Abstract
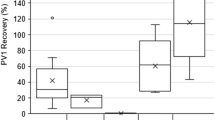
Concentration of viruses in water is necessary for detection and quantification of the viruses present, in order to evaluate microbiological barriers in water treatment plants and detect pathogenic viruses during waterborne outbreaks, but there is currently no standardised procedure. In this study, we implemented a previously described fast and simple lanthanum-based protocol for concentration of norovirus genogroup I (GI), genogroup II (GII) and hepatitis A virus (HAV) in drinking and surface water. We compared the results with those of a widely used skimmed milk flocculation method, followed by nucleic acid extraction and RT-qPCR detection. Three seeding levels, with intended concentrations 5 × 103, 5 × 104 and 5 × 105 genome copies/10 L, were added to drinking water or surface water. All seed levels were detected with both flocculation methods. Samples extracted with skimmed milk flocculation had on average 1.82, 1.86 and 1.38 times higher measured concentration of norovirus GI, GII and HAV, respectively, than those extracted with lanthanum flocculation, across all seeding levels and water types tested. Mengovirus was used as a positive process control. Mengovirus recovery was higher for skimmed milk (40.7% in drinking water, 26.0% in surface water) than for lanthanum flocculation (24.4% in drinking water, 9.7% in surface water). Together, this indicates that skimmed milk flocculation provides higher viral recovery than lanthanum flocculation. However, lanthanum-based flocculation can be performed much faster than skimmed milk flocculation (1.5 h versus 16 h flocculation time) and thus could be a good alternative for rapid monitoring of viruses in water.




Similar content being viewed by others
Data Availability
Available upon request.
Code Availability
Not appilcable.
References
Bitton, G., Feldberg, B. N., & Farrah, S. R. (1979). Concentration of enteroviruses from seawater and tapwater by organic flocculation using non-fat dry milk and casein. Water, Air, and Soil Pollution, 12, 187–195
Borgmastars, E., Jazi, M. M., Persson, S., Jansson, L., Radstrom, P., Simonsson, M., et al. (2017). Improved detection of norovirus and hepatitis a virus in surface water by applying pre-PCR processing. Food Environ Virol, 9(4), 395–405. https://doi.org/10.1007/s12560-017-9295-3
Calgua, B., Fumian, T., Rusinol, M., Rodriguez-Manzano, J., Mbayed, V. A., Bofill-Mas, S., et al. (2013). Detection and quantification of classic and emerging viruses by skimmed-milk flocculation and PCR in river water from two geographical areas. Water Research, 47(8), 2797–2810. https://doi.org/10.1016/j.watres.2013.02.043
Calgua, B., Mengewein, A., Grunert, A., Bofill-Mas, S., Clemente-Casares, P., Hundesa, A., et al. (2008). Development and application of a one-step low cost procedure to concentrate viruses from seawater samples. Journal of Virological Methods, 153(2), 79–83. https://doi.org/10.1016/j.jviromet.2008.08.003
Calgua, B., Rodriguez-Manzano, J., Hundesa, A., Sunen, E., Calvo, M., Bofill-Mas, S., et al. (2013). New methods for the concentration of viruses from urban sewage using quantitative PCR. Journal of Virological Methods, 187(2), 215–221. https://doi.org/10.1016/j.jviromet.2012.10.012
Chlumecka, V., D’Obrenan, P., & Colter, J. S. (1973). Electrophoretic studies on three variants of Mengo encephalomyelitis virus. Canadian Journal of Biochemistry, 51(11), 1521–1526. https://doi.org/10.1139/o73-202
Costafreda, M. I., Bosch, A., & Pinto, R. M. (2006). Development, evaluation, and standardization of a real-time TaqMan reverse transcription-PCR assay for quantification of hepatitis A virus in clinical and shellfish samples. Applied and Environmental Microbiology, 72(6), 3846–3855
da Silva, A. K., Le Saux, J. C., Parnaudeau, S., Pommepuy, M., Elimelech, M., & Le Guyader, F. S. (2007). Evaluation of removal of noroviruses during wastewater treatment, using real-time reverse transcription-PCR: different behaviors of genogroups I and II. Applied and Environmental Microbiology, 73(24), 7891–7897
Feng, Z., Hensley, L., McKnight, K. L., Hu, F., Madden, V., Ping, L., et al. (2013). A pathogenic picornavirus acquires an envelope by hijacking cellular membranes. Nature, 496(7445), 367–371. https://doi.org/10.1038/nature12029
Fernandez-Cassi, X., Timoneda, N., Martinez-Puchol, S., Rusinol, M., Rodriguez-Manzano, J., Figuerola, N., et al. (2018). Metagenomics for the study of viruses in urban sewage as a tool for public health surveillance. Science of the Total Environment, 618, 870–880. https://doi.org/10.1016/j.scitotenv.2017.08.249
Gonzales-Gustavson, E., Cardenas-Youngs, Y., Calvo, M., da Silva, M. F., Hundesa, A., Amoros, I., et al. (2017). Characterization of the efficiency and uncertainty of skimmed milk flocculation for the simultaneous concentration and quantification of water-borne viruses, bacteria and protozoa. Journal of Microbiol Methods, 134, 46–53
Goodridge, L., Goodridge, C., Wu, J., Griffiths, M., & Pawliszyn, J. (2004). Isoelectric point determination of norovirus virus-like particles by capillary isoelectric focusing with whole column imaging detection. Analytical Chemistry, 76(1), 48–52. https://doi.org/10.1021/ac034848s
Guzman-Herrador, B., Carlander, A., Ethelberg, S., Freiesleben de Blasio, B., Kuusi, M., Lund, V., et al. (2015). Waterborne outbreaks in the Nordic countries, 1998 to 2012. Eurosurveillance Weekly. https://doi.org/10.2807/1560-7917.es2015.20.24.21160
Hellmer, M., Paxeus, N., Magnius, L., Enache, L., Arnholm, B., Johansson, A., et al. (2014). Detection of pathogenic viruses in sewage provided early warnings of hepatitis A virus and norovirus outbreaks. Applied and Environment Microbiology, 80(21), 6771–6781. https://doi.org/10.1128/AEM.01981-14
Hoehne, M., & Schreier, E. (2006). Detection of Norovirus genogroup I and II by multiplex real-time RT- PCR using a 3’-minor groove binder-DNA probe. BMC Infectious Diseases, 6, 69
Huang, X. Y., Su, J., Lu, Q. C., Li, S. Z., Zhao, J. Y., Li, M. L., et al. (2017). A large outbreak of acute gastroenteritis caused by the human norovirus GII.17 strain at a university in Henan Province. China. Infect Dis Poverty, 6(1), 6. https://doi.org/10.1186/s40249-017-0236-z
Huggett, J. F., Novak, T., Garson, J. A., Green, C., Morris-Jones, S. D., Miller, R. F., et al. (2008). Differential susceptibility of PCR reactions to inhibitors: an important and unrecognised phenomenon. BMC Research Notes, 1, 70. https://doi.org/10.1186/1756-0500-1-70
ISO (2017). ISO 15216–1 Microbiology of the food chain - Horizontal method for determination of Hepatitis A virus and norovirus using real-time PCR, part 1: Method for quantification. In ISO (Ed.), (First edition 2017–03 ed.). Switzerland.
Jansons, J., & Bucens, M. R. (1986). Virus detection in water by ultrafiltration. Water Research, 20(12), 1603–1606
Kapsch, A. M., Farcet, M. R., Antoine, G., & Kreil, T. R. (2017). A nonenveloped virus with a lipid envelope: hepatitis A virus as used in virus-reduction studies. Transfusion, 57(6), 1433–1439. https://doi.org/10.1111/trf.14091
Kotwal, G., & Cannon, J. L. (2014). Environmental persistence and transfer of enteric viruses. Current Opinion in Virology, 4, 37–43. https://doi.org/10.1016/j.coviro.2013.12.003
Loisy, F., Atmar, R. L., Guillon, P., Le Cann, P., Pommepuy, M., & Le Guyader, F. S. (2005). Real-time RT-PCR for norovirus screening in shellfish. Journal of Virological Methods, 123(1), 1–7
Michen, B., & Graule, T. (2010). Isoelectric points of viruses. Journal of Applied Microbiology, 109(2), 388–397. https://doi.org/10.1111/j.1365-2672.2010.04663.x
Nenonen, N. P., Hannoun, C., Larsson, C. U., & Bergstrom, T. (2012). Marked genomic diversity of norovirus genogroup I strains in a waterborne outbreak. Applied and Environment Microbiology, 78(6), 1846–1852. https://doi.org/10.1128/AEM.07350-11
Pinto, R. M., Costafreda, M. I., & Bosch, A. (2009). Risk assessment in shellfish-borne outbreaks of hepatitis A. Applied and Environmental Microbiology, 75(23), 7350–7355
R Core Team (2020). R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing.
Seitz, S. R., Leon, J. S., Schwab, K. J., Lyon, G. M., Dowd, M., McDaniels, M., et al. (2011). Norovirus infectivity in humans and persistence in water. Applied and Environment Microbiology, 77(19), 6884–6888
Shin, E., Kim, J. S., Oh, K. H., Oh, S. S., Kwon, M., Kim, S., et al. (2017). A waterborne outbreak involving hepatitis A virus genotype IA at a residential facility in the Republic of Korea in 2015. Journal of Clinical Virology, 94, 63–66
Smith, C. M., & Hill, V. R. (2009). Dead-end hollow-fiber ultrafiltration for recovery of diverse microbes from water. Applied and Environment Microbiology, 75(16), 5284–5289
Svraka, S., Duizer, E., Vennema, H., de Bruin, E., van der Veer, B., Dorresteijn, B., et al. (2007). Etiological role of viruses in outbreaks of acute gastroenteritis in The Netherlands from 1994 through 2005. Journal of Clinical Microbiology, 45(5), 1389–1394
Teunis, P. F., Moe, C. L., Liu, P., Miller, S. E., Lindesmith, L., Baric, R. S., et al. (2008). Norwalk virus: how infectious is it? Journal of Medical Virology, 80(8), 1468–1476. https://doi.org/10.1002/jmv.21237
Tornevi, A., Simonsson, M., Forsberg, B., Säve-Söderbergh, M., & Toljander, J. (2016). Efficacy of water treatment processes and endemic gastrointestinal illness - A multi-city study in Sweden. Water Research, 102, 263–270
Vilaginès, P., Sarrette, B., Husson, G., & Vilaginès, R. (1993). Glass wool for virus concentration at ambient water pH level. Water Science and Technology, 27(3–4), 299–306
Yezli, S., & Otter, J. A. (2011). Minimum infective dose of the major human respiratory and enteric viruses transmitted through food and the environment. Food and Environmental Virology, 3(1), 1–30
Zhang, Y., Riley, L. K., Lin, M., & Hu, Z. (2010). Lanthanum-based concentration and microrespirometric detection of microbes in water. Water Research, 44(11), 3385–3392
Zhang, Y., Riley, L. K., Lin, M., Purdy, G. A., & Hu, Z. (2013). Development of a virus concentration method using lanthanum-based chemical flocculation coupled with modified membrane filtration procedures. Journal of Virological Methods, 190(1–2), 41–48. https://doi.org/10.1016/j.jviromet.2013.03.017
Zlot, A., Simckes, M., Vines, J., Reynolds, L., Sullivan, A., Scott, M. K., et al. (2015). Norovirus outbreak associated with a natural lake used for recreation-oregon, 2014. American Journal of Transplantation, 15(7), 2001–2005. https://doi.org/10.1111/ajt.13404
Acknowledgements
This study was funded by the Swedish Civil Contingencies Agency, within the projects “Ökad förmåga till detektion av virus i livsmedel och humanprover för förbättrad smittspårning”, and “Stärkt beredskapskapacitet via rationell laboratoriediagnostik samt förenklad provberedning, pre-PCR processing”. The authors would like to thank Norrvatten (Görväln vattenverk) for surface water samples, Kåre Bondesson at the University Hospital in Uppsala for providing positive norovirus stool samples, and Anna Charlotte Schultz at Technical University of Denmark for cell culture supernatant from mengovirus.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Ethical Approval
Stool samples used in this study have been de-identified and thus no ethical approval was needed according to the Swedish Act concerning the Ethical Review of Research Involving Humans (2003:460).
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Borgmästars, E., Persson, S., Hellmér, M. et al. Comparison of Skimmed Milk and Lanthanum Flocculation for Concentration of Pathogenic Viruses in Water. Food Environ Virol 13, 380–389 (2021). https://doi.org/10.1007/s12560-021-09477-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12560-021-09477-x