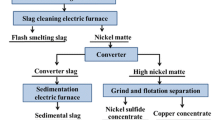
We provide an overview of the current status of research on reprocessing of nickel waste tailings, describe the current condition of the tailings, and set forth an important economic goal — freeing up land occupied by tailings and reducing impacts of human activity on the environment. We study the structure, chemical composition, and phase composition of slag tailings at the Southern Urals Nickel Plant (SUNP). This information was used to design an integrated process for reprocessing of nickel-slag tailings. The first step is to hold the slag/metal melt in a crucible for 15 min at 1300–1400°C, resulting in formation of a nickel-rich (15–18%) matte melt at the bottom. After this melt cools and hardens, it is removed from the crucible, separated from the slag, and inspected in an electron microscope. The resulting matte is suitable for processing into ferronickel via the classical process.
During the second step, the slag from the preceding step was reduced for 30–40 min at 1500–1600°C using crushed electrode, since the low density and porosity of coke mean it is not suitable for this purpose. The slag was vigorously agitated by the release of internal gas. The reduced iron collects to form large inclusions, and settles to the bottom, collecting non-ferrous impurity-metal solutes as it descends. As in the preceding step, the metal was allowed to cool, and the bottom ingot was removed for analysis. We studied the resulting metal, which was used (after preliminary desulfurization) to produce structural steel alloy, and the gangue slag (with Ni and Fe content less than 0.1%) which can be used with blast-furnace slag to produce slag Portland cement. We used these research results to develop a scheme for commercial-scale recycling of SUNP slag waste, with the matte being separated from the slag in an ore smelting furnace, and the iron being separated from the slag in a ladle furnace; in the latter case, the resulting metal can be refined to GOST specifications.





Similar content being viewed by others
References
I. D. Reznik, G. P. Ermakov, and Ya. M. Shneerson, Nickel, Vol. 3: Copper-Nickel Sulfide Ores. Ores and Deposits. Pyrometallurgy. Hydrometallurgy. Nickel Refining. World Nickel Production and Consumption, Nauka i Tekhnologiya, Moscow (2003).
Y. Peng, B. Wang, and D. Bradshaw, “Pentlandite oxidation in the flotation of a complex nickel ore in saline water,” Miner. Eng., 24, 85–87 (2011).
H. Zhu, J. Chen, J. Deng, R. Yu, and X. Xing, “Oxidation behavior and mechanism of pentlandite at 973 K (700 C) in air,” Metall. Mater. Trans. B, B43, 494–502 (2012).
D. Yu, T. A. Utigard, and M. Barati, “Fluidized bed selective oxidation-sulfation roasting of nickel sulfide concentrate: Part I. Oxidation roasting,” Metall. Mater. Trans. B, B45, 653–661 (2014).
R. Pandher, S. Thomas, D. Yu, M. Barati, and T. Utigard, “Sulfate formation and decomposition of nickel concentrates,” Metall. Mater. Trans. B 42, 291–299 (2011).
D. Yu, T. A. Utigard, and M. Barati, “Fluidized bed selective oxidation-sulfation roasting of nickel sulfide concentrate: Part II. Sulfation roasting,” Metall. Mater. Trans., B45, 662–674 (2014).
Y. Ngothai, F. Xia, A. Pring, B. O’Neill, J. Brugger, G. Chen, and C. Colby, Proceedings of the 35th Australasian Chemical Engineering Conference (Chemeca 2007), Sofitel Melbourne, Victoria, Australia (2007).
A. A. Veselovskii, Recycling of Nickel Slag Tailings with Additional Recovery of Metals: A Textbook, Infra-Inzheneriya, Moscow/Vologda (2020).
A. A. Veselovskii and S. A. Laykhan, “Concept for reprocessing of nickel-slag tailings from which additional metal has first been extracted,” Rudy Met., 1, 94–99 (2020).
A. A. Veselovskii, “Processing of nickel slag tailings in which nickel and iron are extracted using chlorine-containing reagents,” Obogashch. Rud (Irkutsk), 372, No. 6, 38–44 (2017).
L. N. Sheludyakov and Eh. A. Kos’yanov, Integrated Reprocessing of Non-Ferrous Metallurgical Slag, Nauka, Alma-Ata (1990).
E. N. Selivanov, S. A. Fedichkin, V. A. Kniss, and A. A. Pankratov, “Forms of occurrence for metals in nickel matte conversion slag,” Rasplavy, 3, 17–23 (2004).
V. Yu. Lozitskii and S. V. Gulyaev, “Forms of occurrence for nickel in converter slag and slag tailings and a technique for reducing irreversible nickel losses when using them,” Tsvetn. Met., 11, 49–53 (2008).
S. A. Fedichkin, A Study of the Depletion Process in Converter Slag from Nickel Production Using Aluminum-Containing Reducing/Sulfiding Complexes, Abstract of dissertation for Candidate in Engineering Sciences, Ekaterinburg (2005).
Yu. A. Gudim and A. A. Golubev, “Efficient techniques for disposition of metallurgical production waste in the Urals,” Ekologiya i Promyshlennost’ Rossii, 12, 4–8 (2008).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Metallurg, Vol. 65, No. 1, pp. 93–98, January, 2021.
Rights and permissions
About this article
Cite this article
Veselovsky, A.A. Recycling of Nickel-Slag Tailings into Liquid Products: A Case Study using Slag from the Southern Urals Nickel Plant. Metallurgist 65, 113–120 (2021). https://doi.org/10.1007/s11015-021-01138-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11015-021-01138-5