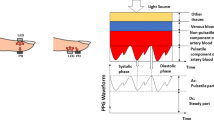
Abstract
Photoplethysmography (PPG) sensor-enabled wearable health monitoring devices can monitor realtime health status. PPG technology is a low-cost, noninvasive optical method used to measure a volumetric change in blood during a cardiac cycle. Continues analysis of change in light signal due to change in the blood helps medical professionals to extract valuable information regarding the cardiovascular system. Traditionally, an electrocardiogram (ECG) has been used as a dominant monitoring technique to detect irregularities in the cardiovascular system. However, in ECG for monitoring cardiac status, several electrodes have to be placed at different body locations, limiting its uses under medical assistantship and in a stationary position. Therefore, to fulfill the market demand for wearable and portable health monitoring devices, researchers are now showing interest in the PPG sensor enable wearable devices. However, the robustness of PPG sensor-enabled wearable devices is highly deviating due to motion artifacts. Therefore before extracting vital sign information like heart rate with PPG sensor, efficient removal of motion artifact is very important. This review orients the research survey on the principles and methods proposed for denoising and heart rate peak detection with PPG. The efficacy of each method related to heart rate peak detection with PPG technologies was compared in terms of mean absolute error, error percentage, and correlation coefficient. A comparative analysis is formulated to estimate heart rate based on the literature survey from the last ten years on PPG technology. This review article aims to explore different methods and challenges mentioned in state-of-the-art research related to motion artifacts removal and heart rate estimation from PPG-enabled wearable devices.






Similar content being viewed by others
References
Kamal AAR, Harness JB, Irving G, Mearns AJ (1989) Skin photoplethysmography—a review. Comput Methods Programs Biomed 28(4):257–269
Vashist SK, Schneider EM, Luong JH (2014) Commercial smartphone-based devices and smart applications for personalized healthcare monitoring and management. Diagnostics 4(3):104–128
Tamura T (2019) Current progress of photoplethysmography and SPO 2 for health monitoring. Biomed Eng Lett 9(1):21–36
Warren KM, Harvey JR, Chon KH, Mendelson Y (2016) Improving pulse rate measurements during random motion using a wearable multichannel reflectance photoplethysmograph. Sensors 16(3):342
Allen J (2007) Photoplethysmography and its application in clinical physiological measurement. Physiol Meas 28(3):R1
Moraes JL, Rocha MX, Vasconcelos GG, VasconcelosFilho JE, De Albuquerque VHC, Alexandria AR (2018) Advances in photopletysmography signal analysis for biomedical applications. Sensors 18(6):1894
Moço AV, Stuijk S, de Haan G (2018) New insights into the origin of remote PPG signals in visible light and infrared. Sci Rep 8(1):1–15
Hartmann V, Liu H, Chen F, Qiu Q, Hughes S, Zheng D (2019) Quantitative comparison of photoplethysmographic waveform characteristics: effect of measurement site. Front Physiol 10:198
Chan ED, Chan MM, Chan MM (2013) Pulse oximetry: understanding its basic principles facilitates appreciation of its limitations. Respir Med 107(6):789–799
Joyner MJ, Casey DP (2015) Regulation of increased blood flow (hyperemia) to muscles during exercise: a hierarchy of competing physiological needs. Physiol Rev 95:549–601
Tamura T, Maeda Y, Sekine M, Yoshida M (2014) Wearable photoplethysmographic sensors—past and present. Electronics 3(2):282–302
Sun Y, Thakor N (2015) Photoplethysmography revisited: from contact to noncontact, from point to imaging. IEEE Trans Biomed Eng 63(3):463–477
Liu J, Yan BPY, Dai WX, Ding XR, Zhang YT, Zhao N (2016) Multi-wavelength photoplethysmography method for skin arterial pulse extraction. Biomed Opt Express 7(10):4313–4326
Spigulis J, Gailite L, Lihachev A, Erts R (2007) Simultaneous recording of skin blood pulsations at different vascular depths by multiwavelength photoplethysmography. Appl Opt 46(10):1754–1759
Hertzman AB (1938) The blood supply of various skin areas as estimated by the photoelectric plethysmograph. Am J Physiol Leg Content 124(2):328–340
Elgendi M (2012) On the analysis of fingertip photoplethysmogram signals. Curr Cardiol Rev 8(1):14–25
Singh N, Moneghetti KJ, Christle JW, Hadley D, Froelicher V, Plews D (2018) Heart rate variability: an old metric with new meaning in the era of using mhealth technologies for health and exercise training guidance. Part two: prognosis and training. Arrhythm Electrophysiol Rev 7(4):247
Hernando A, Peláez-Coca MD, Lozano MT, Aiger M, Izquierdo D, Sánchez A et al (2018) Autonomic nervous system measurement in hyperbaric environments using ECG and PPG signals. IEEE J Biomed Health Inform 23(1):132–142
Castaneda D, Esparza A, Ghamari M, Soltanpur C, Nazeran H (2018) A review on wearable photoplethysmography sensors and their potential future applications in health care. Int J Biosens Bioelectron 4(4):195
Elgendi M, Fletcher R, Liang Y, Howard N, Lovell NH, Abbott D et al (2019) The use of photoplethysmography for assessing hypertension. NPJ Digit Med 2(1):1–11
Chakraborty A, Sadhukhan D, Mitra M (2019) An automated algorithm to extract time plane features from the PPG signal and its derivatives for personal health monitoring application. IETE J Res 1–13. https://doi.org/10.1080/03772063.2019.1604178
Elgendi M, Liang Y, Ward R (2018) Toward generating more diagnostic features from photoplethysmogram waveforms. Diseases 6(1):20
Pilt K, Ferenets R, Meigas K, Lindberg LG, Temitski K, Viigimaa M (2013) New photoplethysmographic signal analysis algorithm for arterial stiffness estimation. Sci World J 2013:1–9
Chakraborty A, Sadhukhan D, Pal S, Mitra M (2020) Automated myocardial infarction identification based on interbeat variability analysis of the photoplethysmographic data. Biomed Signal Process Control 57:101747
Ram MR, Madhav KV, Krishna EH, Komalla NR, Reddy KA (2011) A novel approach for motion artifact reduction in PPG signals based on AS-LMS adaptive filter. IEEE Trans Instrum Meas 61(5):1445–1457
Kim BS, Yoo SK (2006) Motion artifact reduction in photoplethysmography using independent component analysis. IEEE Trans Biomed Eng 53(3):566–568
Ram MR, Madhav KV, Krishna EH, Komalla NR, Sivani K, Reddy KA (2013) ICA-based improved DTCWT technique for MA reduction in PPG signals with restored respiratory information. IEEE Trans Instrum Meas 62(10):2639–2651
Goh CH, Tan LK, Lovell NH, Ng SC, Tan MP, Lim E (2020) Robust PPG motion artifact detection using a 1-D convolution neural network. Comput Methods Programs Biomed 196:105596
Tarvirdizadeh B, Golgouneh A, Tajdari F, Khodabakhshi E (2020) A novel online method for identifying motion artifact and photoplethysmography signal reconstruction using artificial neural networks and adaptive neuro-fuzzy inference system. Neural Comput Appl 32(8):3549–3566
Zhang Y, Song S, Vullings R, Biswas D, Simões-Capela N, Van Helleputte N et al (2019) Motion artifact reduction for wrist-worn photoplethysmograph sensors based on different wavelengths. Sensors 19(3):673
Lee J, Kim M, Park HK, Kim IY (2020) Motion artifact reduction in wearable photoplethysmography based on multi-channel sensors with multiple wavelengths. Sensors 20(5):1493
Motin MA, Karmakar CK, Palaniswami M (2017) Ensemble empirical mode decomposition with principal component analysis: a novel approach for extracting respiratory rate and heart rate from photoplethysmographic signal. IEEE J Biomed Health Inform 22(3):766–774
Khan E, Al Hossain F, Uddin SZ, Alam SK, Hasan MK (2015) A robust heart rate monitoring scheme using photoplethysmographic signals corrupted by intense motion artifacts. IEEE Trans Biomed Eng 63(3):550–562
Biswas A, Roy MS, Gupta R (2019) Motion artifact reduction from finger photoplethysmogram using discrete wavelet transform. In: Bhattacharyya S, Mukherjee A, Bhaumik H, Das S, Yoshida K (eds) Recent trends in signal and image processing. Springer, Singapore, pp 89–98
Ye Y, Cheng Y, He W, Hou M, Zhang Z (2016) Combining nonlinear adaptive filtering and signal decomposition for motion artifact removal in wearable photoplethysmography. IEEE Sens J 16(19):7133–7141
Zhang Z, Pi Z, Liu B (2014) TROIKA: a general framework for heart rate monitoring using wrist-type photoplethysmographic signals during intensive physical exercise. IEEE Trans Biomed Eng 62(2):522–531
Chung H, Ko H, Lee H, Lee J (2020) Deep learning for heart rate estimation from reflectance photoplethysmography with acceleration power spectrum and acceleration intensity. IEEE Access 8:63390–63402
Jarchi D, Casson AJ (2017) Description of a database containing wrist PPG signals recorded during physical exercise with both accelerometer and gyroscope measures of motion. Data 2(1):1
Lee H, Chung H, Lee J (2018) Motion artifact cancellation in wearable photoplethysmography using gyroscope. IEEE Sens J 19(3):1166–1175
Reiss A, Indlekofer I, Schmidt P, Van Laerhoven K (2019) Deep PPG: large-scale heart rate estimation with convolutional neural networks. Sensors 19(14):3079
Zhang Z (2015) Photoplethysmography-based heart rate monitoring in physical activities via joint sparse spectrum reconstruction. IEEE Trans Biomed Eng 62(8):1902–1910
Murthy NKL, Madhusudana PC, Suresha P, Periyasamy V, Ghosh PK (2015) Multiple spectral peak tracking for heart rate monitoring from photoplethysmography signal during intensive physical exercise. IEEE Signal Process Lett 22(12):2391–2395
Sun B, Zhang Z (2015) Photoplethysmography-based heart rate monitoring using asymmetric least squares spectrum subtraction and bayesian decision theory. IEEE Sens J 15(12):7161–7168
Zhang Y, Liu B, Zhang Z (2015) Combining ensemble empirical mode decomposition with spectrum subtraction technique for heart rate monitoring using wrist-type photoplethysmography. Biomed Signal Process Control 21:119–125
Fallet S, Vesin JM (2015) Adaptive frequency tracking for robust heart rate estimation using wrist-type photoplethysmographic signals during physical exercise. In: 2015 computing in cardiology conference (CinC). IEEE, pp 925–928
Salehizadeh S, Dao D, Bolkhovsky J, Cho C, Mendelson Y, Chon KH (2016) A novel time-varying spectral filtering algorithm for reconstruction of motion artifact corrupted heart rate signals during intense physical activities using a wearable photoplethysmogram sensor. Sensors 16(1):10
Mashhadi MB, Asadi E, Eskandari M, Kiani S, Marvasti F (2015) Heart rate tracking using wrist-type photoplethysmographic (PPG) signals during physical exercise with simultaneous accelerometry. IEEE Signal Process Lett 23(2):227–231
Fujita Y, Hiromoto M, Sato T (2017) PARHELIA: particle filter-based heart rate estimation from photoplethysmographic signals during physical exercise. IEEE Trans Biomed Eng 65(1):189–198
Chowdhury SS, Hyder R, Hafiz MSB, Haque MA (2016) Realtime robust heart rate estimation from wrist-type PPG signals using multiple reference adaptive noise cancellation. IEEE J Biomed Health Inform 22(2):450–459
Dubey H, Kumaresan R, Mankodiya K (2018) Harmonic sum-based method for heart rate estimation using PPG signals affected with motion artifacts. J Ambient Intell Humaniz Comput 9(1):137–150
Dao D, Salehizadeh SM, Noh Y, Chong JW, Cho CH, McManus D et al (2016) A robust motion artifact detection algorithm for accurate detection of heart rates from photoplethysmographic signals using time–frequency spectral features. IEEE J Biomed Health Inform 21(5):1242–1253
Farhadi M, Mashhadi MB, Essalat M, Marvasti F (2016) RealTime Heart Rate Monitoring Using photoplethysmographic (PPG) signals during intensive physical exercises. bioRxiv, 092627
Temko A (2017) Accurate heart rate monitoring during physical exercises using PPG. IEEE Trans Biomed Eng 64(9):2016–2024
Zhao D, Sun Y, Wan S, Wang F (2017) SFST: a robust framework for heart rate monitoring from photoplethysmography signals during physical activities. Biomed Signal Process Control 33:316–324
Islam MT, Zabir I, Ahamed ST, Yasar MT, Shahnaz C, Fattah SA (2017) A time-frequency domain approach of heart rate estimation from photoplethysmographic (PPG) signal. Biomed Signal Process Control 36:146–154
Galli A, Narduzzi C, Giorgi G (2017) Measuring heart rate during physical exercise by subspace decomposition and Kalman smoothing. IEEE Trans Instrum Meas 67(5):1102–1110
Islam MS, Shifat-E-Rabbi M, Dobaie AMA, Hasan MK (2017) PREHEAT: Precision heart rate monitoring from intense motion artifact corrupted PPG signals using constrained RLS and wavelets. Biomed Signal Process Control 38:212–223
Ye Y, He W, Cheng Y, Huang W, Zhang Z (2017) A robust random forest-based approach for heart rate monitoring using photoplethysmography signal contaminated by intense motion artifacts. Sensors 17(2):385
Nathan V, Jafari R (2017) Particle filtering and sensor fusion for robust heart rate monitoring using wearable sensors. IEEE J Biomed Health Inform 22(6):1834–1846
Chung H, Lee H, Lee J (2018) Finite state machine framework for instantaneous heart rate validation using wearable photoplethysmography during intensive exercise. IEEE J Biomed Health Inform 23(4):1595–1606
Islam MT, Ahmed ST, Zabir I, Shahnaz C, Fattah SA (2018) Cascade and parallel combination (CPC) of adaptive filters for estimating heart rate during intensive physical exercise from photoplethysmographic signal. Healthc Technol Lett 5(1):18–24
Biagetti G, Crippa P, Falaschetti L, Orcioni S, Turchetti C (2019) Reduced complexity algorithm for heart rate monitoring from PPG signals using automatic activity intensity classifier. Biomed Signal Process Control 52:293–301
Wang M, Li Z, Zhang Q, Wang G (2019) Removal of motion artifacts in photoplethysmograph sensors during intensive exercise for accurate heart rate calculation based on frequency estimation and notch filtering. Sensors 19(15):3312
Motin MA, Karmakar CK, Palaniswami M (2019) PPG derived heart rate estimation during intensive physical exercise. IEEE Access 7:56062–56069
Chung H, Lee H, Lee J (2019) State-dependent Gaussian kernel-based power spectrum modification for accurate instantaneous heart rate estimation. PLoS ONE 14(4):e0215014
Roy B, Gupta R (2020) MoDTRAP: improved heart rate tracking and preprocessing of motion-corrupted photoplethysmographic data for personalized healthcare. Biomed Signal Process Control 56:101676
Arunkumar KR, Bhaskar M (2020) Heart rate estimation from wrist-type photoplethysmography signals during physical exercise. Biomed Signal Process Control 57:101790
Arunkumar KR, Bhaskar M (2020) CASINOR: combination of adaptive filters using single noise reference signal for heart rate estimation from PPG signals. Signal Image Video Process 14:1507–1515
Yousefi R, Nourani M, Ostadabbas S, Panahi I (2013) A motion-tolerant adaptive algorithm for wearable photoplethysmographic biosensors. IEEE J Biomed Health Inform 18(2):670–681
Pang B, Liu M, Zhang X, Li P, Yao Z, Hu X et al (2016) Advanced EMD method using variance characterization for PPG with motion artifact. In: 2016 IEEE biomedical circuits and systems conference (BioCAS). IEEE, pp 196–199
Arenas-Garcia J, Azpicueta-Ruiz LA, Silva MT, Nascimento VH, Sayed AH (2015) Combinations of adaptive filters: performance and convergence properties. IEEE Signal Process Mag 33(1):120–140
Arunkumar KR, Bhaskar M (2019) Heart rate estimation from photoplethysmography signal for wearable health monitoring devices. Biomed Signal Process Control 50:1–9
Sharma H (2019) Heart rate extraction from PPG signals using variational mode decomposition. Biocybern Biomed Eng 39(1):75–86
Biswas D, Everson L, Liu M, Panwar M, Verhoef BE, Patki S et al (2019) CorNET: deep learning framework for PPG-based heart rate estimation and biometric identification in ambulant environment. IEEE Trans Biomed Circuits Syst 13(2):282–291
Roy MS, Gupta R, Chandra JK, Sharma KD, Talukdar A (2018) Improving photoplethysmographic measurements under motion artifacts using artificial neural network for personal healthcare. IEEE Trans Instrum Meas 67(12):2820–2829
Lei R, Ling BWK, Feng P, Chen J (2020) Estimation of heart rate and respiratory rate from PPG signal using complementary ensemble empirical mode decomposition with both independent component analysis and non-negative matrix factorization. Sensors 20(11):3238
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All Authors of this work declare no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pankaj, Kumar, A., Komaragiri, R. et al. A Review on Computation Methods Used in Photoplethysmography Signal Analysis for Heart Rate Estimation. Arch Computat Methods Eng 29, 921–940 (2022). https://doi.org/10.1007/s11831-021-09597-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11831-021-09597-4