Abstract
Purpose
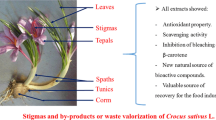
Saffron is widely used for its medicinal and culinary properties. Its stigmas are the most expensive part comparing with the flowers that are discarded during production. For that, the objective of this work was to determine the phytocomplex of stigma and flower material (except stigma) of saffron from Algeria. Crocin, picrocrocin and safranal contents were quantified to classify its quality according to ISO/TS 3632 standards. The antioxidant and antimicrobial properties of extracts were also investigated.
Methods
Crocins, total phenolic content, flavonoids, phenolic acids, and anthocyanins were detected and quantified by HPLC-DAD and spectrophotometric analyses. The antioxidant potential was evaluated by 4 in vitro assays. The antimicrobial activity against seven bacteria and two strains of Candida albicans was also evaluated.
Results
The results revealed that the chromatographic analysis showed the presence of 20 phenolic acids and flavonoids in the plant samples, with the highest concentrations in stigmas. Crocin derivatives were found only in stigmas, except that trans-crocetin (β-D-gentiobiosyl) which was present also in flower material. The highest total phenolic, total flavonoid and total flavonol contents were observed in stigmas and the highest level of anthocyanins and hydrolysable and condensed tannins in flowers. This extract showed a stronger protection effect from β-carotene bleaching and a higher TAC. The both extracts had some antimicrobial effect.
Conclusions
These results point out that flower material could be considered as natural bioresource of polyphenolic compounds, with higher biological activities which remain to be exploited.
Graphic Abstract



Similar content being viewed by others
References
Kamalipour, M., Akhondzadeh, S.: Cardiovascular effects of saffron: an evidence- based review. J. Tehran Heart Cent. 6(2), 59–61 (2011)
Dar, R.A., Shahnawaz, M., Malik, S.B., Sangale, M.K., Ade, A.B., Qazi, P.H.: Cultivation, distribution, taxonomy, chemical composition and medical importance of Crocus sativus. J. Phytopharmacol. 6(6), 356–358 (2017)
Ahmad Tantry, M., Ahmad Dar, B., Singh, S.: An economic analysis of production and marketing of saffron in Jammu and Kashmir. Int. J. Soc. Relev. Concern. 5(10), 12–19 (2017)
Abdullaev, F.: Biological properties and medicinal use of saffron (Crocus sativus L.). Acta Hort. (ISHS) 739, 339–345 (2007). https://doi.org/10.17660/ActaHortic.2007.739.44
Hosseini, A., Razavi, B.M., Hosseinzadeh, H.: Saffron (Crocus sativus) petal as a new pharmacological target: a review. Iran. J. Basic Med. Sci. 21(11), 1091–1099 (2018). https://doi.org/10.22038/IJBMS.2018.31243.7529
Pitsikas, N.: Constituents of saffron (Crocus sativus L.) as potential candidates for the treatment of anxiety disorders and schizophrenia. Molecules. 21(3), 303 (2016). https://doi.org/10.3390/molecules21030303
Rios, J., Recio, M., Giner, R., Manez, S.: An update review of saffron and its active constituents. Phytother. Res. 10(3), 189–193 (1996)
Xi, L., Qian, Z.: Pharmacological properties of crocetin and crocin (digentiobiosyl ester of crocetin) from saffron. Nat. Prod. Commun. 1(1), 65–75 (2006)
Caser, M., Demasi, S., Stelluti, S., Donno, D., Scariot, V.: Crocus sativus L. cultivation in alpine environments: stigmas and tepals as source of bioactive compounds. Agronomy 10, 1473 (2020). https://doi.org/10.3390/agronomy10101473
Soil Science Division Staff: Soil Survey Manual. In: Ditzler, C., Scheffe, K., Monger, H.C. (eds.) USDA Handbook 18. Government Printing Office, Washington, DC (2017)
Walkley, A., Black, I.A.: An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 37, 29–38 (1934)
Bremner, J.: Determination of nitrogen in soil by the Kjeldahl method. J. Agric. Sci. 55, 11–33 (1960)
ISO 3632-2:2010: Spices—Saffron (Crocus sativus L.). In: Part 2: Test Methods, 1st edn. International Organization for Standardization, Genève (2010)
Gismondi, A., Serio, M., Canuti, L., Canini, A.: Biochemical, antioxidant and antineoplastic properties of Italian saffron (Crocus sativus L.). Am J Plant Sci. 3(11), 1573–1580 (2012). https://doi.org/10.4236/ajps.2012.311190
Zanella, L., Gismondi, A., Di Marco, G., Braglia, R., Scuderi, F., Redi, E.L., Galgani, A., Canini, A.: Induction of antioxidant metabolites in Moringa oleifera callus by abiotic stresses. J. Nat. Prod. 82(9), 2379–2386 (2019). https://doi.org/10.1021/acs.jnatprod.8b00801
Singleton, V.L., Rossi, J.A.: Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 16, 144–158 (1965)
Zhishen, J., Mengcheng, T., Jianming, W.: The determination of flavonoid contents in mulberry and their scavenging effects on super oxide radicals. Food Chem. 64(4), 555–559 (1999). https://doi.org/10.1016/S0308-8146(98)00102-2
Kumaran, A., Karunakaran, R.J.: In vitro antioxidant activities of methanol extracts of five Phyllanthus species from India. LWT-Food Sci. Technol. 40(2), 344–352 (2007). https://doi.org/10.1016/j.lwt.2005.09.011
Julkunen-Titto, R.: Phenolic constituents in the levels of northern willows: methods for precursors of clarified apple juice sediment. J. Food Sci. 33, 254–257 (1985)
Mole, S., Waterman, P.: A critical analysis of techniques for measuring tannins in ecological studies. Oecologia 72(1), 137–147 (1987)
Wagner, G.J.: Content and vacuole/extra vacuole distribution of neutral sugars free amino acids, and anthocyanins in protoplast. Plant Physiol. 64, 88–93 (1979). https://doi.org/10.1104/pp.64.1.88
Prieto, P., Pineda, M., Aguilar, M.: Spectrophotometric quantitation of antioxidant capacity through the formation of a phospho-molybdenum complex: specific application to the determination of vitamin E. Anal. Biochem. 269, 337–341 (1999). https://doi.org/10.1006/abio.1999.4019
Sanchez-Moreno, C., Larrauri, J.: Main methods used in lipid oxidation determination. Food Sci. Technol. Int. 4, 391–399 (1998). https://doi.org/10.1177/108201329800400603
Oyaizu, M.: Studies on products of browning reaction prepared from glucose amine. Jpn. J. Nutr. 44, 307–315 (1986). https://doi.org/10.5264/eiyogakuzashi.44.307
Moure, A., Franco, D., Sineiro, J., Dominguez, H., Nunez, M.J., Lema, J.M.: Evaluation of extracts from Gevuina avellana hulls as antioxidants. J. Agri. Food Chem. 48, 3890–3897 (2000). https://doi.org/10.1021/jf000048w
NCCLS, “National Committee for Clinical Laboratory Standards”: Performance standards for antimicrobial susceptibility testing: eleventh informational supplement. M100–S11. NCCLS, Wayne (2001)
NCCLS, “National Committee for Clinical Laboratory Standards”: Reference method for broth dilution antifungal susceptibility testing of yeasts: approved standard. NCCLS, Wayne (1997)
CLSI: Performance standards for antimicrobial disk susceptibility tests; Approved standard Twelfth Edition. CLSI document M02-A12. Clinical and Laboratory Standards Institute, Wayne (2015)
Belyagoubi-Benhammou, N., Belyagoubi, L., Gismondi, A., Di Marco, G., Canini, A., Atik-Bekkara, F.: GC/MS analysis, and antioxidant and antimicrobial activities of alkaloids extracted by polar and a polar solvents from the stems of Anabasis articulata. J. Med. Chem. Res. 28, 754–767 (2019). https://doi.org/10.1007/s00044-019-02332-6
ISO 3632-1: Spices—Saffron (Crocus sativus L.). In: Part 1: Specification, 2nd edn. International Organization for Standardization, Genève (2011)
Lage, M., Cantrell, C.L.: Quantification of saffron (Crocus sativus L.) metabolites crocins, picrocrocin and safranal for quality determination of the spice grown under different environmental Moroccan conditions. Sci. Hortic. 121, 366–373 (2009). https://doi.org/10.1016/j.scienta.2009.02.017
Caballero-Ortega, H., Pereda-Miranda, R., Riveron-Negrete, L., Hernandez, J.M., Medecigo-Rıos, M., Castillo-Villanueva, A.: Chemical composition of saffron (Crocus sativus L.) from four countries. In: Ferna´ ndez, J.A., Abdullaev, F.I. (eds.) Proceedings of the First International Symposium on Saffron Biology and Biotechnology, Acta Horticulture, pp. 321–326. International Society for Horticultural Science, Leuven (2004)
Sujata, V., Ravishankar, A., Venkataraman, L.V.: Methods for the analysis of the saffron metabolites crocin, crocetins, picrocrocin and safranal for the determination of the quality of the spice using thin-layer chromatography, high performance liquid chromatography and gas chromatography. J. Chromatogr. 624, 497–502 (1992). https://doi.org/10.1016/0021-9673(92)85699-T
Atyane, L.H., Molinet, J., Serghini, M.A., Dupuy, N., Maimouni, E.L.: Influence of drying process on safranal content in the Taliouine Saffron (Morocco): quantification by gas chromatography. J. Mater. Environ. Sci. 8(S), 4597–4603 (2017)
Moratalla-López, N., Bouhadida, N., Bagur, M.J., García-Rodríguez, M.V., Oueslati, S., Alonso, G.L.: Comparing Tunisian and Spanish saffron regarding their bioactive metabolites using HPLC and GC methods. Acta Hortic. 1184, 279–286 (2017). https://doi.org/10.17660/ActaHortic.2017.1184.40
Bononi, M., Milella, P., Tateo, F.: Gas chromatography of safranal as preferable method for the commercial grading of saffron (Crocus sativus L.). Food Chem. 176, 15–21 (2015). https://doi.org/10.1016/j.foodchem.2014.12.047
Alonso, G.L., Salinas, M.R., Garijo, J., Sánchez-Fernánde, M.A.: Composition of crocins and picrocrocin from spanish saffron (Crocus sativus L.). J. Food Qual. 24, 219–233 (2001). https://doi.org/10.1111/j.1745-4557.2001.tb00604.x
Menghini, L., Leporini, L., Vecchiotti, G., Locatelli, M., Carradori, S., Ferrante, C., Zengin, G., Recinella, L., Chiavaroli, A., Leone, S., Brunetti, L., Orlando, G.: Crocus sativus L. stigmas and byproducts: Qualitative fingerprint, antioxidant potentials and enzyme inhibitory activities. Food Res. Int. 109, 91–98 (2018). https://doi.org/10.1016/j.foodres.2018.04.028
Vahedi, M., Kabiri, M., Salami, A., Rezadoost, S., Mirzaie, H., Reza Kanani, M.: M.: Quantitative HPLC-based metabolomics of some Iranian saffron (Crocus sativus L.) accessions. Ind Crops Prod. 118, 26–29 (2018). https://doi.org/10.1016/j.indcrop.2018.03.024
Straubinger, M., Bau, B., Eckstein, S., Fink, M., Winterhalter, P.: Identification of novel glycosidic aroma precursors in saffron. J. Agric. Food Chem. 46, 3238–3243 (1998). https://doi.org/10.1021/jf980119f
Carmona, M., Zalacain, A., Pardo, J.E., López, E., Alvarruiz, A., Alonso, G.L.: Influence of different drying and aging conditions on saffron constituents. Agric. Food Chem. 53, 3974–3979 (2005). https://doi.org/10.1021/jf0404748
Chaouqi, S., Moratalla-López, N., Lage, M., Lorenzo, C., Alonso, G.L., Guedira, T.: Effect of drying and storage process on Moroccan saffron quality. Food Biosci. 22, 146–153 (2018). https://doi.org/10.1016/j.fbio.2018.02.003
Tarantilis, P.A., Tsoupras, G., Polissiou, M.: Determination of saffron (Crocus sativus L.) components in crude plant extract using high-performance liquid chromatography-UV–visible photodiode-array detection-mass spectrometry. J. Chromatogr. 699, 107–118 (1995). https://doi.org/10.1016/0021-9673(95)00044-N
Moratalla-Lopez, N., Sanchez, A.M., Lorenzo, C., Lopez-Corcoles, H., Alonso, G.L.: Quality determination of Crocus sativus L. flower by high-performance liquid chromatography. J. Food Compost. Anal. 93, 103613 (2020). https://doi.org/10.1016/j.jfca.2020.103613
Vignolini, P., Heimler, D., Pinelli, P., Ieri, F., Sciullo, A., Romani, A.: Characterization of by-products of saffron (Crocus sativus L.) production. Nat. Prod. Commun. 3(12), 1959–1962 (2008). https://doi.org/10.1177/1934578X0800301203
Termentzi, A., Kokkalou, E.: LC-DAD-MS (ESI+) analysis and antioxidant capacity of Crocus sativus petal extracts. Planta Med. 74, 573–581 (2008). https://doi.org/10.1055/s-2008-1074498
Serrano-Díaz, J., Sánchez, A.M., Maggi, L., Martínez-Tomé, M., Garcia-Diz, L., Murcia, A., Alonso, M.: Increasing the applications of Crocus sativus flowers as natural antioxidants. J. Food Sci. 77(11), C1162–C1168 (2012). https://doi.org/10.1111/j.1750-3841.2012.02926.x
Jadouali, S.M., Atifi, H., Bouzoubaa, Z., Majourhat, K., Gharby, S., Achemchem, F., Elmoslih, A., Laknifli, A., Mamouni, R.: Chemical characterization, antioxidant and antibacterial activity of Moroccan Crocus sativus L. petals and leaves. J. Mater. Environ. Sci. 9(1), 113–118 (2018). https://doi.org/10.26872/jmes.2018.9.1.14
Jadouali, S.M., Atifi, H., Mamouni, R., Majourhat, K., Bouzoubaâ, Z., Laknifli, A., Faouzi, A.: Chemical characterization and antioxidant compounds of flower parts of Moroccan Crocus sativus L. J. Saudi Soc. Agric. Sci. 18, 476–480 (2019). https://doi.org/10.1016/j.jssas.2018.03.007
Azizian–Shermeh, O., Valizadeh, M., Taherizadeh, M., Beigomi, M.: Phytochemical investigation and phytosynthesis of eco–friendly stable bioactive gold and silver nanoparticles using petal extract of saffron (Crocus sativus L.) and study of their antimicrobial activities. Appl. Nanosci. 10, 2921–2922 (2020). https://doi.org/10.1007/s13204-019-01059-5
Babaei, A., Arshami, J., Haghparast, A., Danesh Mesgaran, M.: Effects of Saffron (Crocus sativus) petal ethanolic extract on hematology, antibody response, and spleen histology in rats. Avecina J. Phytomed. 4, 103–109 (2014)
Tanaka, Y., Sasaki, N., Ohmiya, A.: Biosynthesis of plant pigments: anthocyanins, betalains and carotenoids. Plant J 54(4), 733–749 (2008). https://doi.org/10.1111/j.1365-313X.2008.03447.x
Serrano-Díaz, J., Sánchez, A.M., Martínez-Tomé, M., Winterhalter, P., Alonso, G.L.: Flavonoid determinationin the quality control of floral bioresidues from Crocus sativus L. J. Agric. Food Chem. 62, 3125–3133 (2014). https://doi.org/10.1021/jf4057023
Cusano, E., Consonni, R., Petrakis, E.A., Astraka, K., Cagliani, L.R., Polissiou, M.G.: Integrated analytical methodology to investigate bioactive compounds in Crocus sativus L. flowers. Phytochem. Anal. 29(5), 1–11 (2018). https://doi.org/10.1002/pca.2753
Moratalla-López, N., Bagur, M.J., Lorenzo, C., Martínez-Navarro, M.E., Salinas, M.R., Alonso, G.L.: Bioactivity and bioavailability of the major metabolites of Crocus sativus L. flower. Molecules 24(15), 2827 (2019). https://doi.org/10.3390/molecules24152827
Lahmass, I., Ouahhoud, S., Elmansuri, M., Sabouni, A., Elyoubi, M., Benabbas, R., Choukri, M., Saalaoui, E.: Determination of antioxidant properties of six by-products of Crocus sativus L. (Saffron) plant products. Waste Biomass Valor. 9(8), 1349–1357 (2018). https://doi.org/10.1007/s12649-017-9851-y
Lahmass, I., Ouahhoud, S., Elyoubi, M., Benabbas, R., Sabouni, A., Asehraou, A., Saalaoui, E.: Evaluation of antioxidant activities of saffron stigma and spath as by-product of Crocus sativus L. MOJ Biol Med. 3(4), 154–158 (2018). https://doi.org/10.15406/mojbm.2018.03.00091
Asdaq, S.M., Inamdar, M.N.: Potential of Crocus sativus (saffron) and its constituent, crocin, as hypolipidemic and antioxidant in rats. Appl. Biochem. Biotechnol. 162(2), 358–372 (2010). https://doi.org/10.1007/s12010-009-8740-7
Fazeli-Nasab, B.: Evaluation of antibacterial activities of hydroalcoholic extract of saffron petals on some bacterial pathogens. J. Med. Bacteriol. 8(5), 8–20 (2019)
Muzaffar, S., Rather, S.A., Khan, K.Z., Yildiz, F.: In vitro bactericidal and fungicidal activities of various extracts of saffron (Crocus sativus L.) stigmas from Jammu & Kashmir, India. Cogent. Food & Agric. 2(1), 1158999 (2016). https://doi.org/10.1080/23311932.2016.1158999
Wali, A.F., Abou Alchamat, H.A., Hariri, H.K., Hariri, B.K., Menezes, G.A., Zehra, U., Rehman, M.U., Ahmad, P.: Antioxidant, antimicrobial, antidiabetic and cytotoxic activity of Crocus sativus L. petals. Appl. Sci. 10, 1519 (2020). https://doi.org/10.3390/app10041519
De Monte, C., Bizzarri, B., Gidaro, M.C., Carradori, S., Mollica, A., Luisi, G., Granese, A., Alcaro, S., Costa, G., Basilico, N., Parapini, S., Scaltrito, M.M., Masia, C., Sisto, F.: Bioactive compounds of Crocus sativus L. and their semi-synthetic derivatives as promising anti-Helicobacter pylori, anti-malarial and anti-leishmanial agents. J. Enzyme Inhib. Med. Chem. 30(6), 1027–1033 (2015). https://doi.org/10.3109/14756366.2014.1001755
Hussein, R.A., Salih, N.A., Nadhaif, E.T.: Bioactivity of crocin pigment of saffron plant. Plant Arch. 18(1), 357–364 (2018)
Buzzini, P., Arapitsas, P., Goretti, M., Branda, E., Turchetti, B., Pinelli, P., Ieri, F., Romani, A.: Antimicrobial and antiviral activity of hydrolysable tannins. Mini-Rev. Med. Chem. 8(12), 1179–1187 (2008). https://doi.org/10.2174/138955708786140990
Sun, X.H., Zhou, T.T., Wei, C.H., Lan, W.Q., Zhao, Y., Pan, Y.J., Wu, V.C.H.: Antibacterial effect and mechanism of anthocyanin rich Chinese wild blueberry extract on various foodborne pathogens. Food Control. 94, 155–161 (2018). https://doi.org/10.1016/j.foodcont.2018.07.012
Acknowledgements
The authors acknowledge the saffron producer in this region for providing plant material. The authors also thank Dr. BETTIOUI Reda for his help in statistical analysis with PCA.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Belyagoubi, L., Loukidi, B., Belyagoubi-Benhammou, N. et al. Valorization of Algerian Saffron: Stigmas and Flowers as Source of Bioactive Compounds. Waste Biomass Valor 12, 6671–6683 (2021). https://doi.org/10.1007/s12649-021-01454-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-021-01454-6