Abstract
Background
Tumor budding (TB) has been described as an adverse prognostic marker for operable colorectal cancer (CRC); however, a limited number of studies have demonstrated the prognostic significance of TB in patients with drug therapy. This study was conducted to determine the predictive power of TB in stage III CRC patients who received adjuvant chemotherapy.
Methods
We retrospectively collected clinicopathological data including TB of 237 stage III colorectal cancer patients at Hiroshima University Hospital between July 1, 2006 and June 31, 2019. Differential disease-free survival (DFS) was investigated according to TB status.
Results
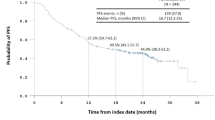
This study included 237 patients with a median age of 67 years, comprising patients who underwent surgery alone (n = 65), 5-fluorouracil (5-FU) monotherapy (n = 129), and oxaliplatin-based chemotherapy (n = 43). Overall, 81 patients developed disease recurrence, and 33 patients died of cancer-related causes. The TB status was categorized into two groups: 99 with low budding (< 5 buds) and 138 with high budding (≥ 5 buds). Overall, the low budding cases demonstrated significantly better DFS. In the 5-FU monotherapy group, low-risk patients (T1, T2, or T3 and N1) with low budding showed a remarkably higher 3-year DFS (91%) compared to high budding (55%).
Conclusion
Our results indicate that TB could play a subsidiary role in selecting patients who could maintain a favorable prognosis with 5-FU monotherapy in stage III CRC.



Similar content being viewed by others
References
Hashiguchi Y, Muro K, Saito Y et al (2019) Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2019 for the treatment of colorectal cancer. Int J Clin Oncol. https://doi.org/10.1007/s10147-019-01485-z
O’Connell MJ, Mailliard JA, Kahn MJ et al (1997) Controlled trial of fluorouracil and low-dose leucovorin given for 6 months as postoperative adjuvant therapy for colon cancer. J Clin Oncol 15(1):246–250
Twelves C, Wong A, Nowacki MP et al (2005) Capecitabine as adjuvant treatment for stage III colon cancer. N Engl J Med 352(26):2696–2704
Grothey A, Sobrero AF, Shields AF et al (2018) Duration of adjuvant chemotherapy for stage III colon cancer. N Engl J Med 378(13):1177–1188
Lieu C, Kennedy EB, Bergsland E et al (2019) Duration of oxaliplatin-containing adjuvant therapy for stage III colon cancer: ASCO clinical practice guideline. J Clin Oncol 37(16):1436–1447
Taieb J, Gallois C (2020) Adjuvant chemotherapy for stage iii colon cancer. Cancers (Basel) 12(9):1–17
Lugli A, Kirsch R, Ajioka Y et al (2017) Recommendations for reporting tumor budding in colorectal cancer based on the International Tumor Budding Consensus Conference (ITBCC) 2016. Mod Pathol 30(9):1299–1311
Maffeis V, Nicolè L, Cappellesso R (2019) RAS, cellular plasticity, and tumor budding in colorectal cancer. Front Oncol. https://doi.org/10.3389/fonc.2019.01255
Beaton C, Twine CP, Williams GL et al (2013) Systematic review and meta-analysis of histopathological factors influencing the risk of lymph node metastasis in early colorectal cancer. Colorectal Dis 15(7):788–797
Cappellesso R, Luchini C, Veronese N et al (2017) Tumor budding as a risk factor for nodal metastasis in pT1 colorectal cancers: a meta-analysis. Hum Pathol 65:62–70
Pai RK, Chen Y, Jakubowski MA et al (2017) Colorectal carcinomas with submucosal invasion (pT1): analysis of histopathological and molecular factors predicting lymph node metastasis. Mod Pathol 30(1):113–122
Lee VWK, Chan KF (2018) Tumor budding and poorly-differentiated cluster in prognostication in stage II colon cancer. Pathol Res Pract 214(3):402–407
Romiti A, Roberto M, Marchetti P et al (2019) Study of histopathologic parameters to define the prognosis of stage II colon cancer. Int J Colorectal Dis 34:905–913
Costas-Chavarri A, Nandakumar G, Temin S et al (2019) Treatment of patients with early-stage colorectal cancer: ASCO resource-stratified guideline. J Glob Oncol 5:1–19
Ueno H, Mochizuki H, Hashiguchi Y et al (2004) Risk factors for an adverse outcome in early invasive colorectal carcinoma. Gastroenterology 127(2):385–394
Yoshida M, Ishiguro M, Ikejiri K et al (2014) S-1 as adjuvant chemotherapy for stage III colon cancer: a randomized phase III study (ACTS-CC trial). Ann Oncol 25(9):1743–1749
Lembersky BC, Wieand HS, Petrelli NJ et al (2006) Oral uracil and tegafur plus leucovorin compared with intravenous fluorouracil and leucovorin in stage II and III carcinoma of the colon: results from National Surgical Adjuvant Breast and Bowel Project protocol C-06. J Clin Oncol 24(13):2059–2064
André T, Boni C, Navarro M et al (2009) Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol 27(19):3109–3116
Haller DG, Tabernero J, Maroun J et al (2011) Capecitabine plus oxaliplatin compared with fluorouracil and folinic acid as adjuvant therapy for stage III colon cancer. J Clin Oncol 29(11):1465–1471
Schmoll HJ, Twelves C, Sun W et al (2014) Effect of adjuvant capecitabine or fluorouracil, with or without oxaliplatin, on survival outcomes in stage III colon cancer and the effect of oxaliplatin on post-relapse survival: a pooled analysis of individual patient data from four randomised controll. Lancet Oncol 15(13):1481–1492
Hoff PM, Saad ED, Costa F et al (2012) Literature review and practical aspects on the management of oxaliplatin-associated toxicity. Clin Colorectal Cancer 11(2):93–100
Ueno H, Murphy J, Jass JR et al (2002) Tumour “budding” as an index to estimate the potential of aggressiveness in rectal cancer. Histopathology 40(2):127–132
Okuyama T, Oya M, Ishikawa H (2003) Budding as a useful prognostic marker in pT3 well- or moderately-differentiated rectal adenocarcinoma. J Surg Oncol 83(1):42–47
Yamadera M, Shinto E, Kajiwara Y et al (2019) Differential survival benefits of 5-fluorouracil-based adjuvant chemotherapy for patients with microsatellite-stable stage III colorectal cancer according to the tumor budding status: a retrospective analysis. Dis Colon Rectum 62(11):1316–1325
Trinh A, Lädrach C, Dawson HE et al (2018) Tumour budding is associated with the mesenchymal colon cancer subtype and RAS/RAF mutations: a study of 1320 colorectal cancers with consensus molecular subgroup (CMS) data. Br J Cancer 119(10):1244–1251
Sabbah M, Emami S, Redeuilh G et al (2008) Molecular signature and therapeutic perspective of the epithelial-to-mesenchymal transitions in epithelial cancers. Drug Resist Updat 11(4–5):123–151
Hwang W, Yang M, Tsai M et al (2011) SNAIL regulates interleukin-8 expression, stem celllike activity, and tumorigenicity of human colorectal carcinoma cells. Gastroenterology 141(1):279–291
Acknowledgements
The authors thank Hisaaki Yoshinaka, Kosuke Ono, Keiso Matsubara and Tetsuya Mochizuki for supporting the medical care and data acquisition.
Author information
Authors and Affiliations
Contributions
SA, WS, YT and HO: designed the study. SA: drafted the manuscript. WS, YT and HE: edited the manuscript. MK, KT, IN and KS: participated in the data acquisition. SA, KS and WY: performed the pathological examination. MH: participated in the statistical work. MH, KS, WY and HO: provided critical revision for the manuscript. All of the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interests to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Akabane, S., Shimizu, W., Takakura, Y. et al. Tumor budding as a predictive marker for 5-fluorouracil response in adjuvant-treated stage III colorectal cancer. Int J Clin Oncol 26, 1285–1292 (2021). https://doi.org/10.1007/s10147-021-01917-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-021-01917-9