minimum of 50 ms between saccade offset and the following saccade onset was imposed in order to control for overshoots and correctional saccades. Blink detection was also performed as in our previous studies (Abeles et al., 2020; Amit et al., 2019; Tal-Perry & Yuval-Greenberg, 2020) and following the recommendations by Hershman et al. (2018). Data points that included blinks along with a 200 ms before and after the blinks were excluded from the analysis. The majority of the resulting saccades were smaller than 1°, demonstrating that participants complied with the instruction to fixate. Saccade rate (SR) per second was calculated for each sample for individual participants and conditions as in previous studies (Abeles et al., 2020; Amit et al., 2019; Dankner et al., 2017; Tal-Perry & Yuval-Greenberg, 2020), by counting the number of saccades detected in that sample beyond trials, divided by the number of samples included in the analysis (after blink rejection) and multiplied by the sampling rate (1000 Hz). Saccade amplitude (size) was calculated as the Euclidean distance between gaze position at the onset of the saccade and gaze position at its offset and is reported in visual degrees.
Statistical analysis
Except when stated otherwise, only trials in which I1 was equal to 1 s were included in the main behavioral and oculomotor analyses. The analyses focused on accuracy rates and saccade rate. Reaction times (RTs) were not analyzed, because the experimental design was not suitable for studying them.
Accuracy rates
Since the distribution of accuracy invalidates the underlying assumptions behind analysis of variance, we analyzed the accuracy rates using a generalized linear mixed model (GLMM) assuming a binomial family of response–that is, a logistic mixed effect model (Jaeger, 2008). Due to the adaptive staircase procedure used to control performance, it was meaningless to compare the two tasks directly and therefore the accuracy rate analysis was performed separately on each of the tasks. The experimental condition (fixed/varied) was used as a predictor in both models. To select the random effect structure best supported by the data, a full model with a random intercept, a random slope for condition and their correlation was first considered, following by a zero-correlation parameter model and an intercept-only model, until finding the model whose estimates converged successfully, with a likelihood-ratio test (χ2 test) used to examine whether the resulting model has a significantly improved fit to the data compared with a reduced model (Bates et al., 2015a). Significance of the fixed factor was tested using the likelihood-ratio test against a null model. In addition, Bayes factor (BF) was approximated using Bayesian information criterion (BIC) approximation (Wagenmakers, 2007) against a null model. For convenience, BF10 is reported when the model is better supported by H1 relative to H0 (indicating how many times the data are more likely under the alternative hypothesis compared with the null hypothesis), BF01 is reported vice versa, and the logarithmic of the BF (logBF) is reported for BF > 100.
Saccade rate (SR) and amplitude
SR and mean saccade amplitude were each analyzed using a two-way repeated-measure analysis of variance (ANOVA), with condition (fixed/varied) and task (tilt/duration) as independent variables. Within-subject standardized effect-size (\( {\eta}_p^2 \) or Cohen’s d) is reported for each significant (p < .05) result along with the 95% confidence interval (CI). In addition, BFs were calculated by comparing the model including the effect with a reduced nested model excluding the effect (Type II sum of squares [SS]), and using a medium-sized Jeffries prior (r = 0.5) for fixed factors and medium-to-large sized Jeffries prior (r = 1) for the random factor (variance due to participants; Rouder et al., 2012), with reporting done as for the accuracy rates along with the estimate’s approximate % error.
To examine the time course of the oculomotor inhibition effect, we conducted an additional exploratory analysis. We divided the time period between -300 ms relative to I1 onset and 0 ms relative to S1 onset to thirteen 100 ms sized bins and contrasted the mean SR between the fixed and varied conditions, separately for each task and bin, while correcting for 26 multiple comparisons using false discovery-rate (FDR) correction (Benjamini & Hochberg, 1995).
Behavioral consequences of saccadic events
Further analysis of accuracy rates was conducted to test whether the inhibition of saccades has a perceptual role in either task. To dissociate the possible effect of temporal expectation from the influence of the saccadic event, only trials of the varied condition were included in the analysis. In this analysis, we collapsed over all trials of the varied condition instead of using only the I1 = 1-s trials as in the rest of the analyses. We compared accuracy rates for trials in which saccades occurred during the interval of −100 to −20 ms relative to S1 or S2 onset to accuracy rates for trials in which no saccades occurred during these intervals. To avoid the effects of saccadic suppression, the degrading of perception during saccades, and the effects of visual obstruction by blinks, we excluded from analysis trials in which saccades or blinks occurred at −20 to 0 ms relative to S1 or S2 onset (~7.11% of all varied trials). This analysis was performed separately for each task using a GLMM with binomial response family, with saccadic event (present/absent) as the predictor. All other details were similar to the procedure reported in the accuracy rate analysis.
Results
Behavior
Accuracy results were analyzed using a GLMM with a binomial family of responses, constructed separately for each task (tilt/duration), allowing for a random intercept by subject for the tilt model, and a full random effects structure for the duration model. Analyses revealed a significant effect of condition for the duration task, χ2(1) = 15.733, p < .001, BF10 = 46.1, with accuracy higher for the fixed as compared to the varied condition (see Fig. 3). This effect is consistent with previous findings (Lapid et al., 2008). No significant condition effect was found for the tilt task, χ2(1) = 2.248, p = .134, BF01 = 18.39, which is also consistent with previous findings using similar task designs (Amit et al., 2019; Griffin et al., 2002; Samaha et al., 2015; Tal-Perry & Yuval-Greenberg, 2020).
Behavioral results. a Percentage of correct responses for each task and condition in I1 = 1-s trials, averaged across participants (N = 20). Data of individual participants are represented as dots. Error bars designate ±1 standard error from the mean. Reported statistics are based on a likelihood-ratio (χ2) test. ***p < .001; ns indicates not significant. b Single-subject condition effect for the duration (circles) and tilt (triangles) tasks, with the diagonal line representing the identity line (where accuracy in fixed trials equals accuracy in varied 1-s trials)
Saccade amplitude
The procedure required fixation throughout the trial, thus the vast majority (92.76%) of all the observed saccades were smaller than 1°, as was the mean saccade amplitude across participants (0.53°±0.14° SD, range: 0.32°–0.89°). Therefore, most of the saccades could be considered microsaccades, following the common definition (Martinez-Conde et al., 2009). As in our previous studies (Abeles et al., 2020; Amit et al., 2019; Tal-Perry & Yuval-Greenberg, 2020) and following the common view in the field that microsaccades and saccades form an oculomotor continuum (Hafed et al., 2009; Ko et al., 2010; Martinez-Conde et al., 2013; Rolfs, 2009), we chose to analyze saccades of all sizes together.
We tested whether saccade amplitude around the time of saccade-rate (SR) analysis differed among tasks and conditions, by extracting the mean saccade amplitude during the time interval of −100 to 0 ms prior to the onset of S1 and analyzing the results using a 2 × 2 repeated-measures ANOVA, with task (tilt/duration) and condition (fixed/varied) as independent factors (see Fig. 4). Four participants had one or more conditions with no saccadic events in this period and were discarded listwise from this analysis. Results showed that saccades performed in the tilt task were, on average, slightly (by 0.07°) but significantly larger than saccades performed in the duration task, F(1, 15) = 8.06, p = .012, \( {\eta}_p^2 \) = .35, 95% CI [.022, .626], BF10 = 5.259 ± 2.3%. This effect was not expected and remains unexplained at this stage. Importantly, consistent with previous findings (Dankner et al., 2017), we found no difference in mean saccade amplitude between the fixed and varied conditions, F(1, 15) = 1.00, p = .334, BF01 = 2.183 ± 1.9%, indicating that the observed SR effects (see below) did not depend on differences in amplitude. In addition, no significant interaction was found between condition and task, suggesting that condition did not affect the observed effect of task, F(1, 15) = 0.30, p = .592, BF01 = 2.739 ± 7.6%.
Saccades amplitude results. a Mean saccade amplitude (in visual degrees) in the period of −100 to 0 ms relative to S1 onset, for participants who had saccadic events in this period (N = 16). Data of individual participants are represented as dots. Error bars designate ±1 standard error from the mean, corrected for within-subject variability (Cousineau & O’Brien, 2014). *p < .05. b Condition effects for the duration (circles) and tilt (triangles) tasks for each participant, with the diagonal line representing the identity line (where mean saccade amplitude in fixed trials equals mean saccade amplitude in varied 1-s trials). c Histogram of mean saccade amplitude of individual subjects, for each condition
Saccade rate
The main goal of this study was to examine the effect of condition on the mean SR prior to the onset of the first stimulus (S1). To answer this question, we performed two analyses: first, a hypothesis-driven analysis on a predefined time region-of-interest, and second, an exploratory analysis on the entire time segment.
In order to reexamine the interpretation of the inhibition effect in previous studies, we focused our hypothesis-driven analysis on the time window of −100 to 0 ms relative to the onset of the first stimulus. This time window was used in our previous studies on oculomotor inhibition (Abeles et al., 2020; Amit et al., 2019; Dankner et al., 2017; Tal-Perry & Yuval-Greenberg, 2020). Analysis was performed using a two-way repeated-measures ANOVA, with task (tilt/duration) and condition (fixed/varied) as the independent variables (see Fig. 5a–b). Results indicated a significant main effect for condition, F(1, 19) = 47.76, p < .0001, \( {\eta}_p^2=.715 \), 95% CI [.449, .835], logBF10 = 16.496 ± 2.4%, resulting from a higher prestimulus SR in the fixed relative to the varied condition. There was no significant difference in SR between tasks, F(1, 19) = 0.59, p = .45, BF01 = 3.114 ± 2.8%, and task did not significantly modulate the effect of condition, F(1, 19) = 1.74, p = .20. Since the temporal expectation hypothesis predicted no interaction and only weak Bayesian support for the lack of interaction was observed (BF01 = 2.010 ± 8.3%) we conducted two additional contrasts to examine the effects of condition separately for the two tasks. This analysis revealed significant and large condition effects for both the duration task, t(19) = 6.242, FDR-corrected p < .0001, Cohen’s d = 1.778, 95% CI [0.958, 2.598], and tilt task, t(19) = 4.610, FDR-corrected p < .0001, Cohen’s d = 1.313, 95% CI = [0.593 2.033], with mean prestimulus SR lower in the fixed as compared with the varied condition for 90% of participants in the duration task and 85% of participants in the tilt task (see Fig. 5c). A supplemental analysis suggested that these effects are independent of saccade sizes. In this analysis, SR was examined separately for small (<.5°) and large (>0.5°) saccades and similar trends were found for both trial groups. Analysis and results are reported in Supplementary Material S1. Another supplemental analysis examined the effects of learning of the fixed interval over the time course of the fixed condition blocks. Consistent with our previous findings (Dankner et al., 2017), we found no evidence for differences between the four block quarters, therefore suggesting that learning of the fixed interval occurred rapidly over the first few trials. Analysis and results are reported in Supplementary Material S2.
Saccade-rate results. a Grand average (N = 20) saccade rate (SR) traces, presented relative to the onset time of the first interval (I1) in 1-s trials. A running window of 50 ms was used to smooth SR traces for display purposes only. The shaded region represents analyzed duration prior to the first stimulus (S1) onset in the main analysis. Significant differences (FDR corrected p < .05) between conditions in the exploratory analysis are designated above in black and gray bars for the duration and tilt task, respectively. b Mean SR averaged across −100 to 0 ms relative to S1 onset. Data for individual participants is illustrated as dots for each condition and task, and error bars depict ±1 standard error from the mean, corrected for within-subject variability (Cousineau & O’Brien, 2014). ***p < .0001; X indicates interaction effect; ns indicates not significant. c Condition effects for single-subject mean pre-S1 SR, reported for the duration (circles) and tilt (triangles) tasks, with overlapping data represented in shaded color, and with the diagonal line representing the identity line (where mean SR in fixed trials equals mean SR in varied 1-s trials).
Next, we conducted an exploratory analysis by dividing the period between −300 ms relative to I1 onset to 0 ms relative to S1 onset to thirteen 100-ms bins and contrasting the mean SR between the fixed and varied conditions, separately for each task and time window. Results revealed pre-cue condition effects in the tilt task: SR in the varied condition was higher than the fixed condition at −300 to −200 ms and at −100 to 0 ms relative to the cue onset. No pre-cue effects were found in the duration task. Early post cue effects were found in both tasks: SR in the varied condition was higher than the fixed condition at 0–100 ms relative to the cue onset in both tasks. Consistent with the hypothesis-driven analysis, we found prestimulus condition effects (varied SR > fixed SR), starting at −400 ms relative to the stimulus onset in the duration task and −300 ms relative to the stimulus onset in the tilt task. These effects lasted for 300–400 ms until the end of the analyzed period at stimulus onset (see Fig. 5a). Full statistical report can be found in the project’s OSF repository (see Data Availability statement).
For the purpose of the study, we focused our analysis on the mean SR in the period prior to the onset of S1 at 1-s trials only. This was obligatory, by design, because only these intervals and trials featured the contrast between the varied and the fixed conditions. Whereas the intervals prior to S1 were either fixed or varied in separate blocks, the intervals prior to S2 were always jittered. Additionally, while the varied condition featured five different pre-S1 intervals, only 1-s interval trials were included in the fixed condition. Therefore, the only way by which it would have been possible to compare fixed and varied, as was our main goal, was to focus on these specific intervals and these specific trials. However, for the completeness of our report, we included supplemental information on the rest of the trials and intervals (see Supplementary Material S3 and S4).
Behavioral consequences of saccadic events
In a previous study that used a similar implicit temporal expectation task, we found no influence of saccades occurring prior to target onset on performance (Amit et al., 2019). Here we examine this question again for the tilt and duration tasks. Trials of the varied condition, collapsed across the different I1 intervals, were divided according to whether a saccade occurred during the −100 to −20 ms period prior to S1 or S2 onset. As expected, there were fewer saccade-present than saccade-absent trials per participant. However, the total number of trials per condition remained high enough to allow this analysis (duration-absent: mean n = 297.75, range: 194−369; duration-present: mean n = 73.45, range: 17–150; tilt-absent: mean n = 297.75, range: 202–365; tilt-present: mean n = 74.1, range: 10–114), and additionally, hierarchical models such as GLMM weighs individual subjects’ contribution according to the contributed number of cases, meaning participants with fewer or noisier data are given less weight in determining the group-level coefficients, thus mitigating possible distortions caused by outlier participants. A GLMM with a binomial family of responses was then constructed separately for each task, with saccadic event (present/absent) as the predictor and accuracy as the predicted response, allowing for a random intercept by subject for each model. Results for both tasks showed no significant difference between trials with and without saccades prior to stimuli onset, with a strong approximated Bayesian support in favor of the null hypothesis (see Fig. 6), tilt task: χ2(1) = 2.987, p = .084, BF01 = 19.37; duration task: χ2(1) = 1.960, p = .162, BF01 = 32.34.
Behavioral consequences of saccadic event. a Percentage of correct responses for the varied condition trials (of all intervals) without (Absent) or with (Present) a saccadic event in the period of −100 to −20 ms prior to S1 or S2 onset, averaged across participants (N = 20). Data of individual participants are represented as dots. Error bars designate ±1 standard error from the mean. Reported statistics are based on a likelihood-ratio (χ2) test against a null model. ns indicates not significant. b Saccadic event effects for single-subject accuracy for the duration (circles) and tilt (triangles) tasks, with the diagonal line representing the identity line (where accuracy in saccade-present trials equals accuracy in saccade-absent trials)
Discussion
In this study, we examined whether the prestimulus oculomotor inhibition effect observed in previous studies reflects temporal expectation, rather than time estimation. Results showed stronger saccadic inhibition (lower SR) in the fixed relative to the varied condition starting around 300–400 ms prior to the onset of the stimulus, in both the implicit temporal expectation (tilt) and explicit time estimation (duration) tasks. This pattern of results supports the hypothesis that oculomotor inhibition is associated with temporal expectation, and not with time estimation.
The oculomotor inhibition effect reflects expectation and not timing
In a series of studies, we have demonstrated prestimulus oculomotor inhibition using various paradigms, modalities, and temporal structures. In these studies, we have manipulated temporal expectation in various ways including rhythms (Dankner et al., 2017), cue associations (Abeles et al., 2020; Amit et al., 2019; Badde et al., 2020; Tal-Perry & Yuval-Greenberg, 2020), and hazard-rate probabilities (Tal-Perry & Yuval-Greenberg, 2020), as well as in different modalities including visual (Amit et al., 2019; Dankner et al., 2017; Tal-Perry & Yuval-Greenberg, 2020), auditory (Abeles et al., 2020), and tactile (Badde et al., 2020). Findings of these studies suggested that prestimulus oculomotor inhibition can be used as a marker of supra-modal temporal expectation. In a recent study, we examined whether the oculomotor inhibition effect originates from temporal orienting for specific targets at specific trials or from a global experience of certainty that is generally elevated throughout the fixed-intervals blocks. Results showed unequivocal support for the trial-by-trial temporal orienting interpretation over the block-wise certainty interpretation (Tal-Perry & Yuval-Greenberg, 2020). The current study provides further evidence in support of the hypothesis that prestimulus oculomotor inhibition starting at around −400–300 ms prior to the onset of a predictable stimulus, reflects attentional processes, namely those involved in the development of temporal expectation.
In the timing literature, tasks where attention is allocated to the interval, such as the duration task used in the current experiment, are called explicit timing tasks; whereas tasks that require attention to be allocated to a predictable target, as in the tilt task, are called implicit timing tasks (Coull & Nobre, 2008; Zelaznik et al., 2002). While the two types of timing processes have some common properties and neuroanatomical regions (Coull et al., 2013; Piras & Coull, 2011), there is evidence to suggest that they are distinct (Coull et al., 2013; Coull & Nobre, 2008; Droit-Volet & Coull, 2016; Zelaznik et al., 2002). The present findings show evidence for the prestimulus oculomotor inhibition effect for both implicit and explicit timing tasks, suggesting that this effect is independent of the type of timing process being used. These findings, together with the previous ones, suggest that prestimulus oculomotor inhibition can be used as a marker for temporal orienting and expectation. Specifically, being a continuous marker, this oculomotor inhibition allows investigation of the period prior to the occurrence of a goal-relevant stimulus, unlike behavioral measures who by definition occur only after the expected event has elapsed and therefore allow only for retrospective examination of expectation processes. In addition, this marker was found to be more sensitive (i.e., have a larger effect size) than both behavioral and common anticipatory electrophysiological markers (Amit et al., 2019). Lastly, since the oculomotor inhibition is involuntary and automatic, it is well suited to study noncooperating populations, such as infants and toddlers.
The time course of the oculomotor inhibition effect
In the present study, we examined the time course of the oculomotor inhibition effect on longer intervals, starting prior to cue onset and until the onset of the stimulus. Using an exploratory analysis, our study shows evidence that the oculomotor inhibition effect is initiated 300–400 ms prior to the onset of the expected event, consistent with previous studies (Abeles et al., 2020; Badde et al., 2020; Dankner et al., 2017; Tal-Perry & Yuval-Greenberg, 2020).
This exploratory analysis revealed evidence for pre-cue effects in the tilt task: there was higher SR for varied relative to fixed trials in the time windows of −300 to −200 and −100 to 0 ms relative to cue onset. This is consistent with a trend found in our previous study using a similar task and interval (Tal-Perry & Yuval-Greenberg, 2020). Finding effects prior to trial onset is not surprising because the conditions were presented in separate blocks, and could therefore trigger sustained cognitive effects. Critically, we found no significant condition effects during the foreperiod following 100 ms post cue onset and until −300 ms prior to the onset of the stimulus (i.e., 700 ms post cue). Furthermore, during a long section of this interval (at ~250–550 ms relative to cue onset) there was not even a trend of this effect and similarly, no trend was found after the target, at ~1,150–1,300 ms relative to I1 onset. This is important because it confirms that the block-wise effects that are evident prior to the cue cannot explain the prestimulus oculomotor inhibition effect, which was the target of this study.
An interesting question is why such pre-cue effects are found in the tilt task (the “implicit timing” task) and not in the duration task (the “explicit timing” task). Arguably, this can be explained by differences between the tasks and condition in the relevance of the cue—in the tilt task, the cue onset is informative regarding S1 onset in the fixed, but not the varied condition, thus participants are expected to attempt forming temporal expectation toward it to a larger extent in the fixed compared to the varied condition, leading to the observed differences in SR. In contrast, in the duration task, participants attend to the cue onset in order to time the duration of the interval, regardless of condition. This leads to no discernable SR differences between conditions.
The oculomotor inhibition effect is independent of a motor response
In addition to providing support for the temporal expectation over the timing hypothesis, another contribution of this study is in examining the oculomotor inhibition effect, for the first time, in a context that does not require immediate motor action. Unlike previous studies, participants in the current study responded only after both stimuli were presented, and long after their prestimulus oculomotor inhibition was measured. Nevertheless, as in the previous studies that used a similar procedure but required an immediate motor response to the target (Amit et al., 2019; Tal-Perry & Yuval-Greenberg, 2020), we found stronger inhibition for the fixed relative to the varied condition. The result suggests that prestimulus oculomotor inhibition occurs regardless of whether a response to the stimulus is required, and is consistent with previous evidence showing no correlation between the oculomotor inhibition effect and RT (Abeles et al., 2020; Amit et al., 2019; Dankner et al., 2017, but see Badde et al., 2020, for a different result). This finding provides additional support for the hypothesis that this marker reflects a general temporal expectation process to an upcoming event, regardless of whether responses were planned or not, making it a useful marker for studying temporal expectation irrespective of its motor consequence.
The functional role of the prestimulus oculomotor inhibition effect
It is still up for debate whether prestimulus oculomotor inhibition has a functional role in perception or not. In the present study, we were able to examine the perceptual effects of inhibiting saccades during an interval both on the perception of the stimulus at the interval offset and on the estimation of the interval duration. Existing evidence regarding the effects of prestimulus saccades on perception is ambiguous. In two previous studies, using visual discrimination (Amit et al., 2019) and auditory discrimination (Abeles et al., 2020) tasks, we found no evidence that performance was modulated by the occurrence or absence of saccades during the interval preceding target onset. In line with these studies, in the present study we observe no effect of saccadic events on the behavioral outcome for both the duration and the tilt tasks. However, in a recent study, using a tactile discrimination task, such a benefit was found, as oculomotor inhibition prior to target onset was associated with enhanced tactile perception (Badde et al., 2020). Thus, the oculomotor inhibition may be advantageous for perception under specific circumstances, but at this stage, it is unknown what are the factors that determine this relation, and consequently whether the oculomotor inhibition effect has a functional role in the perception of an anticipated target.
In addition, these findings show no evidence for perceptual benefit for interval predictability, as manifested by a lack of differences in accuracy rates between the fixed and varied conditions of the tilt task. This lack of accuracy effect is consistent with some previous studies by us and by others, who manipulated temporal expectation using similar designs, and likewise reported no accuracy effects for relatively long intervals as the ones that we have used (Amit et al., 2019; Griffin et al., 2002; Samaha et al., 2015). However, those previous studies consistently report benefits of RT for expected relative to unexpected targets. The present design was unsuitable for examining RTs because its trial procedure was self-paced by the participant, and therefore we remain here with no evidence for perceptual benefits other than the oculomotor inhibition effect.
Although it would have been reassuring to see an accuracy rate effect of interval predictability, we do not find this to be a concern or to affect our interpretation of the findings. Our previous studies have already repeatedly confirmed the link between oculomotor inhibition and perceptual advantages of predictability in similar tasks, and the goal of the present study was to take this finding to the next step and focus on the relation of this effect to timing. Moreover, in a previous study using a similar design (Amit et al., 2019), we showed that oculomotor inhibition is a substantially more sensitive biomarker than behavioral performance or EEG. Thus, in the current study, we feel confident to rely on these findings to further refine the cognitive interpretation of the oculomotor inhibition effect.
Saccades or microsaccades?
Both tasks of this study required fixation and therefore, naturally, the vast majority of saccades performed during the experiment were smaller than one visual degree and could be considered fixational microsaccades (Martinez-Conde et al., 2009). Accumulating evidence in recent years suggests that saccades and microsaccades share the same kinematic properties and are controlled by the same underlying neural structures, and there is, consequently, a wide agreement that they form an oculomotor continuum (Hafed et al., 2009; Ko et al., 2010; Martinez-Conde et al., 2013; Rolfs, 2009). Since our goal was to examine the inhibition of the oculomotor system in general, we did not limit the analyses in the current study to saccades of a certain size. However, an exploratory analysis suggested that the reported temporal expectation effect can be observed for both smaller (<0.5°) and larger (>0.5°) saccades (see Supplementary Material S1). While it remains to be tested, the results described in this study should likely carry to larger (macro-) saccades.
Time compression effects
Our findings in the duration task show that the inhibition of saccades prior to an interval offset does not provide any performance benefits for estimating the interval’s duration. This finding is inconsistent with previous studies that reported time-compression effects when saccades or microsaccades were performed prior to or during an estimated interval (Morrone et al., 2005; Yu et al., 2017). However, the previous studies focused on short intervals of up to a few hundred milliseconds, whereas the present study examined intervals of up to ~3 seconds. Indeed, studies in the timing literature often distinguish between timing tasks that involve subsecond intervals and those that involve supra-second intervals. The former are usually considered to involve ‘automatic processes’ within primary sensorimotor and supplementary motor cortices and the cerebellum, while the latter are reported to be ‘cognitively mediated’ and involve the posterior parietal and prefrontal cortices (Buhusi & Meck, 2005; Hayashi et al., 2014; Lewis & Miall, 2003a, 2003b). Thus, one possibility is that oculomotor-mediated time compression affects only the ‘automatic timing’ processes in subsecond intervals. Alternatively, it is possible that time compression effects did occur in the current study (i.e., in supra-second interval timing), but their effect has been negligible relative to the long overall interval duration, and therefore had little or no effect on performance. Further studies are required in order to make a final verdict on the issue.
Potential underlying mechanisms of the oculomotor inhibition effect
The link between temporal expectation and oculomotor inhibition is likely mediated by an interaction of cortical and subcortical structures, consistent with the possibility of both intentional and unintentional processes. For example, in our previous studies, we suggested a possible involvement of the dorsolateral prefrontal cortex (DLPFC) in realizing this effect (Abeles et al., 2020), as several studies showed it is involved in the development of temporal expectation (Critchley et al., 2001; Tsujimoto & Sawaguchi, 2005; Vallesi et al., 2007), and it projects to various cortical and subcortical oculomotor regions (Johnston & Everling, 2006; Pierrot-Deseilligny et al., 2005; Selemon & Goldman-Rakic, 1988). Another possibility is that the oculomotor inhibition effect is a part of a global motor inhibition process for upcoming targets. During response preparation, the motor system is known to be transiently inhibited (e.g., Duque & Ivry, 2009), and previous transcranial magnetic stimulation (TMS) studies have shown that this inhibition also affects contralateral and nonhomologous motor units (i.e., task-irrelevant muscles; Badry et al., 2009; Greenhouse et al., 2015). The broad inhibition described in these studies may spread not only to motor but also to oculomotor units. As suggested by Greenhouse et al. (2015), such a global preparatory inhibition may serve as a mechanism for improving the signal-to-noise ratio, by reducing the background noise of task-irrelevant muscles in order to facilitate the propagation of the desired response. However, it should be noted that this global inhibition was examined only under conditions when an immediate motor response was required, and in the current study, we show that oculomotor inhibition does not require an immediate motor action. It remains to be explored whether this hypothesis could also explain preparatory inhibition observed in lack of an immediate response, as found in the current experiment.
Conclusions
This study shows that the pretarget oculomotor inhibition effect is associated with temporal expectation, rather than with timing of the interval up to the predictable event. Additionally, the results suggest that this effect is unrelated to motor readiness. These findings elucidate the underlying processes behind this effect and support the notion that it can be used as a biomarker in studies of temporal expectation. The oculomotor inhibition effect is a useful behavioral marker because measuring it requires no cooperation from participants, opening up the possibility of studying these cognitive functions in less-cooperative populations.
Data availability
The data set used for power analysis, the data sets generated by this study, and an R-markdown file that reproduces all the reported statistical analyses, tables, and bar graphs within the paper, are uploaded to the Open Science Foundation repository and are available (https://osf.io/ar683/).
References
Abeles, D., Amit, R., Tal-Perry, N., Carrasco, M., & Yuval-Greenberg, S. (2020). Oculomotor inhibition precedes temporally expected auditory targets. Nature Communications, 11(1), 3524. https://doi.org/10.1038/s41467-020-17158-9
Amit, R., Abeles, D., Carrasco, M., & Yuval-Greenberg, S. (2019). Oculomotor inhibition reflects temporal expectations. NeuroImage, 184(March 2018), 279–292. https://doi.org/10.1016/j.neuroimage.2018.09.026
Badde, S., Myers, C. F., Yuval-Greenberg, S., & Carrasco, M. (2020). Oculomotor freezing reflects tactile temporal expectation and aids tactile perception. Nature Communications, 11(1), 1–9. https://doi.org/10.1038/s41467-020-17160-1
Badry, R., Mima, T., Aso, T., Nakatsuka, M., Abe, M., Fathi, D., Foly, N., Nagiub, H., Nagamine, T., & Fukuyama, H. (2009). Suppression of human cortico-motoneuronal excitability during the Stop-signal task. Clinical Neurophysiology, 120(9), 1717–1723. https://doi.org/10.1016/j.clinph.2009.06.027
Bates, D., Kliegl, R., Vasishth, S., & Baayen, H. (2015a). Parsimonious mixed models. ArXiv. http://arxiv.org/abs/1506.04967
Bates, D., Mächler, M., Bolker, B., & Walker, S. (2015b). Fitting linear mixed-effects models using {lme4} Journal of Statistical Software, 67(1), 1–48. https://doi.org/10.18637/jss.v067.i01
Benjamini, Y., & Hochberg, Y. (1995). Controlling the false discovery rate : A practical and powerful approach to multiple testing. Journal of the Royal Statistical Societ, 57(1), 289–300. https://www.jstor.org/stable/2346101
Brainard, D. H. (1997). The Psychophysics Toolbox. Spatial Vision, 10, 433–436. https://doi.org/10.1163/156856897x00357
Buhusi, C. V., & Meck, W. H. (2005). What makes us tick? Functional and neural mechanisms of interval timing. Nature Reviews Neuroscience, 6(10), 755–765. https://doi.org/10.1038/nrn1764
Caldwell, A., & Lakens, D. (2019). Power analysis with Superpower. https://arcaldwell49.github.io/SuperpowerBook
Clark, A. (2013). Whatever next? Predictive brains, situated agents, and the future of cognitive science. Behavioral and Brain Sciences, 36, 181–204. https://doi.org/10.1017/S0140525X12000477
Coull, J. T., Davranche, K., Nazarian, B., & Vidal, F. (2013). Functional anatomy of timing differs for production versus prediction of time intervals. Neuropsychologia, 51(2), 309–319. https://doi.org/10.1016/j.neuropsychologia.2012.08.017
Coull, J. T., & Nobre, A. C. (2008). Dissociating explicit timing from temporal expectation with fMRI. Current Opinion in Neurobiology, 18(2), 137–144. https://doi.org/10.1016/j.conb.2008.07.011
Cousineau, D., & O’Brien, F. (2014). Error bars in within-subject designs: A comment on Baguley (2012). Behavior Research Methods, 46(4), 1149–1151. https://doi.org/10.3758/s13428-013-0441-z
Critchley, H. D., Mathias, C. J., & Dolan, R. J. (2001). Neural activity in the human brain relating to uncertainty and arousal during anticipation. Neuron, 29(2), 537–545. https://doi.org/10.1016/S0896-6273(01)00225-2
Dankner, Y., Shalev, L., Carrasco, M., & Yuval-Greenberg, S. (2017). Prestimulus inhibition of saccades in adults with and without attention-deficit/hyperactivity disorder as an index of temporal expectations. Psychological Science, 28(7), 835–850. https://doi.org/10.1177/0956797617694863
Denison, R. N., Yuval-Greenberg, S., & Carrasco, M. (2019). Directing voluntary temporal attention increases fixational stability. Journal of Neuroscience, 39(2), 353–363. https://doi.org/10.1523/JNEUROSCI.1926-18.2018
Droit-Volet, S., & Coull, J. T. (2016). Distinct developmental trajectories for explicit and implicit timing. Journal of Experimental Child Psychology, 150, 141–154. https://doi.org/10.1016/j.jecp.2016.05.010
Duque, J., & Ivry, R. B. (2009). Role of corticospinal suppression during motor preparation. Cerebral Cortex, 19(9), 2013–2024. https://doi.org/10.1093/cercor/bhn230
Engbert, R. (2006). Microsaccades: A microcosm for research on oculomotor control, attention, and visual perception. Progress in Brain Research, 154, 177–192.
Engbert, R., & Kliegl, R. (2003). Microsaccades uncover the orientation of covert attention. Vision Research, 43(9), 1035–1045. https://doi.org/10.1016/s0042-6989(03)00084-1
Greenhouse, I., Sias, A., Labruna, L., & Ivry, R. B. (2015). Nonspecific inhibition of the motor system during response preparation. Journal of Neuroscience, 35(30), 10675–10684. https://doi.org/10.1523/JNEUROSCI.1436-15.2015
Griffin, I. C., Miniussi, C., & Nobre, A. C. (2002). Multiple mechanisms of selective attention: Differential modulation of stimulus processing by attention to space or time. Neuropsychologia, 40(13), 2325–2340. https://doi.org/10.1016/S0028-3932(02)00087-8
Hafed, Z. M., Goffart, L., & Krauzlis, R. J. (2009). A neural mechanism for primate superior colliculus. Science, 940(February), 940–943. https://doi.org/10.1126/science.1166112
Hayashi, M. J., Kantele, M., Walsh, V., Carlson, S., & Kanai, R. (2014). Dissociable Neuroanatomical Correlates of Subsecond and Suprasecond Time Perception. Journal of Cognitive Neuroscience, 26(8), 1685–1693. https://doi.org/10.1162/jocn_a_00580
Hershman, R., Henik, A., & Cohen, N. (2018). A novel blink detection method based on pupillometry noise. Behavior Research Methods, 50(1), 107–114. https://doi.org/10.3758/s13428-017-1008-1
Jaeger, T. F. (2008). Categorical data analysis: Away fron ANOVAs (transformation or not) and towards Logit Mixed Models. Journal of Memory and Language, 59(4), 434–446. https://doi.org/10.1016/j.jml.2007.11.007.Categorical
Johnston, K., & Everling, S. (2006). Monkey dorsolateral prefrontal cortex sends task-selective signals directly to the superior colliculus. Journal of Neuroscience, 26(48), 12471–12478. https://doi.org/10.1523/JNEUROSCI.4101-06.2006
Ko, H., Poletti, M., & Rucci, M. (2010). Microsaccades precisely relocate gaze in a high visual acuity task. Nature Neuroscience, 13(12), 1549–1553. https://doi.org/10.1038/nn.2663
Lapid, E., Ulrich, R., & Rammsayer, T. (2008). On estimating the difference limen in duration discrimination tasks: A comparison of the 2AFC and the reminder task. Perception & Psychophysics, 70, 291–305. https://doi.org/10.3758/PP.70.2.291
Lewis, P. A., & Miall, R. C. (2003a). Brain activation patterns during measurement of sub- and supra-second intervals. Neuropsychologia, 41(12), 1583–1592. https://doi.org/10.1016/S0028-3932(03)00118-0
Lewis, P. A., & Miall, R. C. (2003b). Distinct systems for automatic and cognitively controlled time measurement: Evidence from neuroimaging. In Current Opinion in Neurobiology (Vol. 13, Issue 2, pp. 250–255). Elsevier Ltd. https://doi.org/10.1016/S0959-4388(03)00036-9
Lüdecke, D. (2020). sjstats: Statistical Functions for Regression Models (Version 0.17.9). https://doi.org/10.5281/zenodo.1284472
Lüdecke, D., Makowski, D., & Waggoner, P. (2020). performance: Assessment of regression models performance. https://cran.r-project.org/package=performance
Makowski, D., Ben-Shachar, M. S., & Lüdecke, D. (2019). bayestestR: Describing Effects and their Uncertainty, Existence and Significance within the Bayesian Framework. Journal of Open Source Software, 4, 1541. 10.21105/joss.01541
Martinez-Conde, S., Macknik, S. L., Troncoso, X. G., & Hubel, D. H. (2009). Microsaccades: A neurophysiological analysis. Trends in Neurosciences, 32(9), 463–475. https://doi.org/10.1016/j.tins.2009.05.006
Martinez-Conde, S., Otero-Millan, J., & Macknik, S. L. (2013). The impact of microsaccades on vision: towards a unified theory of saccadic function. Nature Reviews. Neuroscience, 14(2), 83–96. https://doi.org/10.1038/nrn3405
Morey, R. D., & Rouder, J. N. (2018). BayesFactor: Computation of Bayes factors for common designs. https://cran.r-project.org/package=BayesFactor
Morrone, M. C., Ross, J., & Burr, D. (2005). Saccadic eye movements cause compression of time as well as space. Nature Neuroscience, 8(7), 950–954. https://doi.org/10.1038/nn1488
Nobre, A. C., & Rohenkohl, G. (2014). Time for the fourth dimension in attention. In A. C. Nobre & S. Kastner (Eds.), The Oxford handbook of attention (pp. 56–75). Oxford University Press.
Nobre, A. C., & van Ede, F. (2017). Anticipated moments: temporal structure in attention. Nature Reviews Neuroscience, 19(1), 34–48. https://doi.org/10.1038/nrn.2017.141
Pierrot-Deseilligny, C., Müri, R. M., Nyffeler, T., & Milea, D. (2005). The role of the human dorsolateral prefrontal cortex in ocular motor behavior. Annals of the New York Academy of Sciences, 1039(May), 239–251. https://doi.org/10.1196/annals.1325.023
Piras, F., & Coull, J. T. (2011). Implicit, predictive timing draws upon the same scalar representation of time as explicit timing. PLOS ONE, 6(3). https://doi.org/10.1371/journal.pone.0018203
R Core Team. (2018). R: A language and environment for statistical computing. https://www.r-project.org/
Rolfs, M. (2009). Microsaccades: Small steps on a long way. Vision Research, 49(20), 2415–2441.
Rouder, J. N., Morey, R. D., Speckman, P. L., & Province, J. M. (2012). Default Bayes factors for ANOVA designs. Journal of Mathematical Psychology, 56(5), 356–374. https://doi.org/10.1016/j.jmp.2012.08.001
Samaha, J., Bauer, P., Cimaroli, S., & Postle, B. R. (2015). Top-down control of the phase of alpha-band oscillations as a mechanism for temporal prediction. Proceedings of the National Academy of Sciences of the United States of America, 112(27), 8439–8444. https://doi.org/10.1073/pnas.1503686112
Schütz, A. C., & Morrone, M. C. (2010). Compression of time during smooth pursuit eye movements. Vision Research, 50(24), 2702–2713. https://doi.org/10.1016/j.visres.2010.07.022
Selemon, L. D., & Goldman-Rakic, P. S. (1988). Common cortical and subcortical targets of the dorsolateral prefrontal and posterior parietal cortices in the rhesus monkey: evidence for a distributed neural network subserving spatially guided behavior. Journal of Neuroscience, 8(11), 4049–4068. https://doi.org/10.1523/jneurosci.08-11-04049.1988
Singmann, H., Bolker, B., Westfall, J., Aust, F., & Ben-Shachar, M. S. (2020). afex: Analysis of factorial experiments. https://cran.r-project.org/package=afex
Tal-Perry, N., & Yuval-Greenberg, S. (2020). Pretarget oculomotor inhibition reflects temporal orienting rather than certainty. Scientific Reports, 10(1), 1–9. https://doi.org/10.1038/s41598-020-78189-2
Tsujimoto, S., & Sawaguchi, T. (2005). Neuronal activity representing temporal prediction of reward in the primate prefrontal cortex. Journal of Neurophysiology, 93(6), 3687–3692. https://doi.org/10.1152/jn.01149.2004
Vallesi, A., Shallice, T., & Walsh, V. (2007). Role of the prefrontal cortex in the foreperiod effect: TMS evidence for dual mechanisms in temporal preparation. Cerebral Cortex, 17(2), 466–474. https://doi.org/10.1093/cercor/bhj163
Wagenmakers, E. J. (2007). A practical solution to the pervasive problems of p values. Psychonomic Bulletin and Review, 14(5), 779–804. https://doi.org/10.3758/BF03194105
Yu, G., Yang, M., Yu, P., & Dorris, M. C. (2017). Time compression of visual perception around microsaccades. Journal of Neurophysiology, 118(1), 416–424. https://doi.org/10.1152/jn.00029.2017
Zelaznik, H. N., Spencer, R. M. C., & Ivry, R. B. (2002). Dissociation of explicit and implicit timing in repetitive tapping and drawing movements. Journal of Experimental Psychology: Human Perception and Performance, 28(3), 575–588. https://doi.org/10.1037/0096-1523.28.3.575
Acknowledgements
We thank Noam Shimoni and Dana Slabbekoornd for their assistance in running the experiment, Omer Solomon for his assistance in the eye-tracking analysis, and Marisa Ourieff for proofreading.
Research software used
MATLAB R2015a (The MathWorks, Natick, MA, USA) was used to code and to control the experiment, with stimuli displayed using Psychtoolbox v3 (Brainard, 1997). Analyses were conducted in R v3.5.2 using R-studio v1.1.463 (R Core Team, 2018). GLMM were analyzed using the lme4 package (Bates et al., 2015b), with model diagnostics done using the performance package (Lüdecke et al., 2020). ANOVAs were performed and bar graphs were produced using the afex package (Singmann et al., 2020). Effect sizes were calculated using the sjstats package (Lüdecke, 2020). BFs were calculated using the BayesFactor package (Morey & Rouder, 2018) for the ANOVA results and using the bayestestR (Makowski et al., 2019) for the GLMM models.
Funding
This study was funded by the Israel Science Foundation grant 1960/19 to S.Y-G. We have no conflict of interest to disclose.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Significance statement
Previous studies have found that eye movements are inhibited prior to expected events. In this study, we show that this behavior is due to temporal expectation to an upcoming event, rather than time-estimating processes of the interval preceding it. We further show evidence that this behavior is independent of motor readiness to the target. These findings indicate that the oculomotor inhibition effect reflects temporal expectation and can be used as a marker to study and assess it.
Supplementary Information
ESM 1
(PDF 897 kb)
Rights and permissions
About this article
Cite this article
Tal-Perry, N., Yuval-Greenberg, S. Prestimulus inhibition of eye movements reflects temporal expectation rather than time estimation. Atten Percept Psychophys 83, 2473–2485 (2021). https://doi.org/10.3758/s13414-021-02319-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13414-021-02319-9