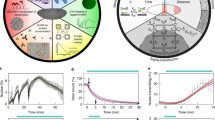
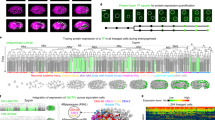
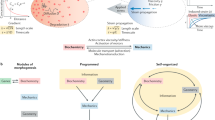
Abstract
The temporal coordination of events at cellular and tissue scales is essential for the proper development of organisms, and involves cell-intrinsic processes that can be coupled by local cellular signalling and instructed by global signalling, thereby creating spatial patterns of cellular states that change over time. The timing and structure of these patterns determine how an organism develops. Traditional developmental genetic methods have revealed the complex molecular circuits regulating these processes but are limited in their ability to predict and understand the emergent spatio-temporal dynamics. Increasingly, approaches from physics are now being used to help capture the dynamics of the system by providing simplified, generic descriptions. Combined with advances in imaging and computational power, such approaches aim to provide insight into timing and patterning in developing systems.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Zaret, K. S. Pioneer transcription factors initiating gene network changes. Annu. Rev. Genet. 54, 367–385 (2020).
Peter, I. S. & Davidson, E. H. Assessing regulatory information in developmental gene regulatory networks. Proc. Natl Acad. Sci. USA 114, 5862–5869 (2017).
Bolouri, H. & Davidson, E. H. Modeling DNA sequence-based cis-regulatory gene networks. Dev. Biol. 246, 2–13 (2002).
Davidson, E. H. & Levine, M. S. Properties of developmental gene regulatory networks. Proc. Natl Acad. Sci. USA 105, 20063–20066 (2008).
DiFrisco, J. & Jaeger, J. Genetic causation in complex regulatory systems: an integrative dynamic perspective. BioEssays 42, 1900226 (2020).
Rodriguez, E. A. et al. The growing and glowing toolbox of fluorescent and photoactive proteins. Trends Biochem. Sci. 42, 111–129 (2017).
Lee, H. N., Mehta, S. & Zhang, J. Recent advances in the use of genetically encodable optical tools to elicit and monitor signaling events. Curr. Opin. Cell Biol. 63, 114–124 (2020).
Shakirova, K. M., Ovchinnikova, V. Y. & Dashinimaev, E. B. Cell reprogramming with CRISPR/Cas9 based transcriptional regulation systems. Front. Bioeng. Biotechnol. 8, 882 (2020).
Santos-Moreno, J., Tasiudi, E., Stelling, J. & Schaerli, Y. Multistable and dynamic CRISPRi-based synthetic circuits. Nat. Commun. 11, 2746 (2020).
Royer, L. A., Lemon, W. C., Chhetri, R. K. & Keller, P. J. A practical guide to adaptive light-sheet microscopy. Nat. Protoc. 13, 2462–2500 (2018).
Reynaud, E. G., Peychl, J., Huisken, J. & Tomancak, P. Guide to light-sheet microscopy for adventurous biologists. Nat. Methods 12, 30–34 (2015).
Liu, T.-L. et al. Observing the cell in its native state: Imaging subcellular dynamics in multicellular organisms. Science 360, eaaq1392 (2018).
Sbalzarini, I. F. Modeling and simulation of biological systems from image data. BioEssays 35, 482–490 (2013).
Marcon, L., Diego, X., Sharpe, J. & Müller, P. High-throughput mathematical analysis identifies Turing networks for patterning with equally diffusing signals. eLife 5, e14022 (2016).
Guckenheimer, J., Holmes, P., Guckenheimer, J. & Sirovich, L. Nonlinear Oscillations, Dynamical Systems, and Bifurcations of Vector Fields (Springer, 2013).
Hohenberg, P. C. & Krekhov, A. P. An introduction to the Ginzburg–Landau theory of phase transitions and nonequilibrium patterns. Phys. Rep. 572, 1–42 (2015).
Schweisguth, F. & Corson, F. Self-organization in pattern formation. Dev. Cell 49, 659–677 (2019).
Hamant, O. & Saunders, T. E. Shaping organs: shared structural principles across kingdoms. Annu. Rev. Cell Dev. Biol. 36, 385–410 (2020).
Ayad, N. M. E., Kaushik, S. & Weaver, V. M. Tissue mechanics, an important regulator of development and disease. Philos. Trans. R. Soc. B Biol. Sci. 374, 20180215 (2019).
Naganathan, S. R. & Oates, A. C. Mechanochemical coupling and developmental pattern formation. Curr. Opin. Syst. Biol. 5, 104–111 (2017).
Hannezo, E. & Heisenberg, C.-P. Mechanochemical feedback loops in development and disease. Cell 178, 12–25 (2019).
Cross, M. C. & Hohenberg, P. C. Pattern formation outside of equilibrium. Rev. Mod. Phys. 65, 851–1112 (1993). This extensive monograph describes the physics of pattern formation.
Deneke, V. E., Melbinger, A., Vergassola, M. & Di Talia, S. Waves of Cdk1 activity in S phase synchronize the cell cycle in drosophila embryos. Dev. Cell 38, 399–412 (2016).
Vergassola, M., Deneke, V. E. & Di Talia, S. Mitotic waves in the early embryogenesis of Drosophila: bistability traded for speed. Proc. Natl Acad. Sci. USA 115, E2165–E2174 (2018). In this work, the authors explain the transition between mitotic phase waves and trigger waves in the syncytial D. melanogaster embryo.
Richardson, K. A., Schiff, S. J. & Gluckman, B. J. Control of traveling waves in the mammalian cortex. Phys. Rev. Lett. 94, 028103 (2005).
Bub, G., Shrier, A. & Glass, L. Global organization of dynamics in oscillatory heterogeneous excitable media. Phys. Rev. Lett. 94, 028105 (2005).
Chang, J. B. & Ferrell, J. E. Jr. Mitotic trigger waves and the spatial coordination of the Xenopus cell cycle. Nature 500, 603–607 (2013).
Cheng, X. & Ferrell, J. E. Apoptosis propagates through the cytoplasm as trigger waves. Science 361, 607–612 (2018). This article describes the discovery and analysis of apoptotic trigger waves.
Süel, G. M., Garcia-Ojalvo, J., Liberman, L. M. & Elowitz, M. B. An excitable gene regulatory circuit induces transient cellular differentiation. Nature 440, 545–550 (2006).
Tyson, J. J. & Keener, J. P. Singular perturbation theory of traveling waves in excitable media (a review). Phys. Nonlinear Phenom. 32, 327–361 (1988).
Zykov, V. S. & Bodenschatz, E. Stabilized wave segments in an excitable medium with a phase wave at the wave back. N. J. Phys. 16, 043030 (2014).
Swaney, K. F., Huang, C.-H. & Devreotes, P. N. Eukaryotic chemotaxis: a network of signaling pathways controls motility, directional sensing, and polarity. Annu. Rev. Biophys. 39, 265–289 (2010).
Palsson, E. et al. Selection for spiral waves in the social amoebae Dictyostelium. Proc. Natl Acad. Sci. 94, 13719–13723 (1997).
Sgro, A. E. et al. From intracellular signaling to population oscillations: bridging size-and time-scales in collective behavior. Mol. Syst. Biol. 11, 779 (2015).
Tayar, A. M., Karzbrun, E., Noireaux, V. & Bar-Ziv, R. H. Propagating gene expression fronts in a one-dimensional coupled system of artificial cells. Nat. Phys. 11, 1037–1041 (2015). In this work, the authors observed transitions between trigger waves and phase waves that are in agreement with theoretical predictions.
Goldstein, R. E. Traveling-wave chemotaxis. Phys. Rev. Lett. 77, 775–778 (1996).
Skoge, M. et al. Cellular memory in eukaryotic chemotaxis. Proc. Natl Acad. Sci. 111, 14448–14453 (2014).
Nakajima, A., Ishihara, S., Imoto, D. & Sawai, S. Rectified directional sensing in long-range cell migration. Nat. Commun. 5, 5367 (2014).
Takahashi, J. S. Transcriptional architecture of the mammalian circadian clock. Nat. Rev. Genet. 18, 164–179 (2017).
Wilds, R. & Glass, L. An atlas of robust, stable, high-dimensional limit cycles. Int. J. Bifurc. Chaos 19, 4055–4096 (2009).
Monk, N. A. M. Oscillatory expression of Hes1, p53, and NF-κB driven by transcriptional time delays. Curr. Biol. 13, 1409–1413 (2003).
Tyson, J. J., Chen, K. C. & Novak, B. Sniffers, buzzers, toggles and blinkers: dynamics of regulatory and signaling pathways in the cell. Curr. Opin. Cell Biol. 15, 221–231 (2003).
Novák, B. & Tyson, J. J. Design principles of biochemical oscillators. Nat. Rev. Mol. Cell Biol. 9, 981–991 (2008).
Strogatz, S. H. Nonlinear Dynamics and Chaos: With Applications to Physics, Biology, Chemistry, and Engineering (Avalon Publishing, 2014).
Pikovsky, A., Rosenblum, M. & Kurths, J. Synchronization: a Universal Concept in Nonlinear Sciences (Cambridge University Press, 2001).
Kuramoto, Y. Cooperative dynamics of oscillator community: a study based on lattice of rings. Prog. Theor. Phys. Suppl. 79, 223–240 (1984). This seminal work describes phase synchronization in a tractable way.
Kuramoto, Y. & Nakao, H. On the concept of dynamical reduction: the case of coupled oscillators. Philos. Trans. R. Soc. Math. Phys. Eng. Sci. 377, 20190041 (2019).
Kotani, K. et al. Nonlinear phase-amplitude reduction of delay-induced oscillations. Phys. Rev. Res. 2, 033106 (2020).
Tigges, M., Marquez-Lago, T. T., Stelling, J. & Fussenegger, M. A tunable synthetic mammalian oscillator. Nature 457, 309–312 (2009).
Elowitz, M. B. & Leibler, S. A synthetic oscillatory network of transcriptional regulators. Nature 403, 335–338 (2000). This article describes proof by synthesis of a delayed-negative-feedback genetic oscillator.
Stricker, J. et al. A fast, robust and tunable synthetic gene oscillator. Nature 456, 516–519 (2008).
Niederholtmeyer, H. et al. Rapid cell-free forward engineering of novel genetic ring oscillators. eLife 4, e09771 (2015).
Tsimring, L. S. Noise in biology. Rep. Prog. Phys. 77, 026601 (2014).
Lax, M. Classical noise. V. Noise in self-sustained oscillators. Phys. Rev. 160, 290–307 (1967).
Pikovsky, A., Rosenblum, M. & Kurths, J. Phase synchronization in regular and chaotic systems. Int. J. Bifurc. Chaos 10, 2291–2305 (2000).
Winfree, A. T. Biological rhythms and the behavior of populations of coupled oscillators. J. Theor. Biol. 16, 15–42 (1967).
Riedel-Kruse, I. H., Muller, C. & Oates, A. C. Synchrony dynamics during initiation, failure, and rescue of the segmentation clock. Science 317, 1911–1915 (2007).
Danino, T., Mondragón-Palomino, O., Tsimring, L. & Hasty, J. A synchronized quorum of genetic clocks. Nature 463, 326–330 (2010).
Tayar, A. M., Karzbrun, E., Noireaux, V. & Bar-Ziv, R. H. Synchrony and pattern formation of coupled genetic oscillators on a chip of artificial cells. Proc. Natl Acad. Sci. USA 114, 11609–11614 (2017).
Lažetic´, V. & Fay, D. S. Molting in C. elegans. Worm 6, e1330246 (2017).
Johnstone, I. L. & Barry, J. D. Temporal reiteration of a precise gene expression pattern during nematode development. EMBO J. 15, 3633–3639 (1996).
Hendriks, G.-J., Gaidatzis, D., Aeschimann, F. & Großhans, H. Extensive oscillatory gene expression during C. elegans larval development. Mol. Cell 53, 380–392 (2014). This article describes the discovery of a genome-wide phase-locked developmental oscillator correlated with moulting.
Meeuse, M. W. et al. Developmental function and state transitions of a gene expression oscillator in Caenorhabditis elegans. Mol. Syst. Biol. 16, e9498 (2020).
Sarrazin, A. F., Peel, A. D. & Averof, M. A segmentation clock with two-segment periodicity in insects. Science 336, 338–341 (2012).
El-Sherif, E., Averof, M. & Brown, S. J. A segmentation clock operating in blastoderm and germband stages of Tribolium development. Development 139, 4341–4346 (2012).
Okuda, K. Variety and generality of clustering in globally coupled oscillators. Phys. Nonlinear Phenom. 63, 424–436 (1993).
Grosskortenhaus, R., Pearson, B. J., Marusich, A. & Doe, C. Q. Regulation of temporal identity transitions in drosophila neuroblasts. Dev. Cell 8, 193–202 (2005).
Gliech, C. R. & Holland, A. J. Keeping track of time: the fundamentals of cellular clocks. J. Cell Biol. 219, e202005136 (2020).
Raff, M. Intracellular developmental timers. Cold Spring Harb. Symp. Quant. Biol. 72, 431–435 (2007).
MacDonald, N. Biological Delay Systems: Linear Stability Theory (Cambridge Univ. Press, 1989).
Lemaire, V., Lee, C. F., Lei, J., Métivier, R. & Glass, L. Sequential recruitment and combinatorial assembling of multiprotein complexes in transcriptional activation. Phys. Rev. Lett. 96, 198102 (2006).
Métivier, R. et al. Estrogen receptor-α directs ordered, cyclical, and combinatorial recruitment of cofactors on a natural target promoter. Cell 115, 751–763 (2003).
May, R. M. & Leonard, W. J. Nonlinear aspects of competition between three species. SIAM J. Appl. Math. 29, 243–253 (1975).
Rabinovich, M. I., Varona, P., Selverston, A. I. & Abarbanel, H. D. I. Dynamical principles in neuroscience. Rev. Mod. Phys. 78, 1213–1265 (2006).
Afraimovich, V., Tristan, I., Huerta, R. & Rabinovich, M. I. Winnerless competition principle and prediction of the transient dynamics in a Lotka–Volterra model. Chaos Interdiscip. J. Nonlinear Sci. 18, 043103 (2008).
Kuznetsov, A. & Afraimovich, V. Heteroclinic cycles in the repressilator model. Chaos Solitons Fractals 45, 660–665 (2012).
Panovska-Griffiths, J., Page, K. M. & Briscoe, J. A gene regulatory motif that generates oscillatory or multiway switch outputs. J. R. Soc. Interface 10, 20120826 (2013).
Balaskas, N. et al. Gene regulatory logic for reading the sonic hedgehog signaling gradient in the vertebrate neural tube. Cell 148, 273–284 (2012). This article describes quantitative modelling of a gradient-driven genetic timer network.
Dessaud, E. et al. Interpretation of the sonic hedgehog morphogen gradient by a temporal adaptation mechanism. Nature 450, 717–720 (2007).
Tufcea, D. E. & François, P. Critical timing without a timer for embryonic development. Biophys. J. 109, 1724–1734 (2015). This study provides a systematic theoretical analysis of a strongly interconnected and repressive GRN.
Averbukh, I., Lai, S.-L., Doe, C. Q. & Barkai, N. A repressor-decay timer for robust temporal patterning in embryonic Drosophila neuroblast lineages. eLife 7, e38631 (2018).
Homem, C. C. F. & Knoblich, J. A. Drosophila neuroblasts: a model for stem cell biology. Development 139, 4297–4310 (2012).
Zhu, X. et al. Speed regulation of genetic cascades allows for evolvability in the body plan specification of insects. Proc. Natl Acad. Sci. USA 114, E8646–E8655 (2017).
Kambadur, R. et al. Regulation of POU genes by castor and hunchback establishes layered compartments in the Drosophila CNS. Genes Dev. 12, 246–260 (1998).
Isshiki, T., Pearson, B., Holbrook, S. & Doe, C. Q. Drosophila neuroblasts sequentially express transcription factors which specify the temporal identity of their neuronal progeny. Cell 106, 511–521 (2001). This article describes the discovery of a sequential genetic timer in D. melanogaster neuroblasts.
Wolpert, L., Tickle, C. & Arias, A. M. Principles of Development (Oxford University Press, 2015).
Li, X. et al. Temporal patterning of Drosophila medulla neuroblasts controls neural fates. Nature 498, 456–462 (2013).
Suzuki, T., Kaido, M., Takayama, R. & Sato, M. A temporal mechanism that produces neuronal diversity in the Drosophila visual center. Dev. Biol. 380, 12–24 (2013).
Le Dréau, G. & Martí, E. Dorsal-ventral patterning of the neural tube: a tale of three signals. Dev. Neurobiol. 72, 1471–1481 (2012).
Cross, M. & Greenside, H. Pattern Formation and Dynamics in Nonequilibrium Systems (Cambridge University Press, 2009).
Oates, A. C., Morelli, L. G. & Ares, S. Patterning embryos with oscillations: structure, function and dynamics of the vertebrate segmentation clock. Development 139, 625–639 (2012).
Gomez, C. et al. Control of segment number in vertebrate embryos. Nature 454, 335–339 (2008).
Cooke, J. & Zeeman, E. C. A clock and wavefront model for control of the number of repeated structures during animal morphogenesis. J. Theor. Biol. 58, 455–476 (1976).
Palmeirim, I., Henrique, D., Ish-Horowicz, D. & Pourquié, O. Avian hairy gene expression identifies a molecular clock linked to vertebrate segmentation and somitogenesis. Cell 91, 639–648 (1997). This article describes the discovery of the molecular segmentation clock in the chick embryo.
Morelli, L. G. et al. Delayed coupling theory of vertebrate segmentation. HFSP J. 3, 55–66 (2009).
Ares, S., Morelli, L. G., Jörg, D. J., Oates, A. C. & Jülicher, F. Collective modes of coupled phase oscillators with delayed coupling. Phys. Rev. Lett. 108, 204101 (2012).
Jörg, D. J., Morelli, L. G., Soroldoni, D., Oates, A. C. & Jülicher, F. Continuum theory of gene expression waves during vertebrate segmentation. N. J. Phys. 17, 093042 (2015).
Webb, A. B. et al. Persistence, period and precision of autonomous cellular oscillators from the zebrafish segmentation clock. eLife 5, e08438 (2016).
Hubaud, A., Regev, I., Mahadevan, L. & Pourquié, O. Excitable dynamics and yap-dependent mechanical cues drive the segmentation clock. Cell 171, 668–682.e11 (2017).
Lauschke, V. M., Tsiairis, C. D., François, P. & Aulehla, A. Scaling of embryonic patterning based on phase-gradient encoding. Nature 493, 101–105 (2013).
Venzin, O. F. & Oates, A. C. What are you synching about? Emerging complexity of Notch signaling in the segmentation clock. Dev. Biol. 460, 40–54 (2020).
Delaune, E. A., François, P., Shih, N. P. & Amacher, S. L. Single-cell-resolution imaging of the impact of notch signaling and mitosis on segmentation clock dynamics. Dev. Cell 23, 995–1005 (2012).
Shih, N. P., Francois, P., Delaune, E. A. & Amacher, S. L. Dynamics of the slowing segmentation clock reveal alternating two-segment periodicity. Development 142, 1785–1793 (2015).
Yoshioka-Kobayashi, K. et al. Coupling delay controls synchronized oscillation in the segmentation clock. Nature https://doi.org/10.1038/s41586-019-1882-z (2020).
Soroldoni, D. et al. A Doppler effect in embryonic pattern formation. Science 345, 222–225 (2014).
Morelli, L. G. & Jülicher, F. Precision of genetic oscillators and clocks. Phys. Rev. Lett. 98, 228101 (2007).
Tsiairis, C. D. & Aulehla, A. Self-organization of embryonic genetic oscillators into spatiotemporal wave patterns. Cell 164, 656–667 (2016).
Dubrulle, J., McGrew, M. J. & Pourquié, O. FGF signaling controls somite boundary position and regulates segmentation clock control of spatiotemporal hox gene activation. Cell 106, 219–232 (2001).
Sawada, A. et al. Fgf/MAPK signalling is a crucial positional cue in somite boundary formation. Dev. Camb. Engl. 128, 4873–4880 (2001).
Bajard, L. et al. Wnt-regulated dynamics of positional information in zebrafish somitogenesis. Development 141, 1381–1391 (2014).
Moreno, T. A. & Kintner, C. Regulation of segmental patterning by retinoic acid signaling during xenopus somitogenesis. Dev. Cell 6, 205–218 (2004).
Sonnen, K. F. et al. Modulation of phase shift between Wnt and Notch signaling oscillations controls mesoderm segmentation. Cell 172, 1079–1090.e12 (2018). This study provides evidence for a phase shift coincidence arrest mechanism in the mouse segmentation clock.
Beaupeux, M. & François, P. Positional information from oscillatory phase shifts: insights from in silico evolution. Phys. Biol. 13, 036009 (2016).
Verd, B. et al. A damped oscillator imposes temporal order on posterior gap gene expression in Drosophila. PLoS Biol. 16, e2003174 (2018).
Verd, B., Crombach, A. & Jaeger, J. Dynamic maternal gradients control timing and shift-rates for drosophila gap gene expression. PLoS Comput. Biol. 13, e1005285 (2017).
Verd, B., Monk, N. A. & Jaeger, J. Modularity, criticality, and evolvability of a developmental gene regulatory network. eLife 8, e42832 (2019). This article demonstrates the use of concepts from dynamical systems to obtain new insight into a well-studied genetic system.
Krotov, D., Dubuis, J. O., Gregor, T. & Bialek, W. Morphogenesis at criticality. Proc. Natl Acad. Sci. USA 111, 3683–3688 (2014).
DiFrisco, J. & Jaeger, J. Beyond networks: mechanism and process in evo-devo. Biol. Philos. 34, 54 (2019).
Salazar-Ciudad, I. & Jernvall, J. The causality horizon and the developmental bases of morphological evolution. Biol. Theory 8, 286–292 (2013).
Bauer, G. et al. The science of living matter for tomorrow. Cell Syst. 6, 400–402 (2018).
Acknowledgements
The authors thank A. Pumir, L. Morelli, A. Bercowsky and L. A. Rohde for critical feedback on the manuscript.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Genetics thanks H. Grosshans and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Glossary
- Syncytial
-
Describes a cell that is multinucleated.
- Kinematic structures
-
Spatial structures that move without transmission of a signal.
- Bistable systems
-
Dynamical units that have two stable states, and the simplest dynamical systems that exhibit memory. The genetic toggle switch, in which two genes mutually repress the expression of each other, is an example of a synthetic gene regulatory network that is bistable.
- Excitable medium
-
Spatially distributed, coupled excitable units that transmit information from one point to another in the form of travelling pulses.
- Critical point
-
Also known as a bifurcation point. A transition point in parameter values (for example, a degradation rate) where a system moves between qualitatively different states; for example, between oscillatory and non-oscillatory states.
- Phase reduction method
-
A mathematical technique that allows the study of limit cycle oscillations (that is, a system with oscillatory dynamics with a fixed amplitude and period that will return to its initial amplitude and period after perturbation, although its phase will drift) by tracking only the time evolution of the phase.
- Heteroclinic cycles
-
A dynamical system that transitions between states in repetitive non-periodic cycles, with the period increasing in each successive cycle.
- Asymmetrical cell division
-
A cell division in which a property or set of factors is present in only one of the daughter cells.
- Type IIIo system
-
In the physics of pattern formation, patterns with a tendency towards a spatially uniform state that oscillates in time.
Rights and permissions
About this article
Cite this article
Negrete, J., Oates, A.C. Towards a physical understanding of developmental patterning. Nat Rev Genet 22, 518–531 (2021). https://doi.org/10.1038/s41576-021-00355-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41576-021-00355-7
This article is cited by
-
Stem Cells and the Microenvironment: Reciprocity with Asymmetry in Regenerative Medicine
Acta Biotheoretica (2022)