Abstract
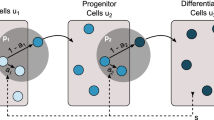
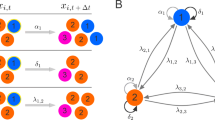
Hematopoiesis is a biological phenomenon (process) of production of mature blood cells by cellular differentiation. It is based on amplification steps due to an interplay between renewal and differentiation in the successive cell types from stem cells to mature blood cells. We will study this mechanism with a stochastic point of view to explain unexpected fluctuations on the mature blood cell number, as surprisingly observed by biologists and medical doctors in a rest hematopoiesis. We consider three cell types: stem cells, progenitors and mature blood cells. Each cell type is characterized by its own dynamics parameters, the division rate and the renewal and differentiation probabilities at each division event. We model the global population dynamics by a three-dimensional stochastic decomposable branching process. We show that the amplification mechanism is given by the inverse of the small difference between the differentiation and renewal probabilities. Introducing a parameter K which scales simultaneously the size of the first component, the differentiation and renewal probabilities and the mature blood cell death rate, we describe the asymptotic behavior of the process for large K. We show that each cell type has its own size and time scales. Focusing on the third component, we prove that the mature blood cell population size, conveniently renormalized (in time and size), is expanded in an unusual way inducing large fluctuations. The proofs are based on a fine study of the different scales involved in the model and on the use of different convergence and average techniques in the proofs.





Similar content being viewed by others
References
Abkowitz JL, Golinelli D, Harrison DE, Guttorp P (2000) In vivo kinetics of murine hemopoietic stem cells. Blood 96(10):3399–3405
Abkowitz J, Catlin S, McCallie M, Guttorp P (2002) Evidence that the number of hematopoietic stem cells per animal is conserved in mammals. Blood 100(7):2665–2667. https://doi.org/10.1182/blood-2002-03-0822
Arino O, Kimmel M (1986) Stability analysis of models of cell production systems. Math Model 7(9–12):1269–1300
Axelrod D, Kimmel M (2002) Branching processes in biology. Springer, New York
Bansaye V, Meleard S (2015) Stochastic models for structured populations: scaling limits and long time behavior. Mathematical biosciences institute lecture series. Springer International Publishing, Cham
Bonnet C, Gou P, Girel S, Bansaye V, Lacout C, Bailly K, Schlagetter MH, Lauret E, Meleard S, Giraudier S (2021) Combined biological and modeling approach of hematopoiesis: from native to stressed erythropoiesis. https://ssrn.com/abstract=3777468 or http://dx.doi.org/10.2139/ssrn3777468
Busch K, Klapproth K, Barile M, Flossdorf M, Holland-Letz T, Schlenner S, Reth M, Höfer T, Rodewald HR (2015) Fundamental properties of unperturbed haematopoiesis from stem cells in vivo. Nature 18(518):542–546. https://doi.org/10.1038/nature14242
Crauste F, Pujo-Menjouet L, Génieys S, Molina C, Gandrillon O (2008) Adding self-renewal in committed erythroid progenitors improves the biological relevance of a mathematical model of erythropoiesis. J Theor Biol 250(2):322–338
Dingli D, Traulsen A, Pacheco J (2007) Compartmental architecture and dynamics of hematopoiesis. PLoS ONE 2:e345
Domen J, Cheshier SH, Weissman IL (2000) The role of apoptosis in the regulation of hematopoietic stem cells: overexpression of BCL-2 increases both their number and repopulation potential. J Exp Med 191(2):253–264. https://doi.org/10.1084/jem.191.2.253
Ethier S, Kurtz T (1986) Markov processes—characterization and convergence. Wiley series in probability and mathematical statistics: probability and mathematical statistics. Wiley, New York
Gonzalez M, Puerto IM, Martinez R, Molina M, Mota M, Ramos A (2010) Workshop on branching processes and their applications, vol 197. Springer, Cham
Ikeda N, Watanabe S (1989) Stochastic differential equations and diffusion processes, 2nd edn. North-Holland, Amsterdam
Joffe A, Metivier M (1986) Weak convergence of sequences of semimartingales with applications to multitype branching processes. Adv Appl Probab 18(1):20–65
Kang HW, Kurtz TG, Popovic L (2014) Central limit theorems and diffusion approximations for multiscale Markov chain models. Ann Appl Probab 24(2):721–759
Khammash M, Munsky B, Peleš S (2006) Reduction and solution of the chemical master equation using time scale separation and finite state projection. J Chem Phys 125(20):204104
Kimmel M, Wazewska-Czyzewska M (1982) Stochastic approach to the process of red cell destruction. Appl Math 2(17):217–225
Kurtz T (1992) Averaging for martingale problems and stochastic approximation. In: Applied stochastic analysis. Springer, pp 186–209
Lei J, Mackey MC (2007) Stochastic differential delay equation, moment stability, and application to hematopoietic stem cell regulation system. SIAM J Appl Math 67(2):387–407
Loeffler M, Wichmann H (1980) A comprehensive mathematical model of stem cell proliferation which reproduces most of the published experimental results. Cell Prolif 13(5):543–561
Marciniak-Czochra A, Stiehl T, Ho AD, Jäger W, Wagner W (2009) Modeling of asymmetric cell division in hematopoietic stem cells—regulation of self-renewal is essential for efficient repopulation. Stem Cells Dev 18(3):377–386
Pazdziorek PR (2014) Mathematical model of stem cell differentiation and tissue regeneration with stochastic noise. Bull Math Biol 76(7):1642–1669
Roeder I, Loeffler M (2002) A novel dynamic model of hematopoietic stem cell organization based on the concept of within-tissue plasticity. Exp Hematol 30(8):853–861
Thirup P (2003) Haematocrit. Sports Med 33(3):231–243
Till JE, McCulloch EA, Siminovitch L (1964) A stochastic model of stem cell proliferation, based on the growth of spleen colony-forming cells. Proc Natl Acad Sci USA 51(1):29
Whichard ZL, Sarkar CA, Kimmel M, Corey SJ (2010) Hematopoiesis and its disorders: a systems biology approach. Blood J Am Soc Hematol 115(12):2339–2347
Acknowledgements
We warmly thank Vincent Bansaye, the hematologist Stéphane Giraudier and the biologist Evelyne Lauret for exciting and fruitful discussions which have motivated this work. We also thank Vincent Bansaye for his precious comments on our paper. This work was supported by a grant from Région Île-de-France.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
Lemma 3
(Lemma 2.9 of Kang et al. 2014) Let \(V^N\) be a sequence of \({\mathbb {R}} _+^{3}\)-valued processes. We consider its occupation measure defined for D a Borelian set by
Let us assume that there exists a function \(\psi : {\mathbb {R}} _+^{3} \rightarrow [1,\infty )\) locally bounded such that \(\lim _{v \rightarrow +\infty } \psi (v)= +\infty \) and such that for each \(t>0\),
Then \({\varGamma _N}\) is relatively compact, and if \(\varGamma _N\) converges in law to \(\varGamma \), then for \(f_1,\dots ,f_m \in D_{\psi }\),
where \(D_{\psi }\) denote the collection of continuous functions f satisfying
Rights and permissions
About this article
Cite this article
Bonnet, C., Méléard, S. Large fluctuations in multi-scale modeling for rest hematopoiesis. J. Math. Biol. 82, 58 (2021). https://doi.org/10.1007/s00285-021-01611-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00285-021-01611-4
Keywords
- Decomposable branching process
- Multiscale approximation
- Stochastic slow–fast dynamical system
- Large fluctuations
- Rest hematopoiesis
- Amplification mechanism