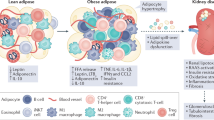
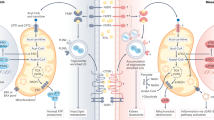
Abstract
Chronic kidney disease (CKD) induces modifications in lipid and lipoprotein metabolism and homeostasis. These modifications can promote, modulate and/or accelerate CKD and secondary cardiovascular disease (CVD). Lipid and lipoprotein abnormalities — involving triglyceride-rich lipoproteins, LDL and/or HDL — not only involve changes in concentration but also changes in molecular structure, including protein composition, incorporation of small molecules and post-translational modifications. These alterations modify the function of lipoproteins and can trigger pro-inflammatory and pro-atherogenic processes, as well as oxidative stress. Serum fatty acid levels are also often altered in patients with CKD and lead to changes in fatty acid metabolism — a key process in intracellular energy production — that induce mitochondrial dysfunction and cellular damage. These fatty acid changes might not only have a negative impact on the heart, but also contribute to the progression of kidney damage. The presence of these lipoprotein alterations within a biological environment characterized by increased inflammation and oxidative stress, as well as the competing risk of non-atherosclerotic cardiovascular death as kidney function declines, has important therapeutic implications. Additional research is needed to clarify the pathophysiological link between lipid and lipoprotein modifications, and kidney dysfunction, as well as the genesis and/or progression of CVD in patients with kidney disease.
Key points
-
HDL and LDL modifications in chronic kidney disease (CKD) increase cardiovascular risk; targeting these modifications might lead to the development of novel therapeutic strategies.
-
In CKD, alterations in proteome content, metabolic solute accumulation and post-translational modifications convert HDL from an anti-inflammatory to a pro-inflammatory molecule and enhance the pro-inflammatory character of LDL.
-
HDL modifications, and the altered correlation between HDL levels and cardiovascular risk in patients with CKD compared with the general population, support the concept that HDL function, rather than HDL cholesterol levels, influences cardiovascular risk.
-
The contribution of non-atherosclerotic cardiovascular disease to cardiovascular risk in patients with CKD increases with declining kidney function, which further contributes to an altered correlation between lipoprotein levels and overall cardiovascular risk in these patients.
-
Deregulated fatty acid metabolism and mitochondrial dysfunction not only negatively affect the heart but also contribute to kidney pathology by promoting inflammation and fibrosis; autophagy has protective effects.
-
Accumulation of saturated fatty acids triggers mitochondrial and cell damage in the kidney, whereas the polyunsaturated fatty acids docosahexaenoic acid and eicosapentaenoic acid are renoprotective.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Soppert, J., Lehrke, M., Marx, N., Jankowski, J. & Noels, H. Lipoproteins and lipids in cardiovascular disease: from mechanistic insights to therapeutic targeting. Adv. Drug Deliv. Rev. 159, 4–33 (2020).
Brewer, H. B. Jr., Remaley, A. T., Neufeld, E. B., Basso, F. & Joyce, C. Regulation of plasma high-density lipoprotein levels by the ABCA1 transporter and the emerging role of high-density lipoprotein in the treatment of cardiovascular disease. Arterioscler. Thromb. Vasc. Biol. 24, 1755–1760 (2004).
Weiner, D. E. & Sarnak, M. J. Managing dyslipidemia in chronic kidney disease. J. Gen. Intern. Med. 19, 1045–1052 (2004).
Ferro, C. J. et al. Lipid management in patients with chronic kidney disease. Nat. Rev. Nephrol. 14, 727–749 (2018).
Lamprea-Montealegre, J. A. et al. Chronic kidney disease, lipids and apolipoproteins, and coronary heart disease: the ARIC study. Atherosclerosis 234, 42–46 (2014).
Lee, P. H. et al. Hypertriglyceridemia: an independent risk factor of chronic kidney disease in Taiwanese adults. Am. J. Med. Sci. 338, 185–189 (2009).
Chu, M., Wang, A. Y., Chan, I. H., Chui, S. H. & Lam, C. W. Serum small-dense LDL abnormalities in chronic renal disease patients. Br. J. Biomed. Sci. 69, 99–102 (2012).
Vickers, K. C. & Remaley, A. T. HDL and cholesterol: life after the divorce? J. Lipid Res. 55, 4–12 (2014).
Duranton, F. et al. Normal and pathologic concentrations of uremic toxins. J. Am. Soc. Nephrol. 23, 1258–1270 (2012).
Gajjala, P. R., Fliser, D., Speer, T., Jankowski, V. & Jankowski, J. Emerging role of post-translational modifications in chronic kidney disease and cardiovascular disease. Nephrol. Dial. Transplant. 30, 1814–1824 (2015).
Chen, H. et al. Combined clinical phenotype and lipidomic analysis reveals the impact of chronic kidney disease on lipid metabolism. J. Proteome Res. 16, 1566–1578 (2017).
Afshinnia, F. et al. Impaired beta-Oxidation and Altered Complex Lipid Fatty Acid Partitioning with Advancing CKD. J. Am. Soc. Nephrol. 29, 295–306 (2018).
Kang, H. M. et al. Defective fatty acid oxidation in renal tubular epithelial cells has a key role in kidney fibrosis development. Nat. Med. 21, 37–46 (2015).
Kruger, C. et al. Proximal tubular cell-specific ablation of carnitine acetyltransferase causes tubular disease and secondary glomerulosclerosis. Diabetes 68, 819–831 (2019).
Nordestgaard, B. G. & Varbo, A. Triglycerides and cardiovascular disease. Lancet 384, 626–635 (2014).
Gordon, T., Castelli, W. P., Hjortland, M. C., Kannel, W. B. & Dawber, T. R. High density lipoprotein as a protective factor against coronary heart disease. The Framingham Study. Am. J. Med. 62, 707–714 (1977).
Rader, D. J. & Hovingh, G. K. HDL and cardiovascular disease. Lancet 384, 618–625 (2014).
Nguyen, T. D. & Schulze, P. C. Lipid in the midst of metabolic remodeling – therapeutic implications for the failing heart. Adv. Drug Deliv. Rev. 159, 120–132 (2020).
Thompson, S. et al. Cause of death in patients with reduced kidney function. J. Am. Soc. Nephrol. 26, 2504–2511 (2015).
Marx, N. et al. Mechanisms of cardiovascular complications in chronic kidney disease: research focus of the Transregional Research Consortium SFB TRR219 of the University Hospital Aachen (RWTH) and the Saarland University. Clin. Res. Cardiol. 107, 120–126 (2018).
Bajaj, A. et al. Lipids, apolipoproteins, and risk of atherosclerotic cardiovascular disease in persons with CKD. Am. J. Kidney Dis. 73, 827–836 (2019).
Lamprea-Montealegre, J. A. et al. Apolipoprotein B, triglyceride-rich lipoproteins, and risk of cardiovascular events in persons with CKD. Clin. J. Am. Soc. Nephrol. 15, 47–60 (2020).
Chang, T. I. et al. Inverse association between serum non-high-density lipoprotein cholesterol levels and mortality in patients undergoing incident hemodialysis. J Am Heart Assoc. 7, e009096 (2018).
Zewinger, S. et al. HDL cholesterol is not associated with lower mortality in patients with kidney dysfunction. J. Am. Soc. Nephrol. 25, 1073–1082 (2014).
Kuma, A. et al. Impact of low-density lipoprotein cholesterol on decline in estimated glomerular filtration rate in apparently healthy young to middle-aged working men. Clin. Exp. Nephrol. 22, 15–27 (2018).
Muntner, P., Coresh, J., Smith, J. C., Eckfeldt, J. & Klag, M. J. Plasma lipids and risk of developing renal dysfunction: the atherosclerosis risk in communities study. Kidney Int. 58, 293–301 (2000).
Schaeffner, E. S. et al. Cholesterol and the risk of renal dysfunction in apparently healthy men. J. Am. Soc. Nephrol. 14, 2084–2091 (2003).
Fox, C. S. et al. Predictors of new-onset kidney disease in a community-based population. JAMA 291, 844–850 (2004).
Rahman, M. et al. Relation of serum lipids and lipoproteins with progression of CKD: The CRIC study. Clin. J. Am. Soc. Nephrol. 9, 1190–1198 (2014).
Haynes, R. et al. Effects of lowering LDL cholesterol on progression of kidney disease. J. Am. Soc. Nephrol. 25, 1825–1833 (2014).
Charytan, D. M. et al. Efficacy and safety of evolocumab in chronic kidney disease in the FOURIER trial. J. Am. Coll. Cardiol. 73, 2961–2970 (2019).
Bowe, B., Xie, Y., Xian, H., Balasubramanian, S. & Al-Aly, Z. Low levels of high-density lipoprotein cholesterol increase the risk of incident kidney disease and its progression. Kidney Int. 89, 886–896 (2016).
Nam, K. H. et al. Association between serum high-density lipoprotein cholesterol levels and progression of chronic kidney disease: results from the KNOW-CKD. J. Am. Heart Assoc. 8, e011162 (2019).
Bardagjy, A. S. & Steinberg, F. M. Relationship between HDL functional characteristics and cardiovascular health and potential impact of dietary patterns: a narrative review. Nutrients 11, 1231 (2019).
Vaziri, N. D., Deng, G. & Liang, K. Hepatic HDL receptor, SR-B1 and Apo A-I expression in chronic renal failure. Nephrol. Dialysis Transplant. 14, 1462–1466 (1999).
Calabresi, L. et al. Acquired lecithin:cholesterol acyltransferase deficiency as a major factor in lowering plasma HDL levels in chronic kidney disease. J. Intern. Med. 277, 552–561 (2015).
Vaziri, N. D., Liang, K. & Parks, J. S. Down-regulation of hepatic lecithin:cholesterol acyltransferase gene expression in chronic renal failure. Kidney Int. 59, 2192–2196 (2001).
Moradi, H. et al. Elevated high-density lipoprotein cholesterol and cardiovascular mortality in maintenance hemodialysis patients. Nephrol. Dialysis Transplant. 29, 1554–1562 (2014).
Rohatgi, A. et al. HDL cholesterol efflux capacity and incident cardiovascular events. N. Engl. J. Med. 371, 2383–2393 (2014).
Bauer, L. et al. HDL cholesterol efflux capacity and cardiovascular events in patients with chronic kidney disease. J. Am. Coll. Cardiol. 69, 246–247 (2017).
Kopecky, C. et al. HDL cholesterol efflux does not predict cardiovascular risk in hemodialysis patients. J. Am. Soc. Nephrol. 28, 769–775 (2017).
Chindhy, S. et al. Impaired renal function on cholesterol efflux capacity, hdl particle number, and cardiovascular events. J. Am. Coll. Cardiol. 72, 698–700 (2018).
Yamamoto, S. et al. Dysfunctional high-density lipoprotein in patients on chronic hemodialysis. J. Am. Coll. Cardiol. 60, 2372–2379 (2012).
Gipson, G. T. et al. Impaired delivery of cholesterol effluxed from macrophages to hepatocytes by serum from CKD patients may underlie increased cardiovascular disease risk. Kidney Int. Rep. 5, 199–210 (2020).
Binder, V. et al. The myeloperoxidase product hypochlorous acid generates irreversible high-density lipoprotein receptor inhibitors. Arterioscler. Thromb. Vasc. Biol. 33, 1020–1027 (2013).
Zewinger, S. et al. Symmetric dimethylarginine, high-density lipoproteins and cardiovascular disease. Eur. Heart J. 38, 1597–1607 (2017).
Speer, T. et al. Abnormal high-density lipoprotein induces endothelial dysfunction via activation of Toll-like receptor-2. Immunity 38, 754–768 (2013).
Barreto, F. C. et al. Serum indoxyl sulfate is associated with vascular disease and mortality in chronic kidney disease patients. Clin. J. Am. Soc. Nephrol. 4, 1551–1558 (2009).
Weichhart, T. et al. Serum amyloid A in uremic HDL promotes inflammation. J. Am. Soc. Nephrol. 23, 934–947 (2012).
Holzer, M. et al. Uremia alters HDL composition and function. J. Am. Soc. Nephrol. 22, 1631–1641 (2011).
Shao, B. et al. A cluster of proteins implicated in kidney disease is increased in high-density lipoprotein isolated from hemodialysis subjects. J. Proteome Res. 14, 2792–2806 (2015).
Moradi, H., Pahl, M. V., Elahimehr, R. & Vaziri, N. D. Impaired antioxidant activity of high-density lipoprotein in chronic kidney disease. Transl. Res. 153, 77–85 (2009).
Tolle, M. et al. High-density lipoprotein loses its anti-inflammatory capacity by accumulation of pro-inflammatory-serum amyloid A. Cardiovasc. Res. 94, 154–162 (2012).
Luo, M. et al. ApoCIII enrichment in HDL impairs HDL-mediated cholesterol efflux capacity. Sci. Rep. 7, 2312 (2017).
Jahangiri, A. High-density lipoprotein and the acute phase response. Curr. Opin. Endocrinol. Diabetes Obes. 17, 156–160 (2010).
Artl, A., Marsche, G., Lestavel, S., Sattler, W. & Malle, E. Role of serum amyloid A during metabolism of acute-phase HDL by macrophages. Arterioscler. Thromb. Vasc. Biol. 20, 763–772 (2000).
Schuchardt, M. et al. Dysfunctional high-density lipoprotein activates toll-like receptors via serum amyloid A in vascular smooth muscle cells. Sci. Rep. 9, 3421 (2019).
Zewinger, S. et al. Serum amyloid A: high-density lipoproteins interaction and cardiovascular risk. Eur. Heart J. 36, 3007–3016 (2015).
Kopecky, C. et al. Quantification of HDL proteins, cardiac events, and mortality in patients with type 2 diabetes on hemodialysis. Clin. J. Am. Soc. Nephrol. 10, 224–231 (2015).
Untersteller, K. et al. HDL functionality and cardiovascular outcome among nondialysis chronic kidney disease patients. J. Lipid Res. 59, 1256–1265 (2018).
Chan, D. T. et al. Chronic kidney disease delays VLDL-apoB-100 particle catabolism: potential role of apolipoprotein C-III. J. Lipid Res. 50, 2524–2531 (2009).
Ooi, E. M., Barrett, P. H., Chan, D. C. & Watts, G. F. Apolipoprotein C-III: understanding an emerging cardiovascular risk factor. Clin. Sci. 114, 611–624 (2008).
Kohan, A. B. Apolipoprotein C-III: a potent modulator of hypertriglyceridemia and cardiovascular disease. Curr. Opin. Endocrinol. Diabetes Obes. 22, 119–125 (2015).
Qu, J., Ko, C. W., Tso, P. & Bhargava, A. Apolipoprotein A-IV: a multifunctional protein involved in protection against atherosclerosis and diabetes. Cells 8, 319 (2019).
Goldberg, I. J., Scheraldi, C. A., Yacoub, L. K., Saxena, U. & Bisgaier, C. L. Lipoprotein ApoC-II activation of lipoprotein lipase. Modulation by apolipoprotein A-IV. J. Biol. Chem. 265, 4266–4272 (1990).
Tan, K. C. B. et al. Carbamylated lipoproteins and progression of diabetic kidney disease. Clin. J. Am. Soc. Nephrol. 15, 359–366 (2020).
Holzer, M. et al. Protein carbamylation renders high-density lipoprotein dysfunctional. Antioxid. Redox Signal. 14, 2337–2346 (2011).
Shao, B. et al. Humans with atherosclerosis have impaired ABCA1 cholesterol efflux and enhanced high-density lipoprotein oxidation by myeloperoxidase. Circul. Res. 114, 1733–1742 (2014).
Kashyap, S. R. et al. Glycation reduces the stability of ApoAI and increases HDL dysfunction in diet-controlled type 2 diabetes. J. Clin. Endocrinol. Metab. 103, 388–396 (2018).
Shao, B., Pennathur, S. & Heinecke, J. W. Myeloperoxidase targets apolipoprotein A-I, the major high density lipoprotein protein, for site-specific oxidation in human atherosclerotic lesions. J. Biol. Chem. 287, 6375–6386 (2012).
Bakillah, A. et al. Plasma nitration of high-density and low-density lipoproteins in chronic kidney disease patients receiving kidney transplants. Med. Inflamm. 2015, 352356 (2015).
Miyazaki, A. et al. N-homocysteinylation of apolipoprotein A-I impairs the protein’s antioxidant ability but not its cholesterol efflux capacity. Biol. Chem. 395, 641–648 (2014).
Speer, T., Zewinger, S. & Fliser, D. Uraemic dyslipidaemia revisited: role of high-density lipoprotein. Nephrol. Dialysis Transplant. 28, 2456–2463 (2013).
Sun, J. T. et al. Increased carbamylation level of HDL in end-stage renal disease: carbamylated-HDL attenuated endothelial cell function. Am. J. Physiol. Renal Physiol. 310, F511–F517 (2016).
Bancells, C., Sanchez-Quesada, J. L., Birkelund, R., Ordonez-Llanos, J. & Benitez, S. HDL and electronegative LDL exchange anti- and pro-inflammatory properties. J. Lipid Res. 51, 2947–2956 (2010).
Yao, S. et al. Oxidized high density lipoprotein induces macrophage apoptosis via toll-like receptor 4-dependent CHOP pathway. J. Lipid Res. 58, 164–177 (2017).
Gao, X. et al. Oxidized high-density lipoprotein impairs the function of human renal proximal tubule epithelial cells through CD36. Int. J. Mol. Med. 34, 564–572 (2014).
Pérez, L. et al. OxHDL controls LOX-1 expression and plasma membrane localization through a mechanism dependent on NOX/ROS/NF-κB pathway on endothelial cells. Lab. Invest. 99, 421–437 (2019).
Sun, J. T. et al. Oxidized HDL, as a novel biomarker for calcific aortic valve disease, promotes the calcification of aortic valve interstitial cells. J. Cardiovasc. Transl. Res. 12, 560–568 (2019).
Honda, H. et al. Oxidized high-density lipoprotein as a risk factor for cardiovascular events in prevalent hemodialysis patients. Atherosclerosis 220, 493–501 (2012).
Florens, N. et al. CKD increases carbonylation of HDL and is associated with impaired antiaggregant properties. J. Am. Soc. Nephrol. 31, 1462–1477 (2020).
Kraus, L. M. & Kraus, A. P. Jr. Carbamoylation of amino acids and proteins in uremia. Kidney Int. Suppl. 78, S102–S107 (2001).
Wang, Z. et al. Protein carbamylation links inflammation, smoking, uremia and atherogenesis. Nat. Med. 13, 1176–1184 (2007).
Chang, C. T. et al. PON-1 carbamylation is enhanced in HDL of uremia patients. J. Food Drug. Anal. 27, 542–550 (2019).
Koeth, R. A. et al. Protein carbamylation predicts mortality in ESRD. J. Am. Soc. Nephrol. 24, 853–861 (2013).
Tsai, M. Y. et al. New automated assay of small dense low-density lipoprotein cholesterol identifies risk of coronary heart disease: the Multi-ethnic Study of Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 34, 196–201 (2014).
Shen, H. et al. Small dense low-density lipoprotein cholesterol was associated with future cardiovascular events in chronic kidney disease patients. BMC Nephrol. 17, 143 (2016).
Oi, K., Hirano, T., Sakai, S., Kawaguchi, Y. & Hosoya, T. Role of hepatic lipase in intermediate-density lipoprotein and small, dense low-density lipoprotein formation in hemodialysis patients. Kidney Int. Suppl. 71, S227–S228 (1999).
Ikewaki, K. et al. Delayed in vivo catabolism of intermediate-density lipoprotein and low-density lipoprotein in hemodialysis patients as potential cause of premature atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 25, 2615–2622 (2005).
Pietzsch, J., Lattke, P. & Julius, U. Oxidation of apolipoprotein B-100 in circulating LDL is related to LDL residence time. In vivo insights from stable-isotope studies. Arterioscler. Thromb. Vasc. Biol. 20, E63–E67 (2000).
Baigent, C. et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet 377, 2181–2192 (2011).
Shlipak, M. G. et al. Cardiovascular mortality risk in chronic kidney disease: comparison of traditional and novel risk factors. JAMA 293, 1737–1745 (2005).
Chawla, V. et al. Hyperlipidemia and long-term outcomes in nondiabetic chronic kidney disease. Clin. J. Am. Soc. Nephrol. 5, 1582–1587 (2010).
Cholesterol Treatment Trialists’ (CTT) Collaboration. Impact of renal function on the effects of LDL cholesterol lowering with statin-based regimens: a meta-analysis of individual participant data from 28 randomised trials. Lancet Diabetes Endocrinol. 4, 829–839 (2016).
Kovesdy, C. P., Anderson, J. E. & Kalantar-Zadeh, K. Inverse association between lipid levels and mortality in men with chronic kidney disease who are not yet on dialysis: effects of case mix and the malnutrition-inflammation-cachexia syndrome. J. Am. Soc. Nephrol. 18, 304–311 (2007).
Liu, Y. et al. Association between cholesterol level and mortality in dialysis patients: role of inflammation and malnutrition. JAMA291, 451–459 (2004).
Ruan, X. Z., Varghese, Z. & Moorhead, J. F. An update on the lipid nephrotoxicity hypothesis. Nat. Rev. Nephrol. 5, 713–721 (2009).
Delporte, C. et al. Impact of myeloperoxidase-LDL interactions on enzyme activity and subsequent posttranslational oxidative modifications of apoB-100. J. Lipid Res. 55, 747–757 (2014).
Hamilton, R. T. et al. LDL protein nitration: implication for LDL protein unfolding. Arch. Biochem. Biophys. 479, 1–14 (2008).
Moore, K. J. & Freeman, M. W. Scavenger receptors in atherosclerosis: beyond lipid uptake. Arterioscler. Thromb. Vasc. Biol. 26, 1702–1711 (2006).
Apostolov, E. O. et al. Carbamylated-oxidized LDL: proatherosclerotic effects on endothelial cells and macrophages. J. Atheroscler. Thromb. 20, 878–892 (2013).
Podrez, E. A. et al. Macrophage scavenger receptor CD36 is the major receptor for LDL modified by monocyte-generated reactive nitrogen species. J. Clin. Invest. 105, 1095–1108 (2000).
Jay, A. G., Chen, A. N., Paz, M. A., Hung, J. P. & Hamilton, J. A. CD36 binds oxidized low density lipoprotein (LDL) in a mechanism dependent upon fatty acid binding. J. Biol. Chem. 290, 4590–4603 (2015).
Le Master, E. et al. Proatherogenic flow increases endothelial stiffness via enhanced CD36-mediated uptake of oxidized low-density lipoproteins. Arterioscler. Thromb.Vasc. Biol. 38, 64–75 (2018).
Meisinger, C., Baumert, J., Khuseyinova, N., Loewel, H. & Koenig, W. Plasma oxidized low-density lipoprotein, a strong predictor for acute coronary heart disease events in apparently healthy, middle-aged men from the general population. Circulation 112, 651–657 (2005).
Drozdz, D. et al. Oxidative stress biomarkers and left ventricular hypertrophy in children with chronic kidney disease. Oxid. Med. Cell. Longev. 2016, 7520231 (2016).
Pawlak, K., Mysliwiec, M. & Pawlak, D. Oxidized LDL to autoantibodies against oxLDL ratio - the new biomarker associated with carotid atherosclerosis and cardiovascular complications in dialyzed patients. Atherosclerosis 224, 252–257 (2012).
Hou, J. S. et al. Serum malondialdehyde-modified low-density lipoprotein is a risk factor for central arterial stiffness in maintenance hemodialysis patients. Nutrients 12, 2160 (2020).
Ok, E., Basnakian, A. G., Apostolov, E. O., Barri, Y. M. & Shah, S. V. Carbamylated low-density lipoprotein induces death of endothelial cells: a link to atherosclerosis in patients with kidney disease. Kidney Int. 68, 173–178 (2005).
Speer, T. et al. Carbamylated low-density lipoprotein induces endothelial dysfunction. Eur. Heart J. 35, 3021–3032 (2014).
Apostolov, E. O., Ray, D., Savenka, A. V., Shah, S. V. & Basnakian, A. G. Chronic uremia stimulates LDL carbamylation and atherosclerosis. J. Am. Soc. Nephrol. 21, 1852–1857 (2010).
Afshinnia, F. et al. Myeloperoxidase levels and its product 3-chlorotyrosine predict chronic kidney disease severity and associated coronary artery disease. Am. J. Nephrol. 46, 73–81 (2017).
Himmelfarb, J., McMenamin, M. E., Loseto, G. & Heinecke, J. W. Myeloperoxidase-catalyzed 3-chlorotyrosine formation in dialysis patients. Free Radic. Biol. Med. 31, 1163–1169 (2001).
Bucala, R. et al. Modification of low density lipoprotein by advanced glycation end products contributes to the dyslipidemia of diabetes and renal insufficiency. Proc. Natl Acad. Sci. USA 91, 9441–9445 (1994).
Hodgkinson, C. P., Laxton, R. C., Patel, K. & Ye, S. Advanced glycation end-product of low density lipoprotein activates the toll-like 4 receptor pathway implications for diabetic atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 28, 2275–2281 (2008).
Ooi, E. M. et al. Plasma apolipoprotein C-III metabolism in patients with chronic kidney disease. J. Lipid Res. 52, 794–800 (2011).
Vaziri, N. D. & Liang, K. Down-regulation of VLDL receptor expression in chronic experimental renal failure. Kidney Int. 51, 913–919 (1997).
Kim, C. & Vaziri, N. D. Down-regulation of hepatic LDL receptor-related protein (LRP) in chronic renal failure. Kidney Int. 67, 1028–1032 (2005).
Koppe, L. et al. Urea impairs beta cell glycolysis and insulin secretion in chronic kidney disease. J. Clin. Invest. 126, 3598–3612 (2016).
Koppe, L. et al. p-Cresyl sulfate promotes insulin resistance associated with CKD. J. Am. Soc. Nephrol. 24, 88–99 (2013).
Spoto, B., Pisano, A. & Zoccali, C. Insulin resistance in chronic kidney disease: a systematic review. Am. J. Physiol. Renal Physiol. 311, F1087–F1108 (2016).
Schwartz, G. G. et al. Fasting triglycerides predict recurrent ischemic events in patients with acute coronary syndrome treated with statins. J. Am. Coll. Cardiol. 65, 2267–2275 (2015).
Matsuura, Y., Kanter, J. E. & Bornfeldt, K. E. Highlighting residual atherosclerotic cardiovascular disease risk. Arterioscler. Thromb. Vasc. Biol. 39, e1–e9 (2019).
Soohoo, M. et al. Serum triglycerides and mortality risk across stages of chronic kidney disease in 2 million U.S. veterans. J. Clin. Lipidol. 13, 744–753.e715 (2019).
Utermann, G. in The Metabolic and Molecular Bases of Inherited Disease 8th edn (eds Scriver, C. R., Beaudet, A. L., Sly, W. S. & Valle, D.) 2753–2787 (McGraw-Hill, 2001)
Kronenberg, F. et al. Lipoprotein(a) serum concentrations and apolipoprotein(a) phenotypes in mild and moderate renal failure. J. Am. Soc. Nephrol. 11, 105–115 (2000).
Hopewell, J. C., Haynes, R. & Baigent, C. The role of lipoprotein (a) in chronic kidney disease. J. Lipid Res. 59, 577–585 (2018).
Bittner, V. A. et al. Effect of alirocumab on Lipoprotein(a) and cardiovascular risk after acute coronary syndrome. J. Am. Coll. Cardiol. 75, 133–144 (2020).
O’Donoghue, M. L. et al. Lipoprotein(a), PCSK9 inhibition, and cardiovascular risk. Circulation 139, 1483–1492 (2019).
Boden, W. E. et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N. Engl. J. Med. 365, 2255–2267 (2011).
Albers, J. J. et al. Relationship of apolipoproteins A-1 and B, and lipoprotein(a) to cardiovascular outcomes: the AIM-HIGH trial (Atherothrombosis Intervention in Metabolic Syndrome with Low HDL/High Triglyceride and Impact on Global Health Outcomes). J. Am. Coll. Cardiol. 62, 1575–1579 (2013).
Landray, M. J. et al. Effects of extended-release niacin with laropiprant in high-risk patients. N. Engl. J. Med. 371, 203–212 (2014).
Parish, S. et al. Impact of Apolipoprotein(a) Isoform Size on Lipoprotein(a) Lowering in the HPS2-THRIVE Study. Circ. Genom. Precis. Med. 11, e001696 (2018).
Tsimikas, S. et al. Lipoprotein(a) reduction in persons with cardiovascular disease. N. Engl. J. Med. 382, 244–255 (2020).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT04023552 (2021).
Czumaj, A. et al. Alterations of fatty acid profile may contribute to dyslipidemia in chronic kidney disease by influencing hepatocyte metabolism. Int. J. Mol. Sci. 20, 2470 (2019).
Khor, B. H. et al. Blood fatty acid status and clinical outcomes in dialysis patients: a systematic review. Nutrients 10, 1353 (2018).
Mantovani, A. et al. Association between increased plasma ceramides and chronic kidney disease in patients with and without ischemic heart disease. Diabetes Metab. 47, 101152 (2020).
Schulze, P. C., Drosatos, K. & Goldberg, I. J. Lipid use and misuse by the heart. Circul. Res. 118, 1736–1751 (2016).
Afshinnia, F. et al. Increased lipogenesis and impaired β-oxidation predict type 2 diabetic kidney disease progression in American Indians. JCI Insight 4, e130317 (2019).
Han, S. H. et al. PGC-1α protects from Notch-induced kidney fibrosis development. J. Am. Soc. Nephrol 28, 3312–3322 (2017).
Ruggiero, C. et al. Albumin-bound fatty acids but not albumin itself alter redox balance in tubular epithelial cells and induce a peroxide-mediated redox-sensitive apoptosis. Am. J. Physiol. Renal Physiol. 306, F896–F906 (2014).
Fierro-Fernandez, M. et al. MiR-95p protects from kidney fibrosis by metabolic reprogramming. FASEB J. 34, 410–431 (2020).
Arif, E. et al. Mitochondrial biogenesis induced by the beta2-adrenergic receptor agonist formoterol accelerates podocyte recovery from glomerular injury. Kidney Int. 96, 656–673 (2019).
Jao, T. M. et al. ATF6alpha downregulation of PPARalpha promotes lipotoxicity-induced tubulointerstitial fibrosis. Kidney Int. 95, 577–589 (2019).
Price, N. L. et al. Genetic deficiency or pharmacological inhibition of miR-33 protects from kidney fibrosis. JCI Insight 4, e131102 (2019).
Chung, K. W. et al. Mitochondrial damage and activation of the STING pathway lead to renal inflammation and fibrosis. Cell Metab. 30, 784–799.e5 (2019).
Xu, S. et al. Palmitate induces ER calcium depletion and apoptosis in mouse podocytes subsequent to mitochondrial oxidative stress. Cell Death Dis. 6, e1976 (2015).
Yamamoto, T. et al. High-fat diet-induced lysosomal dysfunction and impaired autophagic flux contribute to lipotoxicity in the kidney. J. Am. Soc. Nephrol. 28, 1534–1551 (2017).
Soumura, M. et al. Oleate and eicosapentaenoic acid attenuate palmitate-induced inflammation and apoptosis in renal proximal tubular cell. Biochem. Biophys. Res. Commun. 402, 265–271 (2010).
Listenberger, L. L. et al. Triglyceride accumulation protects against fatty acid-induced lipotoxicity. Proc. Natl Acad. Sci. USA 100, 3077–3082 (2003).
Sieber, J. et al. Susceptibility of podocytes to palmitic acid is regulated by stearoyl-CoA desaturases 1 and 2. Am. J. Pathol. 183, 735–744 (2013).
Kampe, K., Sieber, J., Orellana, J. M., Mundel, P. & Jehle, A. W. Susceptibility of podocytes to palmitic acid is regulated by fatty acid oxidation and inversely depends on acetyl-CoA carboxylases 1 and 2. Am. J. Physiol. Renal Physiol. 306, F401–F409 (2014).
Wilfling, F. et al. Triacylglycerol synthesis enzymes mediate lipid droplet growth by relocalizing from the ER to lipid droplets. Dev. Cell 24, 384–399 (2013).
Ackerman, D. et al. Triglycerides promote lipid homeostasis during hypoxic stress by balancing fatty acid saturation. Cell Rep. 24, 2596–2605.e5 (2018).
Praticò, D. & Dogné, J. M. Vascular biology of eicosanoids and atherogenesis. Expert Rev. Cardiovasc. Ther. 7, 1079–1089 (2009).
Chiurchiù, V., Leuti, A. & Maccarrone, M. Bioactive lipids and chronic inflammation: managing the fire within. Front. Immunol. 9, 38 (2018).
Joffre, C., Rey, C. & Laye, S. N-3 polyunsaturated fatty acids and the resolution of neuroinflammation. Front. Pharmacol. 10, 1022 (2019).
Kim, A. S. & Conte, M. S. Specialized pro-resolving lipid mediators in cardiovascular disease, diagnosis, and therapy. Adv. Drug Deliv. Rev. 159, 170–179 (2020).
de Gaetano, M. et al. Specialized pro-resolving lipid mediators: modulation of diabetes-associated cardio-, reno-, and retino-vascular complications. Front. Pharmacol. 9, 1488 (2018).
de Oliveira Otto, M. C. et al. Circulating and dietary omega-3 and omega-6 polyunsaturated fatty acids and incidence of CVD in the multi-ethnic study of atherosclerosis. J. Am. Heart Assoc. 2, e000506 (2013).
Abdelhamid, A. S. et al. Omega-3 fatty acids for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst. Rev. 3, CD003177 (2020).
Bhatt, D. L. et al. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N. Engl. J. Med. 380, 11–22 (2019).
Doshi, R. et al. Meta-analysis comparing combined use of eicosapentaenoic acid and statin to statin alone. Am. J. Cardiol. 125, 198–204 (2020).
Rimm, E. B. et al. Seafood long-chain n-3 polyunsaturated fatty acids and cardiovascular disease: a science advisory from the American heart association. Circulation 138, e35–e47 (2018).
Marklund, M. et al. Biomarkers of dietary omega-6 fatty acids and incident cardiovascular disease and mortality. Circulation 139, 2422–2436 (2019).
Shoji, T. et al. Serum n-3 and n-6 polyunsaturated fatty acid profile as an independent predictor of cardiovascular events in hemodialysis patients. Am. J. Kidney Dis. 62, 568–576 (2013).
Friedman, A. N. et al. Inverse relationship between long-chain n-3 fatty acids and risk of sudden cardiac death in patients starting hemodialysis. Kidney Int. 83, 1130–1135 (2013).
Khor, B. H. et al. Efficacy of nutritional interventions on inflammatory markers in haemodialysis patients: a systematic review and limited meta-analysis. Nutrients 10, 397 (2018).
He, L., Li, M. S., Lin, M., Zhao, T. Y. & Gao, P. Effect of fish oil supplement in maintenance hemodialysis patients: a systematic review and meta-analysis of published randomized controlled trials. Eur. J. Clin. Pharmacol. 72, 129–139 (2016).
Saglimbene, V. M. et al. Effects of omega-3 polyunsaturated fatty acid intake in patients with chronic kidney disease: Systematic review and meta-analysis of randomized controlled trials. Clin. Nutr. 39, 358–368 (2020).
Zeng, Z. et al. Omega-3 polyunsaturated fatty acids attenuate fibroblast activation and kidney fibrosis involving MTORC2 signaling suppression. Sci. Rep. 7, 46146 (2017).
Malhotra, R. et al. Dietary polyunsaturated fatty acids and incidence of end-stage renal disease in the Southern community cohort study. BMC Nephrol. 17, 152 (2016).
Dos Santos, A. L. T. et al. Low linolenic and linoleic acid consumption are associated with chronic kidney disease in patients with type 2 diabetes. PLoS ONE 13, e0195249 (2018).
Cardenas, C., Bordiu, E., Bagazgoitia, J. & Calle-Pascual, A. L. Polyunsaturated fatty acid consumption may play a role in the onset and regression of microalbuminuria in well-controlled type 1 and type 2 diabetic people: a 7-year, prospective, population-based, observational multicenter study. Diabetes Care 27, 1454–1457 (2004).
Shapiro, H., Theilla, M., Attal-Singer, J. & Singer, P. Effects of polyunsaturated fatty acid consumption in diabetic nephropathy. Nat. Rev. Nephrol. 7, 110–121 (2011).
Elajami, T. K. et al. Eicosapentaenoic and docosahexaenoic acids attenuate progression of albuminuria in patients with type 2 diabetes mellitus and coronary artery disease. J. Am. Heart Assoc. 6, e004740 (2017).
de Boer, I. H. et al. Effect of Vitamin D and Omega-3 fatty acid supplementation on kidney function in patients with type 2 diabetes: a randomized clinical trial. JAMA 322, 1899–1909 (2019).
Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. Suppl. 3, 11–50 (2013).
Palmer, S. C. et al. Benefits and harms of statin therapy for persons with chronic kidney disease: a systematic review and meta-analysis. Ann. Intern. Med. 157, 263–275 (2012).
Hou, W. et al. Effect of statin therapy on cardiovascular and renal outcomes in patients with chronic kidney disease: a systematic review and meta-analysis. Eur. Heart J. 34, 1807–1817 (2013).
Rosenson, R. S. et al. HDL and atherosclerotic cardiovascular disease: genetic insights into complex biology. Nat. Rev. Cardiol. 15, 9–19 (2018).
Khera, A. V. et al. Cholesterol efflux capacity, high-density lipoprotein particle number, and incident cardiovascular events: an analysis from the JUPITER Trial (Justification for the Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin). Circulation 135, 2494–2504 (2017).
Gibson, C. M. et al. The CSL112-2001 trial: Safety and tolerability of multiple doses of CSL112 (apolipoprotein A-I [human]), an intravenous formulation of plasma-derived apolipoprotein A-I, among subjects with moderate renal impairment after acute myocardial infarction. Am. Heart J. 208, 81–90 (2019).
Kalim, S. et al. The effects of parenteral amino acid therapy on protein carbamylation in maintenance hemodialysis patients. J. Ren. Nutr. 25, 388–392 (2015).
Kang, A. & Jardine, M. J. SGLT2 inhibitors may offer benefit beyond diabetes. Nat. Rev. Nephrol. 17, 83–84 (2021).
Packer, M. Role of deranged energy deprivation signaling in the pathogenesis of cardiac and renal disease in states of perceived nutrient overabundance. Circulation 141, 2095–2105 (2020).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02364648 (2019).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03960073 (2021).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03579693 (2020).
Mach, F. et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur. Heart J. 41, 111–188 (2020).
Bender, D. A. Introduction to Nutrition and Metabolism 5th edn (CRC, 2014).
Gelber, R. P. et al. Association between body mass index and CKD in apparently healthy men. Am. J. Kidney Dis. 46, 871–880 (2005).
D’Agati, V. D. et al. Obesity-related glomerulopathy: clinical and pathologic characteristics and pathogenesis. Nat. Rev. Nephrol. 12, 453–471 (2016).
Herman-Edelstein, M., Scherzer, P., Tobar, A., Levi, M. & Gafter, U. Altered renal lipid metabolism and renal lipid accumulation in human diabetic nephropathy. J. Lipid Res. 55, 561–572 (2014).
Wu, D. et al. Vaccine against PCSK9 improved renal fibrosis by regulating fatty acid beta-oxidation. J. Am. Heart Assoc. 9, e014358 (2020).
Decleves, A. E. et al. Regulation of lipid accumulation by AMP-activated kinase [corrected] in high fat diet-induced kidney injury. Kidney Int. 85, 611–623 (2014).
Decleves, A. E., Mathew, A. V., Cunard, R. & Sharma, K. AMPK mediates the initiation of kidney disease induced by a high-fat diet. J. Am. Soc. Nephrol. 22, 1846–1855 (2011).
Udi, S. et al. Proximal tubular cannabinoid-1 receptor regulates obesity-induced CKD. J. Am. Soc. Nephrol. 28, 3518–3532 (2017).
Bakker, P. J. et al. Nlrp3 is a key modulator of diet-induced nephropathy and renal cholesterol accumulation. Kidney Int. 85, 1112–1122 (2014).
Yang, P. et al. Inflammatory stress promotes the development of obesity-related chronic kidney disease via CD36 in mice. J. Lipid Res. 58, 1417–1427 (2017).
Acknowledgements
This work was supported by the German Research Foundation (DFG) SFB/TRR219 Project-ID 322900939 (S-03, C-04, M-03, M-05), SFB 1382 Project-ID 403224013 (A-04), by the CORONA foundation and by the Interreg V-A EMR program (EURLIPIDS, EMR23). This project also received funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No 764474 (CaReSyAn).
Author information
Authors and Affiliations
Contributions
All authors made substantial contributions to discussions of the content and wrote the manuscript. J.J. and H.N. reviewed or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Nephrology thanks C. Soulage and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Uraemic retention solutes
-
Substances that accumulate in blood of patients with chronic kidney disease owing to reduced kidney clearance.
- Mitochondrial overload
-
A condition in which mitochondria are presented with an excess of substrate, which overwhelms the mitochondrial capacity for fatty acid β-oxidation, reduces free CoA levels and induces the accumulation of excess acetyl-CoA.
- Cholesterol efflux capacity
-
The capacity of HDL to extract cholesterol from macrophages.
- Pulse wave velocity
-
Velocity at which a blood pressure pulse travels through the vessels; used as a clinical measure of arterial stiffness.
- Acute phase protein
-
A component of the acute phase response, which is the initial systemic response of an organism to inflammation and is characterized by an increase or decrease in acute phase proteins in blood.
- Residence time
-
The length of time present in the circulation.
- Malnutrition–inflammation–cachexia syndrome
-
A condition characterized by malnutrition (protein-energy wasting, defined as loss of body protein mass and energy reserves (fat mass)), inflammation and/or oxidative stress; common in chronic diseases such as chronic kidney disease.
Rights and permissions
About this article
Cite this article
Noels, H., Lehrke, M., Vanholder, R. et al. Lipoproteins and fatty acids in chronic kidney disease: molecular and metabolic alterations. Nat Rev Nephrol 17, 528–542 (2021). https://doi.org/10.1038/s41581-021-00423-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41581-021-00423-5
This article is cited by
-
Post-translational modifications in kidney diseases and associated cardiovascular risk
Nature Reviews Nephrology (2024)
-
Dysregulated palmitic acid metabolism promotes the formation of renal calcium-oxalate stones through ferroptosis induced by polyunsaturated fatty acids/phosphatidic acid
Cellular and Molecular Life Sciences (2024)
-
Increased cardiovascular risk in patients with chronic kidney disease
Herz (2024)
-
Circulating metabolomic markers linking diabetic kidney disease and incident cardiovascular disease in type 2 diabetes: analyses from the Hong Kong Diabetes Biobank
Diabetologia (2024)
-
High-density lipoprotein regulates angiogenesis by long non-coding RNA HDRACA
Signal Transduction and Targeted Therapy (2023)