Abstract
Information on the feeding habits of species is essential to develop appropriate conservation actions. This study aimed to assess spatial and temporal variation in the diet of the Eurasian otter, Lutra lutra in the Anzali Wetland, through fecal and stable isotope analysis. Seven main prey items were observed in the analysis of 300 fresh spraints. The highest feeding index was observed for fish, followed by snakes. Among the fish species consumed, the index of preponderance of Prussian carp (Carassius gibelio) was the highest followed by pike (Esox lucius) and white bream (Blicca bjoerkna). Results of Shannon diversity index suggest spatial variation of species diversity within and between feeding items (p < 0.05); snakes, frogs, and oriental river prawn (Macrobrachium nipponense) showed a seasonal variation. The otter’s trophic level (TL) (3.79) was higher than the TLs of other Anzali Wetland predators, such as pike. Bayesian mixing model showed source proportion contributions of fish 49.5%, reptiles 16.7%, insects 14.8%, crustacean 10.5%, amphibians 4.3%, birds 4.1%, and mollusks 0.1%. When considering only fish species in the Bayesian mixing model, Prussian carp was the main fish prey in the otters’ diet accounting for 47%. Based on the results of this study, the Eurasian otter plays an important role in the ecology of the Anzali Wetland ecosystem even though it preys on exotic species, such as the Prussian carp and the oriental river prawn.



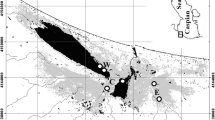
source and discrimination uncertainty (mean ± SD). B The simulated mixing polygon. The position of L. lutra (white crosses) and the average signature of sources (black dots). Probability counter lines are at 5% level (outermost counter line) and at every 10% level


Similar content being viewed by others
References
Abbasi K (2019) Investigation of distribution and ecology of fishes in Anzali Wetland and its rivers (in Persian). Agricultural Research and Education Organization, Inland water aquaculture research center
Abdolmalaki S, Ganinejad D (2015) Bony fishes of the Caspian Sea (Biology, Distribution, Fisheries, Restocking, Strength and Weakness). Iranian Fisheries Science Research Institute Tehran (In Persian)
Beltran RS, Peterson SH, McHuron EA, Reichmuth C, Hückstädt L, Costa DP (2016) Seals and sea lions are what they eat, plus what? Determination of trophic discrimination factors for seven pinniped species. Rapid Commun Mass Spectrom 30:1115–1122
Bonesi L, Chanin P, Macdonald DW (2004) Competition between Eurasian otter Lutra lutra and American mink Mustelavison probed by niche shift. Oikos 106:19–26
Brzeziński M, Jęldrzejewski W, Jędrzejewska B (1993) Diet of otters (Lutra lutra) inhabiting small rivers in the Bialowieza National Park, eastern Poland. J Zool 230:495–501
Cabana G, Rasmussen JB (1996) Comparison of aquatic food chains using nitrogen isotopes. ProcNatlAcadSci 93:10844–10847
Carrasco TS et al (2019) Isotopic niche of the Neotropical otter, Lontra longicaudis (Carnivora, Mustelidae), in different coastal aquatic systems in southern Brazil. Hydrobiologia 835:83–100
Carss D (1995) Foraging behaviour and feeding ecology of the otter Lutra lutra: a selective review. Hystrix Italian J Mamm. https://doi.org/10.4404/hystrix-7.1-2-4069
Carss D, Parkinson S (1996) Errors associated with otter Lutra lutra faecal analysis. I Assessing general diet from spraints. J Zool 238:301–317
Carss D, Kruuk H, Conroy J (1990) Predation on adult Atlantic salmon, Salmo salar L., by otters, Lutra lutra (L.), within the River Dee system Aberdeenshire, Scotland. J Fish Biol 37:935–944
Caut S, Angulo E, Courchamp F (2009) Variation in discrimination factors (Δ15N and Δ13C): the effect of diet isotopic values and applications for diet reconstruction. J Appl Ecol 46:443–453
Clavero M, Prenda J, Delibes M (2005) Amphibian and reptile consumption by otters (Lutra lutra) in a coastal area in southern Iberian Peninsula. Herpetol J 15:125–131
Day CC, Westover MD, McMillan BR (2015) Seasonal diet of the northern river otter (Lontra canadensis): what drives prey selection? Can J Zool 93:197–205
De Grave S, Ghane A (2006) The establishment of the oriental river prawn, Macrobrachium nipponense (de Haan, 1849) in Anzali Lagoon. Iran Aquat Invasions 1:204–208
Delibes M, Adrián I (1987) Effects of crayfish introduction on otter Lutra lutra food in the Doñana National Park. SW Spain BiolConserv 42:153–159
Emmerson W, Philip S (2004) Diets of cape clawless otters at two South African coastal localities. AfrZool 39:201–210
Erlinge S (1969) Food habits of the otter Lutra lutra L. and the mink Mustela vison Schreber in a trout water in southern Sweden. Oikos 20:1–7
Fanelli E, Papiol V, Cartes JE, Rumolo P, Brunet C, Sprovieri M (2011) Food web structure of the epibenthic and infaunal invertebrates on the Catalan slope (NW Mediterranean): evidence from δ13C and δ15N analysis. Deep Sea Res Part I Oceanogr Res Papers 58:98–109
Flint V, Boehme R, Kostin Y, Kuznetsov A, Bourso-Leland N (1984) A field guide to birds of Russia and adjacent territories
Fry B, Wainright SC (1991) Diatom sources of 13 C-rich carbon in marine food webs. Marin Ecol Prog Series 76:149–157
Guest MA, Connolly RM, Loneragan NR (2004) Carbon movement and assimilation by invertebrates in estuarine habitats at a scale of metres. Mar EcolProgSer 278:27–34
Hobson KA, Schell DM, Renouf D, Noseworthy E (1996) Stable carbon and nitrogen isotopic fractionation between diet and tissues of captive seals: implications for dietary reconstructions involving marine mammals. Can J Fish AquatSci 53:528–533
Isely JJ, Grabowski TB (2007) Age and growth Analysis and interpretation of freshwater fisheries data American Fisheries Society. Bethesda, Maryland, p 187228
Jacob U, Mintenbeck K, Brey T, Knust R, Beyer K (2005) Stable isotope food web studies: a case for standardized sample treatment. Mar EcolProgSer 287:251–253
Jacobsen L, Hansen HM (1996) Analysis of otter (Lutra lutra) spraints: Part 1: Comparison of methods to estimate prey proportions; Part 2: Estimation of the size of prey fish. J Zool 238:167–180
Jenkins D, Walker J, McCowan D (1979) Analyses of otter (Lutra lutra) faeces from Deeside. NE Scotland J Zool 187:235–244
Jica D, Moja M (2005) The Study on Integrated Management for Ecosystem Conservation of the Anzali Wetland in the Islamic Republic of Iran. Draft final report Nippon Koei Co., Ltd
Jordaan RK, McIntyre T, Somers MJ, Bester MN (2015) An assessment of spatial and temporal variation in the diet of Cape clawless otters (Aonyx capensis) in marine environments. Afr J Wildl Res 45:342–353
Jordaan RK, Somers MJ, Hall G, McIntyre T (2019) Plasticity and specialisation in the isotopic niche of African clawless otters foraging in marine and freshwater habitats. MammBiol 98:61–72
Jordaan RK, Somers MJ, Hall G, McIntyre T (2020) The diet of spotted-necked otters foraging in trout-stocked waters in Mpumalanga South Africa. AfrZool 55(2):141–148
Karami M, Mirzaei R, Hamzehpour M (2006) Status of Eurasian Otter (Lutra lutra) in Iran. IUCN Otter Spec Group Bull 23:27–33
Kemenes I, Nechay G (1990) The food of otters Lutra lutra in different habitats in Hungary. ActaTheriologica 35:17–24
Kennedy S (2003) Lutra lutra. Animal Diversity Web. http://animaldiversity.org/accounts/Lutra_lutra
Kiabi B (1993) Otters in Iran [in Farsi]. Abzeean Monthly Magazine 4(6):10–15
Krawczyk AJ, Skierczyński M, Tryjanowski P (2011) Diet of the Eurasian otter Lutra lutra on small watercourses in Western Poland. Walter de Gruyter
Kruuk H, Conroy J (1987) Surveying otter Lutra lutra populations: a discussion of problems with spraints. Biol Cons 41:179–183
Kruuk H, Goudswaard P (1990) Effects of changes in fish populations in Lake Victoria on the food of otters (Lutra maculicollis Schinz and Aonyx capensis Lichtenstein). Afr J Ecol 28:322–329
Kruuk H, Moorhouse A (1990) Seasonal and spatial differences in food selection by otters (Lutra lutra) in Shetland. J Zool 221:621–637
Kurle CM (2002) Stable-isotope ratios of blood components from captive northern fur seals (Callorhinus ursinus) and their diet: applications for studying the foraging ecology of wild otariids Canadian. J Zool 80:902–909
Lanszki J, Molnar T (2003) Diet of otters living in three different habitats in Hungary. Folia Zoologica-Praha 52:378–388
Le Loc’h F, Hily C, Grall J (2008) Benthic community and food web structure on the continental shelf of the Bay of Biscay (North Eastern Atlantic) revealed by stable isotopes analysis. J Marin Syst 72:17–34
Lesage V, Hammill MO, Kovacs KM (2002) Diet-tissue fractionation of stable carbon and nitrogen isotopes in phocid seals. Marin MammSci 18:182–193
Mason CF, Macdonald S (2009) Otters: ecology and conservation. Cambridge University Press, Cambridge
Matthews L (1969) The life of mammals, 2 Volumes. Weidenfeld and Nicolson, London, p 1971
McDonald RA, O’Hara K, Morrish DJ (2007) Decline of invasive alien mink (Mustela vison) is concurrent with recovery of native otters (Lutra lutra). Divers Distrib 13:92–98
McMahon J, McCafferty DJ (2006) Distribution and diet of otters (Lutra lutra) in marine areas of Loch Lomond and The Trossachs National Park, Scotland. UK Lutra 49:29
Minagawa M, Wada E (1984) Stepwise enrichment of 15N along food chains: Further evidence and the relation between δ15N and animal age. Geochimica et Cosmochimica Acta 48:1135–1140
Mirzaei R, Karami M, Kar AD, Abdoli A (2010) Habitat quality assessment for the Eurasian otter (Lutra lutra) on the river Jajrood Iran. HystrixItal J Mammal. https://doi.org/10.4404/hystrix-20.2-4447
Mirzaei R, Danehkar A, Abdoli A (2014) The diet of Eurasian otters in the Jajrood River system. Iran Mamm Study 39:33–41
Mirzajani A (1999) Study on common otter (Lutra lutra) status. Environ 25:70–74
Mirzajani A (2009) Limnological survey of Anzali wetland based on ten years data 1990–2003 by use of GIS system. Agricultural Research and Education Organization, Inland water aquaculture research center, Iran
Mirzajani A (2019) The Ecological Effects of Implementing bio Green Management Plan in Anzali wetland (Sorkhankol region). Inland water aquaculture research center
Mirzajani AR, Khodaparast H, Babaei H, Abedini A, Dadai Ghandi A (2010) Eutrophication trend of Anzali wetland based on 1992–2002 data. J Environ Stud 35:65–74
Mirzajani A, Hamidian A, Karami M (2016) Metal bioaccumulation in representative organisms from different trophic levels of the Caspian Sea. Iran J Fish Sci 15:1027–1043
Mirzajani A, Hamidian AH, Pourang N (2020) Use of C and N stable isotopes for determination of the metal flux in the Caspian fishes. Isot Environ Health Stud. https://doi.org/10.1080/10256016.2020.1760265
Mirzajani A, Ghane A, Bagheri S, Abbasi K, Sayadrahim M, Salahi M, Lavajoo F (2020a) Diet survey and trophic position of Macrobrachium nipponense in the food web of Anzali Wetland. Wetlands. https://doi.org/10.1007/s13157-020-01278-5
Moore JW, Semmens BX (2008) Incorporating uncertainty and prior information into stable isotope mixing models. EcolLett 11:470–480
Naderi M, Saatsaz M (2020) Impact of climate change on the hydrology and water salinity in the Anzali Wetland, northern Iran. Hydrol Sci J 65:552–570. https://doi.org/10.1080/02626667.2019.1704761
Naderi S, Mirzajani A, Hadipour E (2017) Distribution of and threats to the Eurasian Otter (Lutra lutra) in the Anzali Wetland. Iran IUCN Otter Spec Group Bull 34:84–94
Naderi S, Mirzajani A, RajabiMaham H, Hadipour E (2017) The mammals of Anzali Wetland in the Southern Caspian Sea Caspian. J Environ Sci 15:223–235
Natarajan A, Jhingran A (1961) Index of preponderance -a method of grading the food elements in the stomach analysis of fishes. Indian J Fish 8:54–59
Newsome SD et al (2009) Using stable isotopes to investigate individual diet specialization in California sea otters (Enhydra lutris nereis). Ecology 90:961–974
Newsome SD, Bentall GB, Tinker MT, Oftedal OT, Ralls K, Estes JA, Fogel ML (2010) Variation in δ13C and δ15N diet–vibrissae trophic discrimination factors in a wild population of California sea otters. Ecol Appl 20:1744–1752
Nikolsky GV, Birkett L (1963) The ecology of fishes, vol 352. Academic press, London
Ozolins J, Rantins M, Kirk A, Miljutin A, Randveer T (1992) The distribution and habitat conditions of the otter (Lutra lutra) in Latvia. In: Proceedings of the First Baltic Theriological Conference, Tartu, pp 186–195
Pauly D, Trites A, Capuli E, Christensen V (1998) Diet composition and trophic levels of marine mammals. ICES J Marin Sci 55:467–481
Peterson BJ, Fry B (1987) Stable isotopes in ecosystem studies. Annu Rev EcolSyst 18:293–320
Pierce GJ, Boyle PR (1991) A review of methods for diet analysis in piscivorous marine mammals. Oceanogr Mar BiolAnnu Rev 29:409–486
Pinnegar J, Polunin N (1999) Differential fractionation of δ13C and δ15N among fish tissues: implications for the study of trophic interactions. Funct Ecol 13:225–231
Post DM (2002) Using stable isotopes to estimate trophic position: models, methods, and assumptions. Ecology 83:703–718
R Core Team (2020) R: a Language and Environment for Statistical Computing. URL:. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/.
Ramsar convention Bureau (2014) Information sheet on Ramsar Wetland. Gland, Switzerland
Rasooli P, Kiabi BH, Abdoli A (2007) On the status and biology of the European Otter, Lutra lutra (Carnivora: Mustelidae), in Iran. Zool Middle East 41:25–29
Reid N, Thompson D, Hayden B, Marnell F, Montgomery WI (2013) Review and quantitative meta-analysis of diet suggests the Eurasian otter (Lutra lutra) is likely to be a poor bioindicato. Ecol Indic 26:5–13
Remonti L, Prigioni C, Balestrieri A, Sgrosso S, Priore G (2008) Trophic flexibility of the otter (Lutra lutra) in southern Italy. Mamm Biol Zeitschrift für Säugetierkunde 73:293–302
Remonti L, Balestrieri A, Prigioni C (2009) Altitudinal gradient of Eurasian otter (Lutra lutra) food niche in Mediterranean habitats Canadian. J Zool 87:285–291
Roos A, Loy A, de Silva P, Hajkova P, Zemanová B (2015) Lutralutra. The IUCN Red List of Threatened Species. https://doi.org/10.2305/IUCN.UK.2015-2.RLTS.T12419A21935287.en
SadeghinejadMasuoleh E (2017) Studies of communities fishes in Anzali wetland. Agricultural Research and Education Organization, Inland water aquaculture research center
Sales-Luís T, Pedroso N, Santos-Reis M (2007) Prey availability and diet of the Eurasian otter (Lutra lutra) on a large reservoir and associated tributaries Canadian. J Zool 85:1125–1135
Schmidt K, Atkinson A, Stübing D, McClelland JW, Montoya JP, Voss M (2003) Trophic relationships among Southern Ocean copepods and krill: some uses and limitations of a stable isotope approach. LimnolOceanogr 48:277–289
Serrano O, Serrano L, Mateo MA, Colombini I, Chelazzi L, Gagnarli E, Fallaci M (2008) Acid washing effect on elemental and isotopic composition of whole beach arthropods: implications for food web studies using stable isotopes. ActaOecologica 34:89–96
Sherwood GD, Rose GA (2005) Stable isotope analysis of some representative fish and invertebrates of the Newfoundland and Labrador continental shelf food web Estuarine. Coast Shelf Sci 63:537–549
Skaren U (1993) Food of Lutralutra in central Finland IUCN otter special group. Bulletin 8:31–34
Somers M (2000) Seasonal variation in the diet of Cape clawless otters (Aonyx capensis) in a marine habitat. Afr Zool 35:261–268
Stock BC, Semmens BX (2016) Unifying error structures in commonly used biotracer mixing models. Ecology 97:2562–2569
Stock BC, Jackson AL, Ward EJ, Parnell AC, Phillips DL, Semmens BX (2018) Analyzing mixing systems using a new generation of Bayesian tracer mixing models. Peer J 6:e5096
Sulkava R (1996) Diet of otters Lutra lutra in central Finland. ActaTheriologica 41:395–408
Tyrrell LP, Newsome SD, Fogel ML, Viens M, Bowden R, Murray MJ (2013) Vibrissae growth rates and trophic discrimination factors in captive southern sea otters (Enhydra lutris nereis). J Mammal 94:331–338
Valipour A, Haghighy D (2000) Study on changes in fishing in Anzali Lagoon (1992-1996). Iran J Fish Sci 8:73–88
Vander Zanden MJ, Rasmussen JB (1999) Primary consumer δ 13 C and δ 15 N and the trophic position of aquatic consumers. Ecology 46:1395–1404
Vander Zanden MJ, Rasmussen JB (2001) Variation in δ15N and δ13C trophic fractionation: implications for aquatic food web studies. LimnolOceanogr 46(8):2061–2066
Vinicombe K (1982) Breeding and population fluctuations on the Little Grebe. British Birds 75:204–218
Zhao L, Schell DM, Castellini MA (2006) Dietary macronutrients influence 13C and 15N signatures of pinnipeds: captive feeding studies with harbor seals (Phoca vitulina). Comp Biochem Physiol Part A Mol Integr Physiol 143:469–478
Ziaie H, Gutleb B (1997) New comments on otters in Iran. IUCN Otter Spec Group Bull 14:91–92
Acknowledgements
The authors would like to thank the Department of Environment (DOE) of Guilan Province and Japan International Cooperation Agency (JICA) for kindly sponsoring and financially supporting both projects; the first assessment of mammals’ fauna in the Anzali Wetland (under Grant number P15/136264); and diet survey and trophic position of Macrobrachium nipponense in the food web of Anzali Wetland (registered in AREEO under the code number 124-73-12-055-95034-950964). We are much grateful to all responsible persons of JICA, DOE and Inland Waters Aquaculture Research Center for logistics preparation of conditions for this research, and to Dr. Keivan Abbasi, Mr. Mostafa Sayadrhaim and Mr. Yaeghoob Rakhsh Bahar, for their cooperation in most of the field and laboratory surveys.
Funding
This work was supported by "Japan International Cooperation Agency (JICA)" (Grant number P15/136264, and the Department of Environment (DOE) of Guilan Province (registered in AREEO under the code number 124-73-12-055-95034-950964). Saeid Naderi has received the first research support, and Alireza Mirzajani has received the second one. The authors have no conflicts of interest to declare that are relevant to the content of this article. All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript. The authors have no financial or proprietary interests in any material discussed in this article. Authors are responsible for correctness of the statements provided in the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Handling Editor: Télesphore Sime-Ngando
Rights and permissions
About this article
Cite this article
Mirzajani, A., Naderi, S., Ganeh, A. et al. Trophic flexibility of Eurasian otter (Lutra lutra) in Anzali Wetland, Iran, assessed by fecal and stable isotope analysis. Aquat Ecol 55, 401–415 (2021). https://doi.org/10.1007/s10452-021-09832-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10452-021-09832-x