Abstract
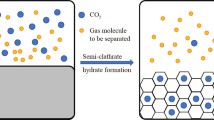
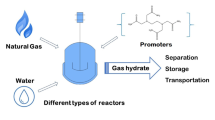
In both natural gas and petroleum reservoirs, the extracted gas is not only composed of methane: a variable and significant quantity of other compounds, such as different hydrocarbons (ethane, butane, pentane, propane, etc.), inert gas (nitrogen), and toxic and corrosive molecules (i.e., carbon dioxide and hydrogen sulfide), are present. In order to reach commercial specifications, natural gas has to be treated, in particular for reaching the minimum gross calorific value required and decreasing CO2 and H2S presence under the respective tolerance values. To do this, several different treatments are commonly applied, like inlet separation, sweetening, mercury removal, dehydration, liquid recovery, and, finally, compression for its transportation. Considering the growing demand and the necessity of exploiting also lower quality natural gas reservoirs, in the present paper, an original solution, for performing a gas treatment, is proposed and analyzed. It consists of promoting hydrates formation for both different compounds separation and gas storage. The greatest part of chemicals commonly present in natural gas is capable to form hydrates, but at different thermodynamic conditions than others. Parameters such as the typology of stored compound and the formation process efficiency are mainly related to partial pressure of each element. Here, the present strategy has been explored and the results achievable were shown. In particular, different possible natural gas compositions were taken into account and specifications required for gas commercialization were considered target of the process. Results led to different possibilities of raw gas treatment: in some cases, gas separation led to contemporary CH4 storage into hydrate structures, while, in the presence of different mixture compositions, contaminants were trapped into water cages and methane (and, eventually, other hydrocarbon compounds) remained in the gas phase.
Similar content being viewed by others
Introduction
Negative effects related to the presence of impurities in natural gas (NG) are increasingly involving researcher attention, due to the necessity of exploiting also reservoirs with a low purity degree. In fact, the world consumption of natural gas will reach over 110 trillion standard cubic feet per year and it is estimated to increase to 200 trillion standard cubic feet per year by 2040 (IEO, 2016). In contrast with the growing demand, the greatest part of new discovered NG fields is poor in quality (Ailin 2018) and has a methane content in the range of 30–90%. Thus, growing attention is involved in developing these low-quality gas reserves. Natural gas is classified as sweet or sour (Demirbas 2010) and that classification depends by the quantity of acid gases present in the mixture. The main component of it is methane, but also other hydrocarbon compounds are commonly present, such as ethane (C2H6), propane (C3H8), isobutene (C4H10), n-butane, pentane (C5H12), and n-pentane. Rather than hydrocarbons, other substances are diffused in NG and are, in every case, considered an issue for human safety, environmental impact, and gross calorific value of the mixture. Among them, carbon dioxide (CO2) and hydrogen sulfide (H2S) are the most widely diffused. Other gases include nitrogen (N2), helium (He), oxygen (O2), hydrogen (H2), mercury (Hg), and mercaptans (IEA, 2010). In particular, mercaptans are responsible of the gas characteristic odor. Both CO2 and H2S represent the major impurities of NG and their removal is necessary. Acid gas removal entity depends on the desired application; i.e., in an internal combustion engine, a concentration of methane higher to 90% is required (Harasimowicz et al. 2007). Carbon dioxide removal is necessary for safety and ease of transport reasons (IEA, 2010). The process of fog liquefying NG to be transported requires a CO2 maximum concentration lower to 50 ppm because, at temperatures necessary for gas liquefaction (commonly lower than − 160 °C), CO2 will freeze, with consequent operational problems, like flowline blockage (Huo 2012). Moreover, high concentrations of CO2 provoke a reduction of energy yielded when burning the gas. Hydrogen sulfide presence is critical not only in terms of energy production but also for human health. The presence of 3500 ppm and over of H2S in gas fuels is capable to cause corrosion of engines (Fang and Zhu 2012) and a quantity of 1000 ppm is potentially fatal to humans. Based on the US pipeline specifications, the minimum gross calorific value required for natural gas is 36.4 MJ per cubic meter, the maximum CO2 amount should be lower than 2%, hydrogen sulfide should not exceed 4ppm, and inert gas must remain below 4% (George et al. 2016). Typical NG treatment, for reaching commercial specifications, includes inlet separation, sweetening, mercury removal, dehydration, natural gas liquid recovery, and compression for transportation (Zulhairun et al. 2020). In particular, CO2 and H2S are usually separated from other compounds by using amine solvents, absorption equipment, and membranes (Duo, 2012; George. et al., 2016). The remaining contaminants are commonly removed via distillation and absorption. The main problem of NG treatment stays in the process complexity and in its costs. Moreover, the necessity of exploiting reservoirs having a more significant quantity of impurities will lead to a higher production of CO2 (mainly for separation), making necessary a more intensive and expensive geological storage activity. The present work deals with the possibility of adopting the hydrates formation process as a strategy for gas mixture separation. Natural gas hydrates are solid crystalline compounds, which naturally occur in the presence of NG, water, and necessary conditions of relatively high pressure and low temperature (Rossi et al. 2019). The interest of researchers for hydrates arisen from NG pipeline blockage they usually provoked, also at temperatures higher than 0 °C. Previous works tested and proved the possibility of removing single compounds from a gas mixture by hydrate formation, in function of partial pressure that each species assumes inside the mixture (Gambelli et al. 2019a; Castellani et al. 2019). Almost all NG components are able to form hydrate and, in particular, methane, ethane, propane, isobutane, carbon dioxide, hydrogen sulfide, and also nitrogen and hydrogen, even if at completely different thermodynamic conditions from other compounds, thus the possibility of simplifying the gas treatment process and reducing its costs by separating some elements from other via hydrate formation. The number and typology of gaseous species involved in the process is a function of their respective quantities in the whole mixture and pressure and temperature used for the process: in some cases, contaminants may be entrapped into water cages and then removed, in order to increase the gaseous mixture purity degree and, in other cases, methane and other hydrocarbons may be involved in the formation. This second solution also allows to provide the solid storage of methane, which represents a solution of interest for NG transportation, considering the average density of methane contained in hydrates (Gambelli et al. 2019b). Different NG compositions were taken into account and, according to pressure and temperature required for hydrate formation of each species described in Sloan Jr and Koh 2008), the results achievable by varying the process thermodynamic conditions were shown.
A brief overview on natural gas treatment
The treatment of natural gas consists of a sum of different processes (Alcheikhhamdon and Hoorfar 2016), which are (i) phase separation; (ii) acid gas removal; (iii) natural gas dehydration; (iv) natural gas liquid (NGL) recovery, and (v) liquefaction of natural gas.
In every NG treatment facility, phase separation is the first step. Before further processes, natural gas has to be separated from condensate and water, which might be already present or produced inside the pipelines, due to the heavy component condensation. The most used method consists in the gravity separation process; it focused on the relative density of separated fluids. The gas–liquid separation efficiency is given by the maximum size of liquid droplets allowed to be entrained with the separated gas.
The acid gas removal units are present at the early stages of gas handling due to the elevated costs related to the use of corrosion-resistant facilities. The goal is moving acid gas quantities below the required limits. Processes adopted for acid gas removal are grouped with the term “sweetening.” The present term is also used for CO2 removal because processes used are the same. Carbon dioxide removal is needed for the prevention of corrosion problems, increasing the NG calorific value, and avoiding frost formation in refrigerating units. The content of carbon dioxide is reduced as a function of pipelines required for specifications (usually 2–3% v/v) (Alcheikhhamdon and Hoorfar 2016). More restrictive requirements occur in the presence of cryogenic units, in order to avoid the formation of frost CO2. Here, the presence of such gas should not exceed 50 ppmv (Klinkenbijl et al. 1999). After CO2, the most dangerous compound, in terms of pipeline corrosion, is hydrogen sulfide. However, the prior reason for its removal stays in its toxicity for humans and for the environment (Beitler et al. 2011). The H2S content inside the treated gas must be lower than 4–10 ppmv (Centre for Energy Economics 2004). Also, other contaminants, such as mercaptans (organosulfur) and carbonyl sulfide need to be removed. In fact, an elevated presence of organosulfur elements is associated with an incomplete combustion and to sulfurous acid rains. Because of its toxicity, once removed, H2S cannot be released in the atmosphere; that gas is usually flared to produce sulfur oxides (SOx), which are less dangerous for both humans and the environment (U.S. Environmental Protection Agency Office 2015). Firstly, the residual gas is enriched in H2S by scrubbing carbon dioxide, then one-third of H2S is combusted for SO2 production and, immediately after, it reacts with the remaining quantity of H2S to produce elemental sulfur.
Technologies adopted for gas sweetening are liquid desiccant absorption, solid desiccant adsorption, and membrane separation. The optimal strategy is chosen time per time as a function of maximum acid gas concentration tolerated, technology’s reliability, process capacity, and others (Tennyson and Schaaf 1977). However, the most adopted strategy for acid gas removal consists of liquid desiccant processes (Arnold and Stewart 2008).
Dehydration consists in liquid water removal from NG, which is naturally present inside reservoirs with oil and gas. Water provokes humidification of the gas, with an equilibrium quantity of water vapor. That equilibrium is a function of pressure, temperature, and gas composition; in particular, higher temperature or lower pressure and a higher acid gas concentration are related to a higher NG water saturation limit (Zirrahi et al. 2010). Dehydration is necessary for avoiding condensation in pipelines and gas handling facilities, where it would contribute to causing pressure drop and corrosion. Another significant issue associated with water present in natural gas stays in the potential formation of hydrate compound, which are capable to completely avoid the gas flow into pipelines (Gambelli 2018; Gambelli et al. 2019c). Different strategies for gas dehydration have been performed in the past decades and the appropriate technology is chosen by taking into account the maximum quantity of water which can be tolerated in the gas, rather than gas processing capacity, process conditions, and facility location. The most used methods are liquid desiccant absorption and solid desiccant adsorption (Anyadiegwu et al. 2014).
In the major part of cases, natural gas is saturated with an equilibrium quantity of heavy hydrocarbons, having a high molecular weight. That quantity is a function of the reservoir oil properties and is also related to pressure and temperature reached during the gas treatment phases. In particular, NG absorbs a greater amount of such hydrocarbons in presence of relative high temperature and low-pressure values. Unlike the previously described contaminants, heavy hydrocarbons also provide some positive contribution to the whole mixture. They have a higher calorific value than methane; thus, their presence enriches the specific NG and increases its commercial price. The main problem associated to heavy components is their tendency to condensate and so lead to two-phase flow and slugging regime in gas transport facilities. That phenomenon is avoided by a hydrocarbon dewpoint verification process: when necessary, a partial heavy component removal from NG is performed to reduce the dewpoint below the maximum tolerated value for gas transport. Another reason for heavy component limitation in NG is the necessity of not exceeding with the mixture calorific value. In fact, lots of turbines and gas burners are designed to work with fuel gases having a specific calorific value range (usually they are designed to work with fuels having the same specification required for NG production) (Farag et al. 2011; Kurz et al. 2004). The hydrocarbon dewpoint control is an alternative possibility to define the NGL recovery process. The separation of NGL is carried out in an external refrigeration unit, where appropriate thermodynamic conditions promote the high dewpoint hydrocarbons condensation.
Finally, natural gas liquefaction is necessary for its transportation via pipelines. The gas transport phase is accompanied by technical, economical, and political problems (National Petroleum Council 2011) and the liquefaction of NG may contribute to reduce their entity. Firstly, the gas to liquid volume ratio for a unit mass is 600:1; moreover, the energy density of liquefied natural gas is comparable with other fuels (i.e., it is at least 60% diesel oil). Natural gas liquefaction requires cryogenics conditions (− 162 °C) (Songhurst 2014). The liquified natural gas units need a more restrictive raw gas treatment before the liquefaction process (Klinkenbijl et al. 1999). In particular, carbon dioxide content must be lower than 50 ppmv, in order to avoid frost formation. About hydrogen sulfide, its contents must be reduced to 0–4 ppmv. Also, the dehydration process has to respect more restrictive requirements: the maximum water content accepted is 1 ppmv. The contemporary mercury removal (if present) is needed for avoiding metal embrittlement corrosion and consequent gas losses (Wilhelm 2009). The maximum gas mercury tolerated is equal to 10 ng/Nm3 and that results is usually reached by using solid desiccants. A last consideration about inert gases contribution is needed. Their presence strongly reduces the NG heating value, rather than increasing transport phase costs (greater volumes) and gas volatility. Nitrogen is the most diffused inert gas in NG. It is usually separated from the remaining mixture upstream of the liquefaction unit, or downstream, by exploiting the difference in boiling point between methane and nitrogen for gas-liquid separation (Mitariten 2001). In fact, natural gas liquefies at − 162 °C, while nitrogen needs lower temperatures (− 195.8 °C) (Finn and Nitrogen 2007).
Methods
Hydrate formation conditions, for each species present in NG mixtures and capable of being involved in that process, were defined by taking into account data reported in Sloan Jr and Koh 2008) and published elsewhere in the literature. In a temperature range of 273–285 K, for each degree, the pressure value required for hydrate formation was defined for methane, ethane, propane, butane, hydrogen sulfide, nitrogen, and carbon dioxide. Then, six different NG compositions were proposed, different from each other in particular for CH4, CO2, and H2S respective percentages. Five possible mixture pressure values were taken into account for hydrate formation: 20, 40, 60, 80, and 100 bar. For each value, different possible results of hydrate formation, as a function of different temperature values present in the range describe previously, were discussed for all NG mixtures defined. In the “Results and discussion” section, five tables for each different mixture are shown: every table is related to specific pressure values and shows which compounds are capable of forming hydrates at different temperature values. Hydrate formation was evaluated by considering partial pressure of all species, calculated with the Dalton law.
Results and discussion
Once evaluated the possible NG compositions, six different mixtures were assumed. In Table 1, only elements capable to form hydrates were shown in terms of percentage composition. If their sum is different from 100%, the reason stays in the assumption that some other compounds are present in the gaseous mixture but are not capable to form hydrate. Also, nitrogen and hydrogen were added to this last group; even if they theoretically might produce hydrates, pressure, and temperature required conditions and their respective continued presence in the mixture, makes their involvement in the formation process unfeasible.
Considering that raw natural gas commonly consists of 30–90% methane (Zulhairun A.K., 2020), these six compositions move from 38 to 88 % of it. Other hydrocarbons and contaminant quantities ware widely varied from Hp. 1 to Hp. 6. In particular, CO2 moved from 3 to 20%, while H2S was in the range of 3–7 %. Heavy hydrocarbons moved from 1–2% to 10–11%. As previously explained, other compounds, such as nitrogen, C5+ hydrocarbons, hydrogen, oxygen, and mercury, were not considered because, due to their chemical structure or to their very contained presence, they could not form hydrates.
Thermodynamic conditions for hydrate formation
In this section, a graphical description of gas hydrate formation in presence of a single type of guest is shown. The diagrams are based on a data collection of equilibrium values previously published in the literature. Finally, the last diagram proposed describes thermodynamic equilibrium conditions for hydrate containing natural gas mixtures. In that case, the specific composition of each mixture has been reported, in order to provide a clear idea about the weight of each gaseous species contained in the mixture.
Figures 1, 2, 3, 4, 5 and 6 show two different trends: methane, carbon dioxide, and nitrogen assume the same behavior, or a relatively gradual increase in pressure needed for hydrate formation with the increasing temperature; conversely, ethane, propane, and butane have an almost constant trend of pressure, until reaching a critical temperature value, after which pressure immediately and drastically increases. Obviously, even if the trend is similar, each compound is characterized by different specific equilibrium values. In particular, carbon dioxide equilibrium occurs milder conditions than methane hydrate, even if the thermodynamic region between the two respective equilibrium curves can be considered relatively narrow, while nitrogen hydrates require significantly more severe conditions to be formed.
Phase equilibrium diagram for pure ethane hydrate (Sloan and Koh, 2008; Sundramoorthy et al. 2016)
About the other compounds, pressure-temperature equilibrium values are close to each other and extremely lower than the previous compounds. Conversely, once the critical temperature value is reached, pressure increases drastically and hydrate formation requires more severe conditions than other compounds.
If natural gas mixtures are directly involved in hydrate formation, the equilibrium trend assumes a different configuration if compared with single species and it is a function of the specific concentration of each compound, as it is possible to see in Fig. 7.
In Fig. 7, seven different natural gas mixtures have been shown, having the following concentrations, here expressed in molar fraction: NG1: 0.8721 CH4, 0.0126 C2H6, 0.001 C3H8, 0.0508 CO2, 0.0635 N2; NG2: 0.7213 CH4, 0.01 C2H6, 0.0007 C3H8, 0.2186 CO2, 0.0496 N2; NG3: 0.82 CH4, 0.113 C3H8, 0.042 C4H10, 0.05 CO2; NG4: 0.7354 CH4, 0.175 CO2, 0.0696 H2S, 0.02 N2; NG5: 0.8502 CH4, 0.0501 CO2, 0.0947 H2S, 0.05 N2; NG6: 0.8251 CH4, 0.11 CO2. 0.0349 H2S, 0.03 N2; and NG7: 0.9112 CH4, 0.0503 C2H6, 0.0149 C3H8, 0.069 C4H10, 0.0048 CO2, 0.0046 N2.
In all cases, the main component is methane, which ranged from 0.7213 to 0.9112 mf; for that reason, the equilibrium behavior of hydrate with those natural gas mixtures is very similar to that of methane. However, data present in the literature only describe mixtures having a high percentage of methane. Conversely, in the following sections, methane concentration was significantly lowered than those values, varying from 88 to 38%.
Mixture having a CH4 content of 88 %
Results reached for mixture 1 are shown in Tables 2, 3, 4, 5, and 6. In all cases, tables are ordered in function of mixture’s pressure, from 100 to 20 bar.
Seeing Tables 2, 4, 5, and 6 clearly appears how, in the function of different pressure and temperature values, several solutions are possible. With the present natural gas composition, three different solutions are feasible: (i) hydrate formation involving only methane; (ii) CH4 and H2S hydrate formation, and (iii) CH4, C4H10, and H2S hydrate formation. In every case, irrespective of the selective NG mixture, that strategy has to be accompanied with other further operations. For example, in the case of contemporary CH4 and H2S entrapment inside water cages, a consequent step for H2S removal will be necessary. The only exception is given by pure methane hydrate formation. In that case, also the presence of other compounds usually diffused in natural gas, but non considered in this section, may be neglected, because of the unfeasibility of hydrates formation for them. Thus, when only CH4 hydrate formation occurs, two targets can be reached at the same time: methane separation from other contaminants and its storage in solid form (which is a potential definitive solution for gas transportation). Even in this case, some further operation will be necessary for the remaining gas treatment. Hydrogen sulfide must be separated from other gases and converted in SO2 or in pure sulfur, before being released. To do this, the same method should be applied, in particular by considering its different behavior in forming hydrates than other contaminants. However, this further aspect, or the contaminant mixture treatment carried out without a selective hydrate formation process, will be deepened in future works. Pure methane hydrates may be produced at the following different thermodynamic conditions (related to the whole mixture): 100 bar and 284 K, 80 bar and 283–285 K, 60 bar and 279–280 K, and, finally, 40 bar and 276 K. Hydrates involving both methane and hydrogen sulfide are possible in presence of 100 bar and 274–283 K, 80 bar 273–280 K, 60 bar and 273–280 K, and, finally, 40 bar and 273–275 K. Even if that case requires a second step for pure methane production, it may be preferred to the previous solution, due to the considerably higher range of possibility to reach it and to the significant difference in pressure–temperature conditions between hydrates formation about the two different compounds. In fact, gas mixture separation via hydrate formation in the presence of only two different species is absolutely easier and more efficient than in the presence of several different elements. Finally, a mixture of CH4, C4H10, and H2S hydrates is possible only with 100 bar and 273 K. In conclusion, with NG compositions similar to the present one, hydrate formation is a particularly effective strategy for heavy hydrocarbons, carbon dioxide, and inert gas removal and, in the presence of specific pressure, and temperature conditions are also able to perform hydrogen sulfide separation.
Mixture having a CH4 content of 78 %
This second mixture leads to another different possibility, or the H2S removal from NG via its entrapment inside water cages. The formation of only H2S hydrates occurs at several different thermodynamic conditions: 100 bar and 284–285 K, 80 bar and 282–285 K, 60 bar and 280–283 K, 40 bar and 276–280 K, and, finally, 20 bar and 273–275 K (Tables 7, 8, 9, 10, and 11). Even in this case, methane may be entrapped into hydrates; the main difference with the previous composition is the unfeasibility of entrapping only methane. Three different mixtures into hydrates are possible: (i) CH4 and H2S; (ii) CH4, C4H10, and H2S; and (iii) CH4, C3H8, C4H10, and H2S. Even here, the contemporary formation of CH4 and H2S hydrates cover the greatest combination of analyzed pressure and temperature values. Unlike the first mixture, also propane may be entrapped, in particular at 100 bar and 273–276 K, 80 bar and 273–274 K, and 60 bar and 273 K. Thus, with gas mixture composition similar to this one, the immediate production of pure methane hydrates is no longer possible. However, hydrogen sulfide removal in solid form is now feasible, and also propane can be involved in the formation process.
Mixture having a CH4 content of 68% (Tables 12, 13, 14, 15, and 16)
With the constant reduction of methane presence in the NG mixture, several further options become feasible. Even in this case, carbon dioxide is not involved in hydrate formation and a promising option consists of forming only H2S hydrates, in order to separate this compound from the NG mixture. For the first time, also ethane may be involved in the formation process. With this kind of gas composition, two further options of interest are feasible: pure carbon dioxide removal and heavy hydrocarbon removal. In the presence of specific conditions, all species are able to form hydrates, rather than carbon dioxide. In particular, it occurs at 100 bar and 273–276 K and 80 bar and 273–274 K. In the presence of such conditions, CO2 may be removed in its gaseous phase, and then the remaining mixture needs of further treatments for acid gas removal and so on. On the contrary, some particular conditions lead to the formation of hydrates containing only H2S and some heavy hydrocarbons. In the presence of 40 bar and 275 K, only H2S and C4H10 are capable to form hydrates, while in the presence of 40 bar and 274 K, also propane can be added to the previous. This second solution permits to carry out a gas sweetening operation and, at the same time, to decrease natural gas dewpoint by removing a part of heavy hydrocarbons present inside.
Mixture having a CH4 content of 58%
Even with this kind of mixture, three main options are possible: H2S removal, CO2 separation in gaseous phase, and H2S and heavy hydrocarbon removal. H2S removal occurs at 100 bar and 281–285 K (Table 17), 80 bar and 280–285 K (Table 18), 60 bar and 278–283 K (Table 19), and 40 bar and 277–280 K (Table 20). Carbon dioxide separation in gaseous form is possible at 100 bar and 273–276 K and at 80 bar and 273–274 K. A further possibility consists in removing both CO2 and C2H6 in gaseous form and is possible only at 60 bar and 273–276 K. The last option consents the separation of all heavy hydrocarbon compounds, with the only exception of ethane; it may be performed at 60 bar and 277 K, 40 bar and 273–276 K, and, finally, at 20 bar and 273–275 K (Table 21).
Mixture having a CH4 content of 48%
For the first time, the partial pressure of carbon dioxide is enough elevated to permit hydrates formation, that occurs only at 100 bar and 273 K (Table 22). This particular case is not interesting because also all other species are involved in the process, without any exception. Here, the possibility of heavy hydrocarbon removal, accompanied with H2S separation, is more pronounced than in previous NG compositions. In particular, it occurs at 80 bar and 278 K (Table 23), 60 bar and 275–278 K (Table 24), 40 bar and 273–276 K (Table 25), and 20 bar and 273 and 275 K (Table 26). Carbon dioxide separation in gaseous form is possible at 100 bar and 274–277 K, 80 bar and 273–276 K, and, finally, 60 bar and 273–274 K. A little further option consists in removing both carbon dioxide and butane in gaseous form; it is possible only at 100 bar and 277–279 K and 80 bar and 277 K.
Mixture having a CH4 content of 38%
Natural gas mixtures, having a methane concentration equal to 38%, were described in Tables from 27, 28, 29, 30, and 31.
This last NG composition has the lowest methane content; here, CO2 is about 20% and its entrapping into water cages occurs in a more extended range of thermodynamic conditions. Only hydrogen sulfide and H2S with heavy hydrocarbon separation are the two options of interest. About this second possibility, some differences occur if comparing this case with the previous NG mixtures. For the first time, the hydrate formation process can be used for removing all heavy hydrocarbons capable of forming hydrates (ethane, propane, and butane), together with hydrogen sulfide. It occurs in correspondence of 80 bar and 276 K and 60 bar and 273–275 K.
In conclusion, with elevated concentration of methane in natural gas mixtures, the main possibility related to hydrate formation consists in separating methane from all other compounds and, at the same time, storing it in solid form. With the decrease of its concentration, firstly also the possibility of involving only H2S in hydrate formation and, thus, removing it occurs. Then, also carbon dioxide removal in gaseous form and heavy hydrocarbon separation by their entrapment into hydrates, together with hydrogen sulfide, become feasible. In particular, with the reduction of methane presence, this last option becomes more and more pronounced and extended to a wider range of pressure–temperature conditions, until reaching the possibility of contemporary removing all heavy hydrocarbon present into natural gas and capable to form hydrate (this last consideration excludes C5+ hydrocarbons because, due to their always limited presence, they cannot be involved in the process).
Conclusions
The term natural gas indicates a gaseous mixture mainly composed of methane, and also comprising some different compounds, such as heavy hydrocarbons, acid, and inert gases. While the first group may represent a valid opportunity for increasing the NG calorific value, the other two groups only bring several issues in terms of gas transportation, pipeline corrosion, gas calorific value reduction, toxicity for humans and for the environment, and so on. Even heavy hydrocarbons may constitute a problem, due to their higher tendency to condensate than methane, thus necessitating gas treatment before its commercialization. After a brief overview of the most important phases related to the gas treatment process, the present work suggests a further option, consisting of the use of the hydrate formation process as a strategy for different NG compound separation. Based on data presented elsewhere in the literature, six different NG compositions were supposed and their capability of forming hydrates was verified at five different pressure values, i.e., 100, 80, 60, 40, and 20 bar and in a temperature range of 273–285 K. Starting from the assumption that some elements are not capable to produce hydrates (because of their chemical structure or their very contained presence) and that also contaminant mixtures must be treated before being released in the external environment, the hydrate formation process cannot be only used, but may replace some alternative and more energy-intensive and costly operations. Results reached for these different mixtures highlighted the possibility of performing different targets, depending on the percentage of methane present in the NG mixture. In case of high CH4 concentrations, the most effective solution consists in pure methane separation and its entrapment in a solid form that makes it immediately ready for transportation. With the decreasing of CH4 concentration in NG, other options become more feasible: (i) hydrogen sulfide removal in solid form; (ii) pure carbon dioxide (or carbon dioxide with part of heavy hydrocarbon separation in gaseous form); and (iii) contemporary hydrogen sulfide and heavy hydrocarbon removal in solid form. This last possibility is the most feasible in correspondence of mixtures having the lowest CH4 content and, in some cases, brings to all heavy hydrocarbon removal (obviously, only the species capable to form hydrates), together with hydrogen sulfide.
References
Ailin J (2018) Progress and prospects of natural gas development technologies in China. Nat Gas Ind B 5:547–557
Alcheikhhamdon Y, Hoorfar M (2016) Natural gas quality enhancement: a review of the conventional treatment processes, and the industrial challenges facing emerging technologies. Journal of Natural Gas Science and Engineering 34:689–701
Anyadiegwu C, Kerunwa A, Oviawele P (2014) Natural gas dehydration using triethylene glycol TEG. Petroleum Coal 56:409
Arnold K, Stewart M (2008) Surface Production Operations. Elsevier, Amsterdam
Bavoh CB, Partoon B, Lal B, Keong LK (2017) Methane hydrate-liquid-vapor-equilibrium phase condition measurements in the presence of natural amino acids. Journal of Natural Gas Science and Engineering 37:425–434
Beitler C, Fisher K, McIntush K, Tyndall K, Lundeen J (2011) When is CO2 more hazardous than H2S. Hydrocarb Process 90:45–48
Bishnoi PR, Dholabhai PD (1999) Equilibrium conditions for hydrate formation for a ternary mixture of methane, propane and carbon dioxide, and a natural gas mixture in the presence of electrolytes and methanol. Fluid Phase Equilib 158-160:821–827
Bottger A, Kamps APS, Maurer G (2016) An experimental investigation on the phase equilibrium of the binary system (methane + water) at low temperatures: solubility of methane in water and three-phase (vapour + liquid + hydrate) equilibrium. Fluid Phase Equilib 407:209–216
Buleiko VM, Grigoriev BA, Mendoza J (2018) Calorimetric investigation of hydrates of pure isobutane and iso- and normal butane mixtures. Fluid Phase Equilib 462:14–24
Castellani B, Gambelli AM, Nicolini A, Rossi F (2019) Energy and environmental analysis of membrane-based CH4-CO2 replacement processes in natural gas hydrates. Energies 12:850
Centre for Energy Economics at Bureau of Economic Geology (2004) Interstate natural GasdQuality Specifications & Interchangeability. Texas
Chen L, Sun C, Chen G, Nie Y, Sun Z, Liu Y (2009) Measurements of hydrate equilibrium conditions for CH4, CO2, and CH4+C2H6+C3H8 in various systems by step-heating method. Chin J Chem Eng 17:635–641
Demirbas A (2010) Natural Gas: Methane Gas Hydrate, Springer, Ch. 2, pp. 57 – 76. http://www.springer.com.
Fang M, Zhu D (2012). Chemical Absorption. In: Handbook of climate change mitigation, Springer, New York Dordrecht Heidelberg London.
Farag H, Ezzat M, Amer H, Nashed A (2011) Natural gas dehydration by desiccant materials. Alexandria Engineering Journal 50:431–439
Finn A, Nitrogen A (2007) rejection strategies. Hydrocaron. Engineering:49–52
Gambelli AM (2018) Natural gas recovery from hydrate compounds using CO2 replacement strategies: experimental study on thermal stimulation. Energy Procedia 148:647–654
Gambelli AM, Rossi F (2019) Natural gas hydrates: Comparison between two different applications of thermal stimulation for performing CO2 replacement. Energy 172:423–434
Gambelli AM, Rossi F (2020) The use of sodium chloride as strategy for improving CO2/CH4 replacement in natural gas hydrates promoted with depressurization methods. Arab J Geosci 13:898
Gambelli AM, Castellani B, Nicolini A, Rossi F (2019a) Gas hydrate formation as a strategy for CH4/CO2 separation: experimental study on gaseous mixtures produced via Sabatier reaction. Journal of Natural Gas Science and Engineering 71:102985
A.M. Gambelli, B. Castellani, A. Nicolini, F. Rossi. Experimental study on natural gas hydrate exploitation: optimization of methane recovery, carbon dioxide storage and deposit structure preservation. J Pet Sci Eng, 177 (22019b) 594-601.
Gambelli AM, Filipponi M, Nicolini A, Rossi F (2019c) International Multidisciplinary GeoConference: SGEM; Sofia Vol. 19, Fasc. 4.1 : 333 – 343. Sofia: Surveying Geology & Mining Ecology Managment (SGEM). DOI: 10.5593/sgem2019/4.1/S17.043.
Gambelli AM, Castellani B, Nicolini A, Rossi F (2020) Water salinity as potential aid for improving the carbon dioxide replacement process’ effectiveness in natural gas hydrate reservoirs. Processes 8:1298
George G, Bhoria N, AlHallaq S, Abdala A, Mittal V (2016) Polymer membranes for acid gas removal from natural gas. Sep Purif Technol 158:333–356
Harasimowicz M, Orluk P, Zakrzewska-Trznadel G, Chmielewski A (2007) Application of polyimide membranes for biogas purification and enrichment. J Hazard Mater 144(3):698–702
Herri JM, Bouchemoua A, Kwatersky M, Fezoua A, Ouabbas Y, Cameirao A (2011) Gas hydrate equilibria for CO2-N2 and CO2-CH4 gas mixtures – Experimental studies and thermodynamic modelling. Fluid Phase Equilib 301:171–190
Huo D (2012) The Global Sour Gas Problem, Energy Resource Engineering, Stanford University.
Jarrahian A, Nakhaee A (2019) Hydrate-liquid-vapor equilibrium condition for N2 + CO2 + H2O system: Measurement and modelling. Fuel 237:769–774
Kassim Z, Khan MS, Lal B (2019) Thermodynamic modelling on methane hydrate equilibrium condition in the presence of electrolyte inhibitor. Materials Today: Proceedings 19:1395–1402
Khan MS, Partoon B, Bavoh CB, Lal B, Mellon BM (2017a) Influence of tetramethylammonium hydroxide on methane and carbon dioxide gas hydrate phase equilibrium conditions. Fluid Phase Equilib 440:1–8
Khan MS, Bavoh C, Partoon B, Lal B, Bustam MA, Shariff AM (2017b) Thermodynamic effect of ammonium based ionic liquids on CO2 hydrates phase boundary. J Mol Liq 238:533–539
Klinkenbijl, J., Dillon, M., Heyman, E., (1999). Gas pre-treatment and their impact on liquefaction processes. In: GPA Nashville TE Meeting, p. 2.
Kurz R, Etheridge C, Kaiser R, (2004) On fuel suitability for gas turbines. In: Thirty Third Turbomachinery Symposium, Houston, Texas.
Kyung D, Lee K, Kim H, Lee W (2014) Effect of marine environmental factors on the phase equilibrium of CO2 hydrate. International Journal of Greenhouse Gas Control 20:285–292
Liu H, Guo P, Du J, Wang Z, Chen G, Li Y (2017) Experiments and modelling of hydrate phase equilibrium of CH4/CO2/H2S/N2 quaternary sour gases in distilled water and methanol-water/solutions. Fluid Phase Equilib 432:10–17
Mitariten M (2001) New technology improves nitrogen-removal economics. Oil Gas J 17:99
Mooijer-van den Heuvel MM, Peters CJ, Arons JS (2002) Gas hydrate phase equilibria for propane in the presence of additive components. Fluid Phase Equilib 193:245–249
Mu L, Cui Q (2019) Hydrate phase equilibrium condition of the synthetic natural gas with high content of CO2 in the electrolyte solutions containing methanol. J Chem Thermodyn 132:383–389
Nema Y, Ohmura R, Senaha I, Yasuda K (2017) Quadruple point determination in carbon dioxide hydrate forming system. Fluid Phase Equilib 441:49–53
Rossi F, Gambelli AM, Sharma DK, Castellani B, Nicolini A, Castaldi MJ (2019) Simulation of methane hydrates formation in seabed deposit and gas recovery adopting carbon dioxide replacement strategies. Appl Therm Eng 148:371–381
Saberi A, Alamdari A, Shariati A, Mohammadi AH (2018) Experimental measurement and thermodynamic modelling of equilibrium condition for natural gas hydrate in MEG aqueous solution. Fluid Phase Equilib 459:110–118
Sadeq D, Iglauer S, Lebedev M, Smith C, Barifcani A (2017) Experimental determination of hydrate phase equilibrium for different gas mixtures containing methane, carbon dioxide and nitrogen with motor current measurements. Journal of Natural Gas Science and Engineering 38:59–73
Semenov AP, Medvedev VI, Guschin PA, Yakushev V (2015) Effect of heating rate on the accuracy of measuring equilibrium conditions for methane and argon hydrates. Chem Eng Sci 137:161–169
Shicai S, Yong Z, Changling L, Yufeng L (2015) Preliminary study on measurement technology for hydrate phase equilibrium. Fluid Phase Equilib 403:60–69
Shu S, Tiwikrama AH, Yang C, Lee M (2019) Phase equilibrium and dynamic behavior of methane hydrates decomposition via depressurization in the presence of a promoter tert-butanol. J Taiwan Inst Chem Eng 95:119–130
Sloan ED Jr, Koh CA (2008) Clathrate hydrates of the natural gases, 3ed edn. CRC Press, Boca Raton, FL
B. Songhurst, (2014). LNG Plant Cost Escalation j Oxford Institute for Energy Studies. Oxford Institute for Energy Studies. Available: http://www. oxfordenergy.org/2014/02/lng-plant-cost-escalation.
Sundramoorthy JD, Hammonds P, Lal B, Philips G (2016) Gas hydrate equilibrium measurement and observation of gas hydrate dissociation with/without a KHI. Procedia Engineering 148:870–877
Tennyson R, Schaaf R (1977) Guidelines can help choose proper process for gas treating plants. Oil Gas J 10:75
Thakore JL, Holder GD (1987) Solid-Vapor Azeotropes in Hydrate-Forming Systems. Ind Eng Chem Res 26:462–469
The National Petroleum Council NPC (2011) Natural Gas Pipelines Challenges
U.S. Environmental Protection Agency Office of Air Quality Planning and Standards (2015) Standards of performance for petroleum refineries background information for final standards. North Carolina
Wilhelm S (2009) Risk analysis for operation of aluminum heat exchangers contaminated by mercury. Process Saf Prog 28:259–266
Xu S, Fan S, Yao H, Wang Y, Lang X, Lv P, Fang S (2017) The phase equilibria of multicomponent gas hydrate in methanol/ethylene glycol solution based formation water. J Chem Thermodyn 104:212–217
Yasuda K, Oto Y, Shen R, Uchida T, Ohmura R (2013) Phase equilibrium condition measurement in nitrogen and air clathrate hydrate forming systems at temperatures below freezing point of water. J Chem Thermodyn 67:143–147
Zirrahi M, Azin R, Hassanzadeh H, Moshfeghian M (2010) Prediction of water content of sour and acid gases. Fluid Phase Equilib 299:171–179
Zulhairun AK, Wijiyanti R, Widiastuti N, Goh PS, Ismail AF (2020) Prospects of nanocomposite membranes for natural gas treatment. Chapter 14. Nanocomposite membranes for water and gas separation. https://doi.org/10.1016/B978-0-12-816710-6.00014-6
Funding
Open access funding provided by Università degli Studi di Perugia within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
A.M. Gambelli was involved in conceptualization, data curation and writing, while F. Rossi took care of supervision and writing.
Additional information
Responsible Editor: Santanu Banerjee
This paper was selected from the 3rd Conference of the Arabian Journal of Geosciences (CAJG), Tunisia 2020
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gambelli, A.M., Rossi, F. Hydrate formation as a method for natural gas separation into single compounds: a brief analysis of the process potential. Arab J Geosci 14, 846 (2021). https://doi.org/10.1007/s12517-021-07165-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12517-021-07165-5